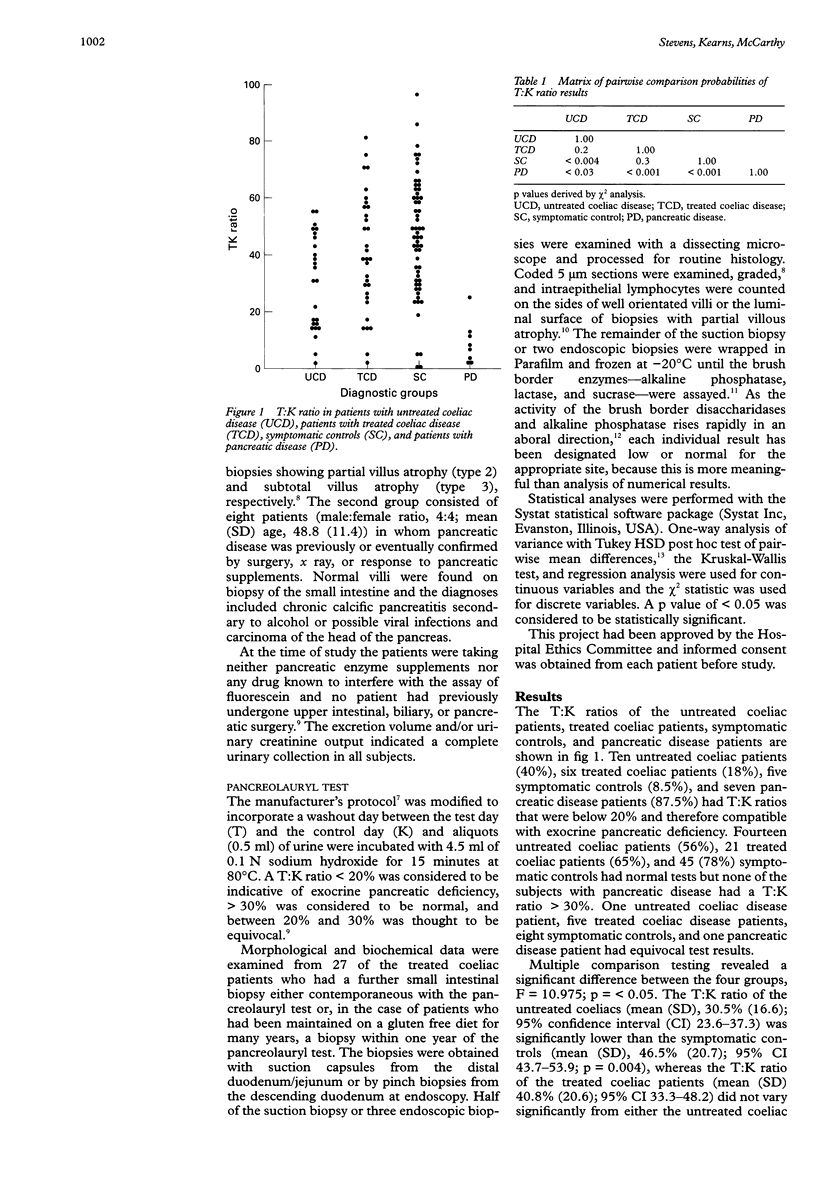
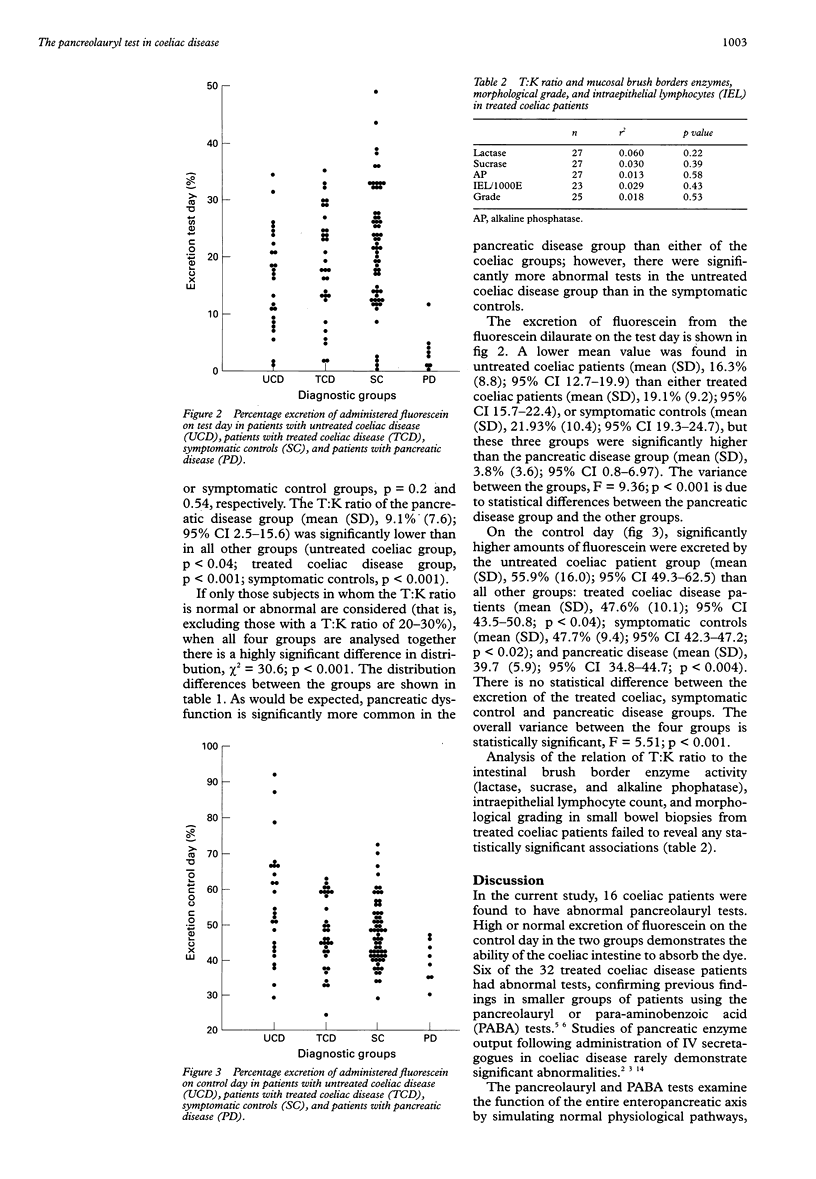
Abstract
AIMS: To determine the frequency of abnormal pancreolauryl tests in untreated and treated adults with coeliac disease and to see whether abnormalities in treated coeliac patients correlate with the degree of recovery of intestinal morphology or brush border enzyme activity. METHODS: Pancreolauryl tests were performed in a study population of 57 adult coeliac patients (25 on gluten containing diets and 32 on gluten free diets), 59 symptomatic controls, and eight patients with pancreatic disease. Brush border enzyme activity and morphological assessment were performed on small intestinal biopsies in 27 of the treated coeliac patients. RESULTS: Forty per cent of untreated coeliac patients and 18% of treated coeliac patients had abnormal tests. In treated coeliac patients, no significant correlation was detected between the pancreolauryl test result and either brush border enzyme activity or morphological parameters. CONCLUSION: Abnormal pancreolauryl test results are common in untreated and treated adult coeliac disease patients. Abnormalities in treated coeliac patients do not correlate with the degree of recovery of small intestinal morphology or brush border enzymes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ansaldi N., Oderda G. Exocrine pancreatic insufficiency in celiac sprue. Gastroenterology. 1981 Apr;80(4):883–883. [PubMed] [Google Scholar]
- Barry R. E., Barry R., Ene M. D., Parker G. Fluorescein dilaurate--tubeless test for pancreatic exocrine failure. Lancet. 1982 Oct 2;2(8301):742–744. doi: 10.1016/s0140-6736(82)90924-2. [DOI] [PubMed] [Google Scholar]
- Carroccio A., Iacono G., Montalto G., Cavataio F., Di Marco C., Balsamo V., Notarbartolo A. Exocrine pancreatic function in children with coeliac disease before and after a gluten free diet. Gut. 1991 Jul;32(7):796–799. doi: 10.1136/gut.32.7.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chey W. Y. Regulation of pancreatic exocrine secretion. Int J Pancreatol. 1991 Summer;9:7–20. doi: 10.1007/BF02925574. [DOI] [PubMed] [Google Scholar]
- Collins B. J., Bell P. M., Boyd S., Kerr J., Buchanan K. D., Love A. H. Endocrine and exocrine pancreatic function in treated coeliac disease. Pancreas. 1986;1(2):143–147. doi: 10.1097/00006676-198603000-00006. [DOI] [PubMed] [Google Scholar]
- DREILING D. A. The pancreatic secretion in the malabsorption syndrome and related malnutrition states. J Mt Sinai Hosp N Y. 1957 May-Jun;24(3):243–250. [PubMed] [Google Scholar]
- Ferguson A., Murray D. Quantitation of intraepithelial lymphocytes in human jejunum. Gut. 1971 Dec;12(12):988–994. doi: 10.1136/gut.12.12.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey K. Statistics in practice. Comparing the means of several groups. N Engl J Med. 1985 Dec 5;313(23):1450–1456. doi: 10.1056/NEJM198512053132305. [DOI] [PubMed] [Google Scholar]
- Gould S. R., Chinn G. L., Nobbs B. T., Lewis G. A. Evaluation of a tubeless pancreatic function test in patients with steatorrhoea in a district general hospital. J R Soc Med. 1988 May;81(5):270–273. doi: 10.1177/014107688808100511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karttunen T., Niemelä S. Lymphocytic gastritis and coeliac disease. J Clin Pathol. 1990 May;43(5):436–437. doi: 10.1136/jcp.43.5.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane R., O'Grady J. G., Sheil J., Stevens F. M., Egan-Mitchell B., McNicholl B., McCarthy C. F., Fottrell P. F. Intestinal lactase, sucrase and alkaline phosphatase in relation to age, sex and site of intestinal biopsy in 477 Irish subjects. J Clin Pathol. 1983 Jan;36(1):74–77. doi: 10.1136/jcp.36.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnestad P., Erichsen A., Fausa O., Flaten O., Hanssen L. E., Schrumpf E. The release of human pancreatic polypeptide, gastrin, gastric inhibitory polypeptide, and somatostatin in celiac disease related to the histological appearance of jejunal mucosa before and 1 year after gluten withdrawal. Scand J Gastroenterol. 1983 Mar;18(2):169–175. doi: 10.3109/00365528309181579. [DOI] [PubMed] [Google Scholar]
- Marsh M. N. Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity ('celiac sprue'). Gastroenterology. 1992 Jan;102(1):330–354. [PubMed] [Google Scholar]
- O'Grady J. G., Stevens F. M., Keane R., Cryan E. M., Egan-Mitchell B., McNicholl B., McCarthy C. F., Fottrell P. F. Intestinal lactase, sucrase, and alkaline phosphatase in 373 patients with coeliac disease. J Clin Pathol. 1984 Mar;37(3):298–301. doi: 10.1136/jcp.37.3.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan P. T., DiMagno E. P. Exocrine pancreatic insufficiency in celiac sprue: a cause of treatment failure. Gastroenterology. 1980 Mar;78(3):484–487. [PubMed] [Google Scholar]
- Scharpé S., Iliano L. Two indirect tests of exocrine pancreatic function evaluated. Clin Chem. 1987 Jan;33(1):5–12. [PubMed] [Google Scholar]
- Weizman Z., Hamilton J. R., Kopelman H. R., Cleghorn G., Durie P. R. Treatment failure in celiac disease due to coexistent exocrine pancreatic insufficiency. Pediatrics. 1987 Dec;80(6):924–926. [PubMed] [Google Scholar]


