Abstract
Chronic stress is a risk factor in the development of cognitive decline and even Alzheimer’s disease (AD), although its underlying mechanism is not fully understood. Our previous data demonstrated that the level of homocysteine (Hcy) was significantly elevated in the plasma of stressed animals, which suggests the possibility that Hcy is a link between stress and cognitive decline. To test this hypothesis, we compared the cognitive function, plasma concentrations of Hcy, and the brain beta-amyloid (Aβ) level between rats with or without chronic unexpected mild stress (CUMS). A lower performance by rats in behavioral tests indicated that a significant cognitive decline was induced by CUMS. Stress also disturbed the normal processing of Aβ precursor protein (APP) and resulted in the accumulation of Aβ in the brains of rats, which showed a positive correlation with the hyperhomocysteinemia (HHcy) that appeared in stressed rats. Hcy-targeting intervention experiments were used to verify further the involvement of Hcy in stress-induced APP misprocessing and related cognitive decline. The results showed that diet-induced HHcy could mimic the cognitive impairment and APP misprocessing in the same manner as CUMS, while Hcy reduction by means of vitamin B complex supplements and betaine could alleviate the cognitive deficits and dysregulation of Aβ metabolism in CUMS rats. Taken together, the novel evidence from our present study suggests that Hcy is likely to be involved in chronic stress-evoked APP misprocessing and related cognitive deficits. Our results also suggested the possibility of Hcy as a target for therapy and the potential value of vitamin B and betaine intake in the prevention of stress-induced cognitive decline.
Keywords: Chronic unexpected mild stress, Homocysteine, Beta-amyloid, Cognitive decline
Introduction
Stressful life events can trigger a cascade of physiological alterations in our body, which enable us to cope with and adapt to novel challenging and unexpected situations. However, when stress response is inadequate or excessive and prolonged, many parts of our physiological systems are negatively affected by long exposures to stress mediators (Sandi 2004). As a key organ of stress reactivity and coping processes, the brain expresses receptors for stress hormones abundantly in regions underpinning cognition and emotion, which also makes the brain the primary target of the negative effects of prolonged stress (McEwen and Gianaros 2010). Accumulating evidence indicates that chronic exposure to stress might have immediate or long-term deleterious effects on brain structure and cognitive function (Lupien et al. 2009; Marin et al. 2011). Severe or sustained stress experiences could induce dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis and has been found to be closely associated with loss of neurons, impairment of synaptic plasticity, and atrophy in the hippocampus complex, leading to the development of cognitive decline and even neuropsychiatric or neurodegenerative disorders (de Kloet et al. 2005; Kim and Diamond 2002). Amnestic cognitive decline is the predominant symptom of Alzheimer’s disease (AD), while mild cognitive impairment is considered as a predementia early stage of AD (Kidd 2008). In this regard, the relationship between stress and AD also attracted many concerns in large numbers of studies. In fact, chronic stress itself was considered a risk factor for the development of AD (Machado et al. 2014). Higher levels of life stressors were proved to be associated with higher incidence of AD (Johansson et al. 2010) and increased susceptibility to AD (Wang et al. 2012). Traumatic stress events were also found to lower the age of AD onset (Rothman and Mattson 2010). Although chronic stress contributes so importantly to the development of cognitive decline and even AD, the mediators and mechanisms underlying the deleterious effects of stress are still not completely understood.
Our previous studies indicated that chronic stress could increase the plasma concentration of homocysteine (Hcy) in rats by down-regulating the expression of cystathionine β-synthase (CBS), a key enzyme of Hcy metabolism (Zhao et al. 2013). We have also reported the association between stress-induced hyperhomocysteinemia (HHcy) and the development of some stress-related diseases, including depression, atherosclerosis, and irritable bowel syndrome (Liu et al. 2009; Xinxing et al. 2014; Zhao and Qian 2014), which suggests that Hcy could be a potential link between stress and the onset of cognitive decline. In fact, HHcy has been proposed as a modifiable risk factor for mild cognitive impairment and even AD in many epidemiological and clinical studies (de Jager 2014; Seshadri 2006). A sixfold increase in the rate of brain volume loss, a definite marker of cognitive decline, was identified in those community-dwelling older people with HHcy (Williams et al. 2002). The risk for dementia and AD would also double in the subjects with plasma Hcy > 15 μmol/L (Ravaglia et al. 2005). The role of Hcy in cognitive decline was also confirmed in animal studies, which demonstrated the anti-Hcy treatment-induced resistance to cognitive impairment and brain pathological changes in transgenic AD mice (Hasegawa et al. 2010). However, it is still unknown whether and how Hcy was involved in stress-induced cognitive decline and even AD.
Beta-amyloid peptides (Aβ) are generated under normal physiological conditions, while its overproduction is responsible for the formation of senile plaques that constitute one of the main neuropathological hallmarks of AD (Querfurth and LaFerla 2010). Aβ is produced through a sequential proteolytic processing of its amyloid precursor protein (APP) by β- and γ-secretases (Hardy and Selkoe 2002) and is removed by the degradation of some proteases, such as insulin-degrading enzyme (IDE) and so on (LaFerla et al. 2007). Disturbance of the normal metabolic processing of APP could lead to a progressive accumulation of Aβ, whose neurotoxic potency was suspected to play a pivotal role in the development of cognitive decline and AD (Catania et al. 2009).
In the present study, we evaluated the relationship between plasma levels of Hcy and cognitive functions in rats that received chronic unexpected mild stress (CUMS) by behavioral and biochemical measurements. Using a CUMS model that contains both physiologically and psychologically stressful stimulations to mimic adverse stress from negative life events, we identified the cognitive decline and brain Aβ accumulation in stressed rats. That implied that although CUMS was not a full-blown AD model, it has some potential links to the very early stages of this neurodegenerative disease. The effects of stress-induced HHcy on the proteolytic processing of APP were also detected by western blots in the present study to reveal the mechanism underlying the Aβ accumulation in the brains of stressed rats. Finally, Hcy-targeting interventions were performed to confirm the involvement of Hcy on stress-induced APP misprocessing and related cognitive deficits.
Materials and methods
Animals
The experiments were performed on male Sprague-Dawley albino rats weighing from 180 to 220 g (purchased from the Laboratory Animal Center of the Beijing Institute for Basic Medical Sciences, IBMS, Beijing, China). The animals were housed in plastic cages with access to food and water ad libitum and maintained on a 12-h light/dark cycle at room temperature (22–26 °C). Behavioral evaluations were carried out between 9:00 and 18:30. The rats were acclimated to test boxes for 5 days before the first testing day and for more than 30 min on each subsequent testing day. The experimental protocols were approved by the Institutional Animal Care and Use Committee of IBMS (Permit Number: 2012-D-3098) and were in accordance with the NIH Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023) and the ARRIVE guidelines from NC3Rs. All of the surgical procedures were performed under sodium pentobarbital (1 %, 50 mg/kg, i.p.) anesthesia, and efforts were made to minimize suffering.
Treatment
CUMS was employed to mimic adverse stress stimulations from negative life events. During the CUMS protocol, rats were raised in isolation (one rat per cage) as part of the protocol. One of the following stressors was administered daily (in random unpredictable order) over a period of 8 weeks: food and water deprivation for 24 h, 45° cage tilt for 24 h, shaker stress (horizontal shakes at high speed, 200 rpm) for 40 min, soiled cage (200 ml water in the sawdust bedding) for 20 h, continuous overnight illumination, restraint in a small tube with breathing holes for 6 h, tail pinch for 5 min, and 4 °C cold swimming for 5 min. Control rats were raised normally (five rats per cage) and were subjected to no stress stimulation. There were ten rats in each group at each time point (weeks 2, 4, 6, and 8).
To evaluate the role of Hcy in stress-induced cognitive decline, 1 % supplementary methionine diets were feed to normal rats (n = 15) to induce HHcy which also appeared in the CUMS situation. The diet was custom-made, prepared by a commercial vendor (Hua-Fu-Kang, China) and matched for calories. Vitamin B and betaine complex (including vitamin B6 24 mg/kg/day, vitamin B12 20 μg/kg/day, folate 10 mg/kg/day, betaine 100 mg/kg/day), which had been proved to remedy HHcy in our previous study (Zhao and Qian 2014), were administrated intragastrically in the control and CUMS rats daily to validate the potential involvement of Hcy in stress-induced cognitive decline (n = 15, respectively). The intragastrically infusion was operated daily by a disposable semi-soft polyethylene catheter tube attached to a 5-ml syringe. All rats were preacclimated to the gentle restraint and handling operations. A skilled researcher was designated to do the intragastrical infusion to minimize the stress from the operation of intragastrically infusion in rats.
Open-field test
Locomotor activity and exploratory behavior were evaluated using an open-field test. The open-field apparatus was a plywood box measuring 100 cm × 100 cm. All of the walls were painted black. The floor was divided into 25 equal squares. The rats were placed individually in one corner of the apparatus, and their ambulation (number of squares crossed) and immobility frequency were observed for 5 min. An open-field test score was calculated as the sum of ambulation and immobility frequency.
Object recognition test
Tests were performed as previously described with minor modification (Ennaceur and Delacour 1988). The open field consisted of a square open field (65 cm × 65 cm × 45 cm) made of black wood. On the day before the experiment, animals were familiarized with the field for 10 min. During the first trial of the experiment, two identical objects equidistant from the sides (10 cm) were placed within the chamber. The animal was placed in the center of the open field and allowed to explore freely for several minutes in 30-s increments of object exploration time, which was defined as the length of time that a rat was sniffing, directing its nose to and pawing the object. During the second 10-min exploration phase, after an interval of 1 h, the rat was returned to the same box, in which a familiar object had been switched to a novel one. To preclude the existence of olfactory cues, each rat had its own lining paper in the box and the paper was wiped thoroughly before each testing. A cognitive index was calculated according to the following formula: (the time exploring the novel object − the time exploring the old object) / the time exploring two objects.
Step-down test
The test was performed in a passive avoidance reaction tank (45 cm × 45 cm × 45 cm) with an energized copper grid bottom. An insulated rubber mat with diameter and height of 8.5 cm was set as the safe platform for rats to avoid electric shocks. After a 3-min acclimation period, 45 V alternating current was applied to train the rats to learn to stay on the platform. After a 24-h interval, the rats were placed on the platform again and an electric current was applied for observation over a 5-min period. The length of time taken for the rats to jump from the safe platform to the copper grid was observed as the step-down latency. The step-down latency and the number of step-downs in 5 min were considered as the memory performance.
Water maze test
The test was performed following the standard procedure with minor modifications (Morris 1984). A black circular plastic tank (120 cm in diameter, 75 cm in depth) with water at 22–25 °C was located in a test room containing various prominent visual cues as in the Morris water maze. Rats were trained to find a hidden platform submerged 2 cm beneath the surface of the water in five consecutive half-days, two trials per half-day with a 30-s interval. If they failed to find the platform within 2 min, they were manually guided to the platform and allowed to remain there for 10 s. Rats were assessed in the probe trial 24 h after the last training session, which consisted of two 3-min free swims in the pool with and without the platform, respectively. The latency to find the hidden platform and the number of entries to the original platform were recorded to evaluate space memory in rats.
Sample preparation for biochemical analysis
After behavioral observation, rats were sacrificed by anesthesia with sodium pentobarbital (1 %, 50 mg/kg, i.p.). Blood was collected from the heart into a precooled anticoagulation tube containing 0.2 ml EDTANa2 solution (60 mg/ml). Plasma was collected after centrifugation (900g in 4 °C for 20 min) and was stored at −80 °C until being measured for Hcy concentration. The whole brain was quickly removed onto an ice plate and washed with cold 0.9 % saline. The cerebral cortex and hippocampus complex were carefully dissected from the brain, homogenized and lysed in an ice-cold RIPA lysis buffer (Applygen Inc., China) of 1 % NP-40, 0.1 % sodium dodecyl sulfate (SDS) in 50 mM Tris-HCl, pH 7.4, and containing a protease inhibitor. Extract was collected with centrifugation for 15 min at 10,000g and was stored at −80 °C for enzyme-linked immune-sorbent assay (ELISA) and Western blot detection.
Measurement of the plasma Hcy
The total plasma Hcy values were determined using high-performance liquid chromatography (HPLC) with fluorimetric detection and isocratic elution (Xinxing et al. 2014). This method involves the reduction of thiol groups, protein precipitation, and derivatization with 7-fluorobenzene-2-oxy-1, 3-diazolic- 4-ammonium sulfate—SBD-F. The HPLC system included a WATERS LC2695 apparatus and a WATERS 2475 fluorescence detector. Chromatographic separation was performed using a C18 model Symmetry Shield RP18 column (3.9 mmi.d. × 150 mm, 5 μm microparticles). The fluorescence of the separated compounds was detected with a detector adjusted for excitation at 390 nm and emission at 470 nm. The Hcy content was calculated with a calibration curve using a known HCT concentration and N-acetyl-L-cysteine as the internal standard.
ELISA for Aβ
Aβ1–40 and Aβ1–42 levels in hippocampal and cerebral extracts were assayed by sensitive sandwich ELISA kits (Wako Chemicals, Richmond, VA) according to the protocol described by the manufacturer (Li et al. 2014).
Immunohistochemistry
Immunostaining was performed as described in our previous study (Xie et al. 2013). Briefly, rats were anesthetized with pentobarbital sodium (100 mg/kg, i.p) and transcardially perfused with 0.9 % saline, followed by 4 % paraformaldehyde in 0.1 M phosphate-buffered saline (PBS). Whole brain was dissected, post-fixed at 4 °C for additional 90 min in the same fixative solution, and transferred into 30 % sucrose 0.01 M PBS overnight. Coronal frozen sections at 40 μm of thickness were cut on CM1900 freezing microtome (Leica, Germany), incubated for 1 h in 0.05 % Triton X-100 and 10 % goat serum in 0.01 M PBS at room temperature, followed by incubation with anti-Aβ1–39 (1:200, Cell Signal, Boston, MA) at 4 °C overnight. After three washes with PBS, sections were incubated with biotinylated secondary antibodies and then developed by using the avidin-biotin complex method (ZSGQ-Bio Inc., China) with 3,3′-diaminibenzidine as a chromogen. Micrographic images were obtained under a microscope (Olympus, Japan) with an auto-exposure option.
Western blot analysis
Hippocampal and cortical extracts were used for Western blot analysis as previously described (Xie et al. 2013). Briefly, protein samples (40 μg) were electrophoresed by 10 % SDS-PAGE gel and transferred onto polyvinylidene difluoride membrane with a SDidry Transfer System (Bio-Rad, USA). Membranes were blocked at room temperature for 1 h at 5 % milk in PBS containing 0.2 % Tween-20, followed by incubation with an appropriate primary antibody. After three washes with PBS, membranes were incubated with HPR-labeled secondary antibodies (ZSGQ-Bio Inc.) at room temperature for 2 h. Membranes were developed with Pierce ECL Western blotting substrate kit (Thermo Scientific, USA) and the signals were captured with an ImageQuant LAS 4000 (GE, USA). Images were analyzed by Image Pro-Plus 6.0, and the GAPDH was used as an internal loading control.
Data analysis
All data were shown as mean ± SD. Parametric one-way ANOVA followed by Fisher’s PLSD post hoc analysis were used for comparisons between groups. Correlation between the brain Aβ level and the plasma concentration of Hcy was analyzed by Pearson’s coefficients, and multiple comparisons were corrected by Bonferroni’s analysis. All analyses were performed using the SPSS statistical package, and P < 0.05 was considered to be significantly different.
Results
Chronic stress induced time-dependent cognitive decline in rats
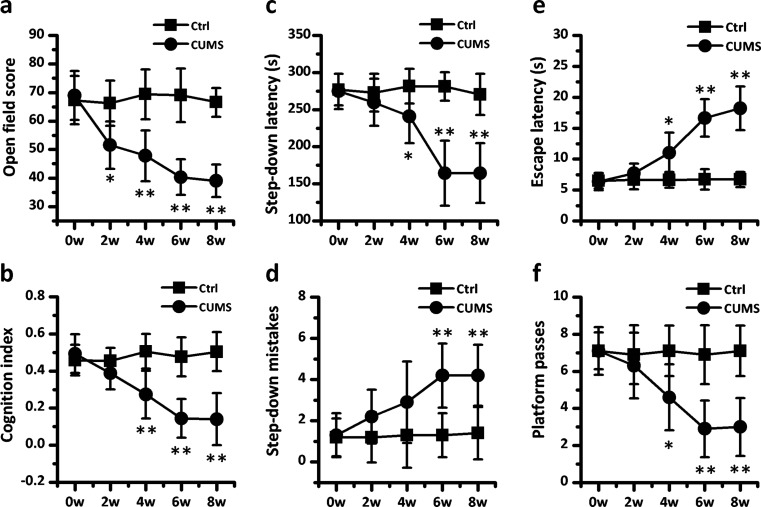
To investigate the effect of chronic stress on cognition in rats, we chose the CUMS model to mimic adverse stress stimulation from negative life events. Four different behavioral paradigms were employed to evaluate the alteration of cognitive function in rats (Fig. 1). Exploring behavior was assessed in an open-field test, where we observed that the open-field score of rats decreased statistically beginning from the second week of CUMS modeling. The open-field score dropped nearly 42 % in 6- and 8-week CUMS rats. In an object recognition test, CUMS rats had a lower cognition index than the control group beginning with the fourth week. We examined the passive avoidance memory function of rats by means of a step-down test, in which shortened step-down latencies and increased numbers of mistakes were identified in rats that had received over 4 or over 6 weeks CUMS, respectively. Finally, in a Morris water maze test, the effect of stress on spatial memory function was evaluated. All rats in the control and CUMS groups were similarly proficient swimmers and showed a gradual improvement in finding the hidden platform within the training period (data not shown). However, in the probe test, more than 4 weeks of CUMS induced a significant delay in escape latency and a decrease in the number of platform location crosses.
Fig. 1.
Cognitive decline can be induced by chronic stress in rats. a The open-field scores were calculated as the sum of ambulation and immobility frequency in rats receiving no (Ctrl) or weeks of chronic unexpected mild stress (CUMS). b The cognition index for novel objective reorganization was measured in Ctrl and CUMS rats. c, d The step-down latencies and the number of step-down mistakes were used to evaluate passive avoidance memory in the two groups of rats. e, f The escape latency and the number of platform passes for rats in a Morris water maze test were measured to estimate spatial memory in Ctrl and CUMS rats after five half-day training sessions. Values represent mean ± SD, n = 10; * P < 0.05, ** P < 0.01
Chronic stress induced APP misprocessing and accumulation of Aβ in the brains of rats
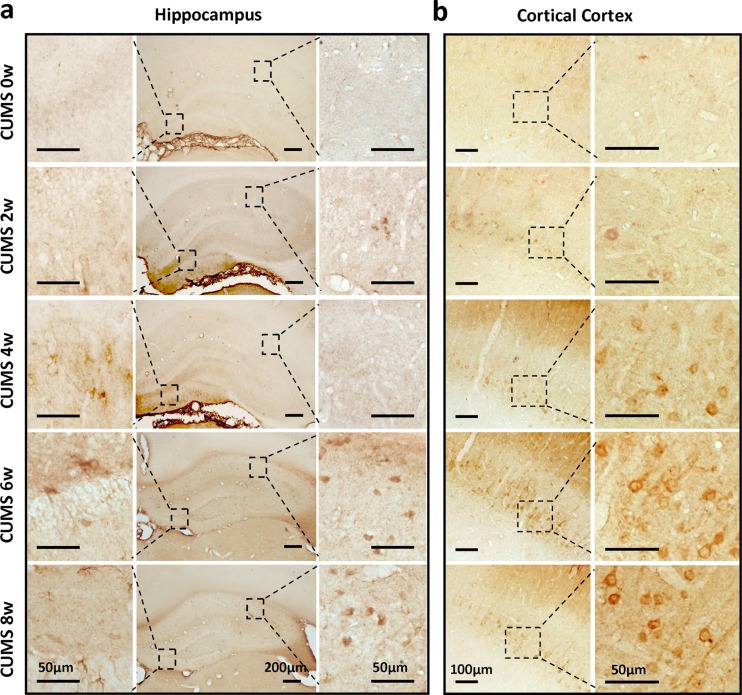
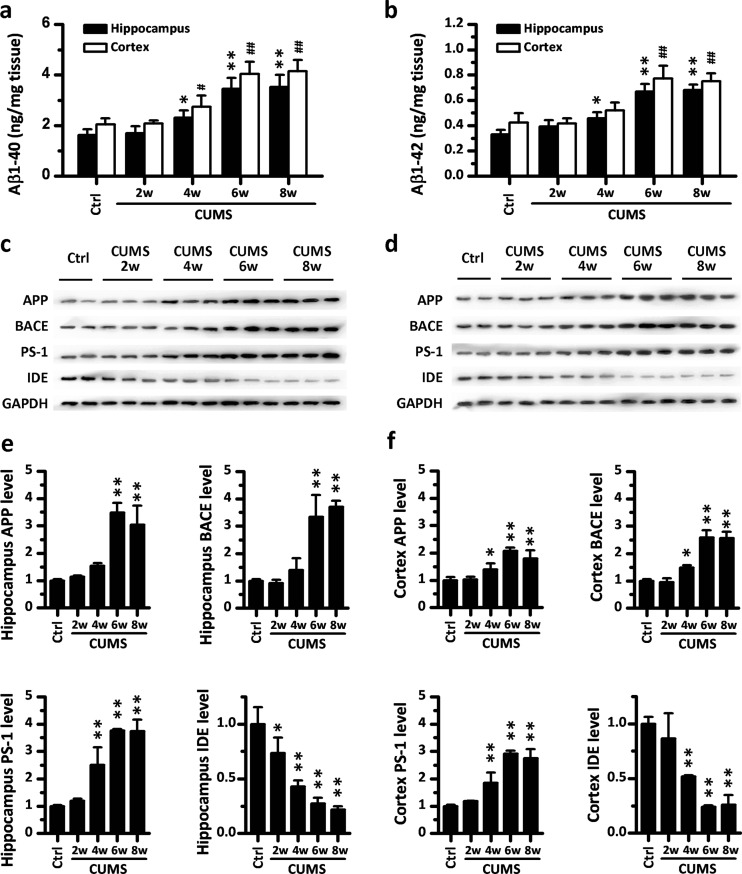
To reveal the potential changes in the brain underlying stress-induced cognitive decline, immunohistochemical staining for Aβ was performed on sections of rat hippocampus structure and cerebral cortex. As shown in Fig. 2a, b, we found that compared with the control group, CUMS could induce a time-dependent up-regulation and mild accumulation of Aβ in the brains of rats. In the hippocampus, an increased Aβ by CUMS was mainly distributed in the cell bodies, which showed in the small round staining in the hippocampal cornu ammonis 1 (CA1) and neuroglia-like staining in the dentate gyrus. In the cerebral cortex, however, elevated Aβ immunopositive staining mainly appeared in the pyramid neuron within layer IV. In order to confirm that CUMS induced an increase of Aβ in the brain, we also measured the level of Aβ1–40 and Aβ1–42 in hippocampal and cerebral extracts using an ELISA assay, which showed a significantly higher level of Aβ1–40 and Aβ1–42 in the homogenate of both the hippocampus and cerebral cortex of rats receiving 6 or 8 weeks of CUMS (Fig. 3a, b).
Fig. 2.
Chronic unexpected mild stress (CUMS) induced the up-regulation of β-amyloid (Aβ) in the hippocampus structure and cerebral cortex of rats. a Immunohistochemical detection for Aβ in the sections of hippocampus structure from CUMS rats. Scale bar = 200 μm in lower magnification images; scale bar = 50 μm in higher magnification images. b Representative images of cerebral cortex sections from CUMS rats with antibody to Aβ. Scale bar = 100 μm in lower magnification images; scale bar = 50 μm in higher magnification images
Fig. 3.
Chronic unexpected mild stress (CUMS) evokes APP misprocessing in the hippocampus and cerebral cortex of rats. a, b The levels of Aβ 1–40 and Aβ 1–42 in the hippocampus and brain cortex of CUMS rats (n = 6) were measured by a sandwich enzyme-linked immunosorbent assay. c, d Representative Western blots of antibodies APP, BACE, PS-1, IDE, and GAPDH in the hippocampus and cortex homogenates from rats in CUMS are shown, respectively. e, f Densitometry analyses of the immunoreactivities to antibodies was shown in the previous panel. Values represent mean ± SD, n = 3; * P < 0.05, ** P < 0.01
To investigate the mechanism responsible for the up-regulation of Aβ, we examined the expression of its precursor, the Aβ precursor protein (APP), and the key metabolic enzymes of Aβ, including β-secretase (β-site APP-cleaving enzyme, BACE), γ-secretase component (presenilin, PS-1), and IDE, using Western blot analyses. The results indicated that CUMS alters APP processing in a time-dependent manner, and all the molecules concerned showed statistical changes in expression after 6 weeks of CUMS (Fig. 3c–f). In detail, six or more weeks of CUMS induced a significant increase in the level of hippocampal and cerebral APP, BACE, and PS-1, while the expression of IDE was down-regulated by more than 4 weeks of CUMS stimulation both in the hippocampus and in the cerebral cortex.
HHcy appeared in CUMS rats and showed a positive correlation with levels of Aβ in the brain
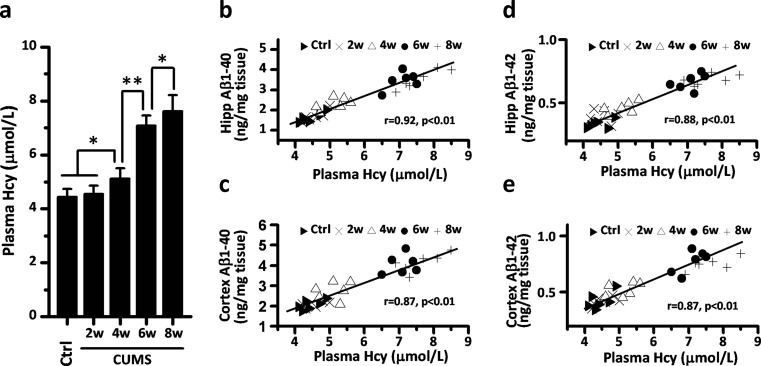
First, we wanted to check whether CUMS administrated to the rats could result in higher levels of plasma Hcy. The HPLC results confirmed that a time-dependent elevation of Hcy level in plasma could be induced by weeks of CUMS (Fig. 4a). Compared with the control group, the concentrations of plasma Hcy in 6- and 8-week CUMS rats increased 59.8 and 72.0 %, respectively (both P < 0.01). Next, we investigated whether stress-induced HHcy was correlated with the accumulation of Aβ in the brain by Pearson’s correlation analyses. The results are shown in Fig. 4b–e, which indicated that both the hippocampal and the cerebral levels of Aβ, irrespective of the levels of Aβ1–40 or Aβ1–42, were positively correlated with the concentration of Hcy in plasma (all correlation coefficients were greater than 0.87, and all P values were smaller than 0.01).
Fig. 4.
The up-regulation of β-amyloid (Aβ) in the brains of stressed rats is correlated with chronic unexpected mild stress (CUMS)-induced hyperhomocysteinemia. a The level of plasma homocysteine (Hcy) in CUMS rats was measured by high-performance liquid chromatography. Values represent mean ± SD, n = 6; * P < 0.05, ** P < 0.01. b–e The positive correlation between the increase of plasma Hcy levels and the up-regulation of β-amyloid (Aβ) 1–40, Aβ 1–42 in the hippocampus and cerebral cortex, respectively, was analyzed by Pearson correlations
Hcy-targeting intervention mimicked or reversed stress-induced cognitive deficits
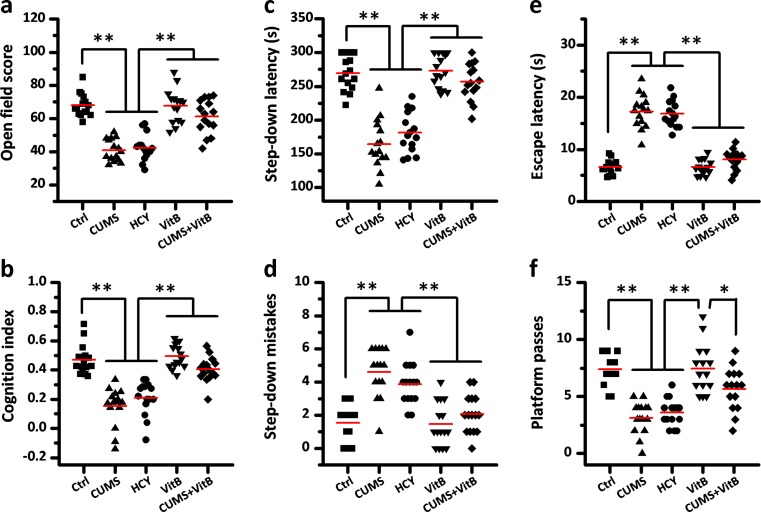
To evaluate the role of Hcy on stress-induced cognitive decline, we employed Hcy-targeting intervention to control the level of Hcy in rats’ plasma. Based on the results of CUMS modeling, 6 weeks was set as the length of time for our next experiments. Rats were divided randomly into five groups. In addition to the control and CUMS rats, the HCY group received 6 weeks of diet with 1 % supplementary methionine to mimic the CUMS-induced HHcy. Vitamin B complex (including vitamin B6 24 mg/kg/day, vitamin B12 20 μg/kg/day, folate 10 mg/kg/day, betaine 100 mg/kg/day) was administrated intragastrically to naïve rats (VitB group) and CUMS rats (CUMS + VitB group) for HHcy remedy. Measurement for the level of plasma Hcy among the groups confirmed the effectiveness of Hcy-targeting intervention. Compared to the level of plasma Hcy in the control group (4.43 ± 0.30 μmol/L), both 6 weeks of CUMS and 6 weeks of methionine supplementation diet induced significant HHcy in rats. The plasma Hcy levels in the CUMS and HCY groups were 7.37 ± 0.44 and 8.18 ± 0.61 μmol/L, respectively (both P < 0.01 vs. control). Vitamin B complex administration decreased the level of Hcy both in naïve and CUMS rats, which were 3.99 ± 0.30 μmol/L (P < 0.05 VitB vs. control) and 5.30 ± 0.61 μmol/L (P < 0.01 CUMS + VitB vs. CUMS).
Open-field, object recognition, step-down, and water maze tests were performed to measure the changes in cognitive functions. As shown in Fig. 5, we observed that HHcy (which was induced by 6 weeks of methionine supplementation diet) led to similar behavioral changes as those in rats receiving 6 weeks of CUMS, including a lower open-field score, cognition index, step-down latency,and water maze platform passes, and elevated step-down mistakes and water maze escaping latency. Compared with CUMS group, vitamin B complex supplementation in CUMS rats reversed the CUMS-induced behavioral alterations almost to the control normal. However, compared with the control group, no statistical changes in behavior were caused by vitamin B complex supplementation in naïve rats.
Fig. 5.
Homocysteine-targeting intervention mimics or reverses stress-induced cognitive decline in rats. a The open-field scores were calculated as the sum of ambulation and immobility frequency in rats. b The cognition index for novel objective reorganization was measured in all groups. c, d The step-down latencies and the number of step-down mistakes were used to evaluate the learning and memory of fear in the five groups of rats. e, f The escape latency and the number of platform passes for rats in a Morris water maze test were measured to estimate the space memory in all rats after five half-day trainings. The gray short lines represent means, n = 15; * P < 0.05, ** P < 0.01
Hcy-targeting intervention mimicked or reversed stress-induced APP misprocessing
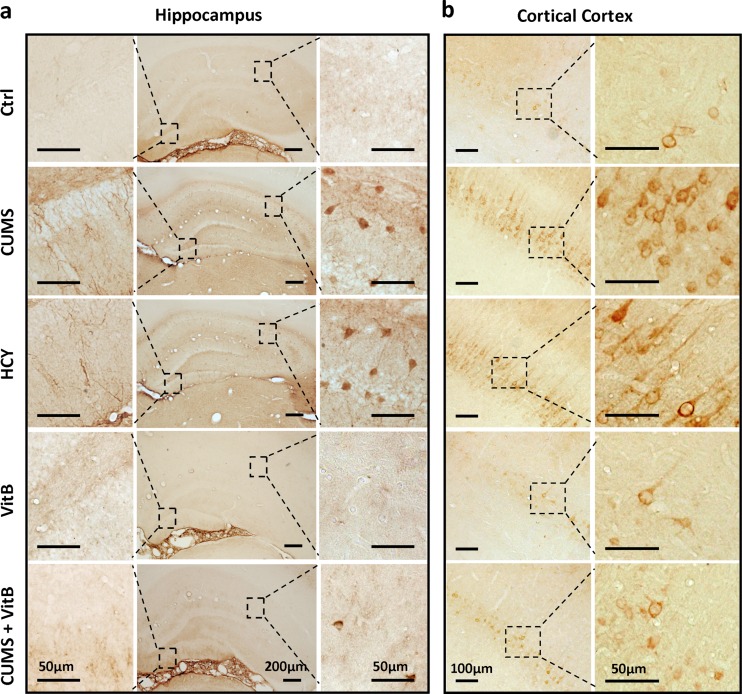
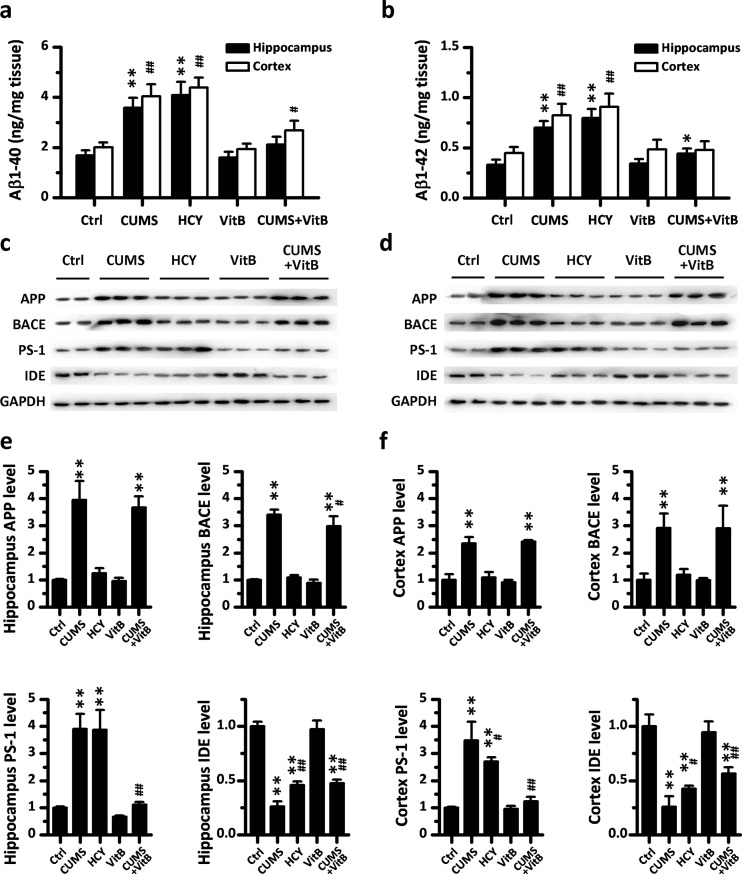
To study the mechanisms underlying the role of Hcy on stress-induced cognitive deficits, we next examined the effects of Hcy-targeting intervention on Aβ accumulation in rats’ brains. The immunohistochemistry for Aβ revealed that the area of Aβ immunopositive staining in the hippocampus and cerebral cortex enlarged significantly in 6-week CUMS rats and 6-week diet-induced HHcy rats (Fig. 6). Compared with the CUMS group, vitamin B complex supplementation in CUMS rats alleviated the CUMS-induced accumulation of Aβ immunostaining in the brains of rats (Fig. 6). The results of an ELISA assay for Aβ showed statistically higher levels of brain Aβ1–40 and Aβ1–42 in the HHcy group rats, which was similar to results in the CUMS rats (Fig. 7a, b). Vitamin B complex supplementation reduced the level of Aβ1–40 and Aβ1–42 significantly in the brain of the CUMS group, but had no Aβ-reducing effect in naïve rats.
Fig. 6.
Chronic unexpected mild stress (CUMS)-induced accumulation of β-amyloid (Aβ) in the brains of rats is reduced by homocysteine (Hcy) control. a Immunohistochemical detection for Aβ in the sections of hippocampus structure from rats in five groups. Scale bar = 200 μm in lower magnification images; scale bar = 50 μm in higher magnification images. b Representative images of cerebral cortex sections from rats with antibody to Aβ. Scale bar = 100 μm in lower magnification images; scale bar = 50 μm in higher magnification images
Fig. 7.
APP processing in rats’ hippocampus and cerebral cortex is regulated by homocysteine-targeting intervention. a, b The level of Aβ 1–40 and Aβ 1–42 in the hippocampus and brain cortex of five groups of rats (n = 10) were measured by a sandwich enzyme-linked immunosorbent assay. c, d Representative Western blots of antibodies APP, BACE, PS-1, IDE, and GAPDH in hippocampus and cortex homogenates from rats in five groups were shown respectively. e, f Densitometry analyses of the immunoreactivities to antibodies were shown in the previous panel. Values represent mean ± SD, n = 3; * P < 0.05, ** P < 0.01
We also examined the APP processing using Western blot analyses (Fig. 7c–f) The results indicated that the rats with diet-induced HHcy had an increase in the level of PS-1 and a decrease in the level of IDE, which was similar to the situation in CUMS rats. However, the expression of APP and BACE, which was also up-regulated in CUMS rats, was not influenced by HHcy. Vitamin B complex supplementation reversed the expressed alterations of PS-1 and IDE in CUMS rats, whereas the expressions of APP and BACE were unchanged. In the rats without CUMS stimulation, no effect of vitamin B complex supplementation on the expression of APP and metabolic enzymes of Aβ was found.
Discussion
Although stress has been proved to be a strong risk factor for cognitive decline and even AD by numerous epidemiological studies (Machado et al. 2014; Seshadri 2006), the mechanisms underlying it are still not fully understood. Our previous study showed that the dysregulation of Hcy metabolism could be induced by chronic stress, and this mechanism played an important role in the development of some stress-associated diseases (Xinxing et al. 2014; Zhao et al. 2013). Therefore, we speculate that stress-induced HHcy could be the potential link between stress and cognitive decline and even AD. To test this hypothesis, we explored the role of Hcy in cognitive changes using the CUMS rat model in the current study.
One of the interesting aspects of the present findings was that six or more weeks of CUMS could induce the APP misprocessing and related cognitive decline in rats, which was suggested by both the changes in behavior and the accumulation of Aβ in the hippocampus and cerebral cortex. As a commonly used psychological stress model, CUMS had been reported to impair the cognitive performance of rats or mice in a water maze test (Gu et al. 2014; Liu et al. 2014), novelty recognition test (Liu et al. 2014), and passive avoidance test (Zhang et al. 2012). Furthermore, chronic stress had also been shown to induce Aβ accumulation and plaque deposits in the brain of transgenic AD model mice (Rothman et al. 2012; Seo et al. 2011). However, the evidence for the up-regulation of Aβ in stressed non-transgenic normal rodents was rarely reported. Some clues came from the deposits of Aβ in the brain of stress-level glucocorticoid-treated wild-type mice (Wang et al. 2011) and rats (Catania et al. 2009; Martisova et al. 2012). Our study showed that the level of Aβ could be up-regulated by CUMS, which was similar to the data reported in stress-induced anhedonia rats (Briones et al. 2012). These results added some new support to the causative effects of stress in the development of cognitive decline and AD. However, we must emphasize that only mild accumulation of Aβ occurred, and no plaque was found in the brains of our CUMS rats. This means that CUMS-induced cognitive decline is an early-stage alteration and is very far from the actual AD. It is unclear whether senile plaques could be induced by long enough stress in aged rats, and further studies are needed to assess it.
Although several potential mechanisms responsible for the deleterious effects of chronic stress on cognitive function have been proposed, its roles in Aβ pathogenesis and the development of cognitive decline remain to be fully investigated. The major results of the present work highlight the involvement of Hcy in chronic stress-induced APP misprocessing and related cognitive decline. The results from the Hcy-targeting intervention experiment strongly suggested that the stress-induced HHcy and subsequent up-regulation of Aβ could be one of the mechanisms of stress affecting the development of cognitive decline. A similar role for Hcy in Aβ pathogenesis has also been proved in transgenic AD mice with diet-induced HHcy (Li et al. 2014) and in normal rats receiving daily Hcy injections (Chai et al. 2013; Zhang et al. 2009). These studies reported that Hcy could disturb the metabolism of Aβ and induce Aβ accumulation and cognition impairment both in AD or normal rodents. Here, our discovery showed evidence for the first time that Hcy is a potential mediating factor of adverse stress effects on cognition. On the other hand, stress is an extremely complex biological process and can affect nearly every system of our body through the non-specific effects of stress hormones. Thus, it seems impossible to imagine that HHcy is the only mediating factor of stress effects on cognition. In fact, we noticed that diet-induced HHcy failed to mimic the increase of APP and BACE caused by CUMS, which indicated that even just concerning Aβ pathogenesis, other mechanisms exist in stress-induced cognitive decline besides the involvement of Hcy.
It is well known that Aβ is produced by the cutting of APP by β- and γ-secretase (Hardy and Selkoe 2002) and is removed by the degradation of some proteases, such as IDE (LaFerla et al. 2007). Our results showed that CUMS induced the up-regulation of APP, BACE, PS-1, and the down-regulation of IDE, which indicated APP misprocessing. Hcy-targeting intervention mimicked or reversed the expression alteration of PS-1 in CUMS rats, and partially mimicked or reversed the change of IDE, while the APP and BACE remained unaffected. These results indicated that the PS-1 could probably be the target of Hcy effects in stress situations and that IDE could also partially be regulated by Hcy levels. It also suggested that stress has non-Hcy mediators to control the expression of APP, BACE, and even IDE. Our observation is in line with previous reports showing that elevated Hcy could increase Aβ production via the enhanced expression of γ-secretase and cause memory deficits in rodents or humans (Hasegawa et al. 2012; Li et al. 2014; Zhang et al. 2009). A previous paper showed no significant change of IDE and other Aβ catabolic enzymes in Tg2576 mice receiving a diet deficient in folate and vitamin Bs (Zhuo and Pratico 2010). The reasons for the discrepancy between this report and our data are not fully understood, but are likely attributed to the difference in animal models and species. Given that Hcy intervention had only a partial effect on the expression of IDE in our study, the role of IDE in stress-induced Aβ elevation needs to be explored in future studies.
Hcy is produced as an intermediate of methionine metabolism, whose normal level in the body is maintained by its catabolism depending on folate, vitamin B12, B6, and betaine (Obeid and Herrmann 2006). In the present study, we fed rats with supplementary vitamin B complex and betaine to remedy the stress-induced HHcy. The results indicated that the benefits of vitamin B and betaine to cognition were stress-specific and Hcy-dependent. Numerous studies have focused on vitamin B status and its relationship to cognitive decline, and some of them have shown evidence that vitamin B can prevent or delay cognitive decline (Cacciapuoti 2013; de Jager 2014). However, confusing and conflicting results from these studies made it difficult to summarize the role of vitamin B (Morris 2012). The stress-specific benefits of vitamin B that appeared in our experiment provide some new clues to interpreting the conflict in the vitamin B and cognitive decline literature. Our results also suggested the potential value of vitamin B and betaine intake in the prevention of stress-associated cognitive decline.
There were several limitations of the present study. First, only plasma Hcy was measured here, and the alteration of Hcy level in cerebrospinal fluid was unknown. Because Hcy can penetrate the blood-brain barrier through carrier-mediated transport (Kamath et al. 2006), we therefore speculated that the Hcy in the cerebrospinal fluid went through changes similar to those in plasma. However, further studies would be warranted to assess it. Second, considering that CUMS is also a commonly used animal model for depression (Chengfeng et al. 2014), we cannot exclude the interference of emotional effects on cognition in our study. Further studies are necessary to explore the interactions between emotion and cognition, and we would also like to check the role of Hcy in other stress models with fewer components of emotional changes.
In summary, our studies establish for the first time to our knowledge a key role for Hcy in the adverse effects of stress on cognitive function. The PS-1-associated Aβ metabolic dysregulation could be the target of Hcy, which is likely to contribute to the development of stress-related cognitive decline. Our data also suggest that supplementary vitamin B and betaine intake could be a valid approach for individuals controlling their stress-associated risk factors for cognitive decline.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No. 81401041, No. 81302602, No. 31571173, No. 31400948, and No. 81500454).
Abbreviations
- Aβ
beta-amyloid peptides
- AD
Alzheimer’s disease
- APP
Aβ precursor protein
- BACE
β-site APP-cleaving enzyme
- CBS
cystathionine β-synthase
- CUMS
chronic unexpected mild stress
- Hcy
homocysteine
- HHcy
hyperhomocysteinemia
- HPA
hypothalamic pituitary adrenal
- IDE
insulin-degrading enzyme
- PS-1
presenilin
- VitB
vitamin B
Compliance with ethical standards
Conflicts of interest
The authors declare that they have no competing interests.
Footnotes
Fang Xie and Yun Zhao contributed equally to this work.
References
- Briones A, et al. Stress-induced anhedonia is associated with an increase in Alzheimer’s disease-related markers. Br J Pharmacol. 2012;165:897–907. doi: 10.1111/j.1476-5381.2011.01602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacciapuoti F. Lowering homocysteine levels with folic acid and B-vitamins do not reduce early atherosclerosis, but could interfere with cognitive decline and Alzheimer’s disease. J Thromb Thrombolysis. 2013;36:258–262. doi: 10.1007/s11239-012-0856-x. [DOI] [PubMed] [Google Scholar]
- Catania C, Sotiropoulos I, Silva R, Onofri C, Breen KC, Sousa N, Almeida OF. The amyloidogenic potential and behavioral correlates of stress. Mol Psychiatry. 2009;14:95–105. doi: 10.1038/sj.mp.4002101. [DOI] [PubMed] [Google Scholar]
- Chai GS, et al. Betaine attenuates Alzheimer-like pathological changes and memory deficits induced by homocysteine. J Neurochem. 2013;124:388–396. doi: 10.1111/jnc.12094. [DOI] [PubMed] [Google Scholar]
- Chengfeng S, Wei L, Xinxing W, Lei W, Rui Z, Lingjia Q. Hyperhomocysteinemia is a result, rather than a cause, of depression under chronic stress. PLoS One. 2014;9:e106625. doi: 10.1371/journal.pone.0106625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jager CA. Critical levels of brain atrophy associated with homocysteine and cognitive decline. Neurobiol Aging. 2014;35(Suppl 2):S35–S39. doi: 10.1016/j.neurobiolaging.2014.03.040. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: behavioral data. Behav Brain Res. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-X. [DOI] [PubMed] [Google Scholar]
- Gu HF, et al. Epigallocatechin-3-gallate attenuates impairment of learning and memory in chronic unpredictable mild stress-treated rats by restoring hippocampal autophagic flux. PLoS One. 2014;9:e112683. doi: 10.1371/journal.pone.0112683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Hasegawa T, et al. Urinary homocysteic acid levels correlate with mini-mental state examination scores in Alzheimer’s disease patients. J Alzheimers Disease JAD. 2012;31:59–64. doi: 10.3233/JAD-2012-120022. [DOI] [PubMed] [Google Scholar]
- Hasegawa T, Mikoda N, Kitazawa M, LaFerla FM. Treatment of Alzheimer’s disease with anti-homocysteic acid antibody in 3xTg-AD male mice. PLoS One. 2010;5:e8593. doi: 10.1371/journal.pone.0008593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson L, Guo X, Waern M, Ostling S, Gustafson D, Bengtsson C, Skoog I. Midlife psychological stress and risk of dementia: a 35-year longitudinal population study. Brain J Neurol. 2010;133:2217–2224. doi: 10.1093/brain/awq116. [DOI] [PubMed] [Google Scholar]
- Kamath AF, Chauhan AK, Kisucka J, Dole VS, Loscalzo J, Handy DE, Wagner DD. Elevated levels of homocysteine compromise blood-brain barrier integrity in mice. Blood. 2006;107:591–593. doi: 10.1182/blood-2005-06-2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd PM. Alzheimer’s disease, amnestic mild cognitive impairment, and age-associated memory impairment: current understanding and progress toward integrative prevention. Altern Med Rev J Clin Ther. 2008;13:85–115. [PubMed] [Google Scholar]
- Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nat Rev Neurosci. 2002;3:453–462. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- LaFerla FM, Green KN, Oddo S. Intracellular amyloid-beta in Alzheimer’s disease. Nat Rev Neurosci. 2007;8:499–509. doi: 10.1038/nrn2168. [DOI] [PubMed] [Google Scholar]
- Li JG, Chu J, Barrero C, Merali S, Pratico D. Homocysteine exacerbates beta-amyloid pathology, tau pathology, and cognitive deficit in a mouse model of Alzheimer disease with plaques and tangles. Ann Neurol. 2014;75:851–863. doi: 10.1002/ana.24145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, et al. Resveratrol prevents impaired cognition induced by chronic unpredictable mild stress in rats. Prog Neuro-Psychopharmacol Biol Psychiatry. 2014;49:21–29. doi: 10.1016/j.pnpbp.2013.10.017. [DOI] [PubMed] [Google Scholar]
- Liu X, et al. Proteomic analysis of homocysteine induced proliferation of cultured neonatal rat vascular smooth muscle cells. Biochim Biophys Acta. 2009;1794:177–184. doi: 10.1016/j.bbapap.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Machado A, et al. Chronic stress as a risk factor for Alzheimer’s disease. Rev Neurosci. 2014;25:785–804. doi: 10.1515/revneuro-2014-0035. [DOI] [PubMed] [Google Scholar]
- Marin MF, et al. Chronic stress, cognitive functioning and mental health. Neurobiol Learn Mem. 2011;96:583–595. doi: 10.1016/j.nlm.2011.02.016. [DOI] [PubMed] [Google Scholar]
- Martisova E, Solas M, Gerenu G, Milagro FI, Campion J, Ramirez MJ. Mechanisms involved in BACE upregulation associated to stress. Curr Alzheimer Res. 2012;9:822–829. doi: 10.2174/156720512802455368. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Gianaros PJ. Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Ann N Y Acad Sci. 2010;1186:190–222. doi: 10.1111/j.1749-6632.2009.05331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris MS. The role of B vitamins in preventing and treating cognitive impairment and decline. Adv Nutr. 2012;3:801–812. doi: 10.3945/an.112.002535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Obeid R, Herrmann W. Mechanisms of homocysteine neurotoxicity in neurodegenerative diseases with special reference to dementia. FEBS Lett. 2006;580:2994–3005. doi: 10.1016/j.febslet.2006.04.088. [DOI] [PubMed] [Google Scholar]
- Querfurth HW, LaFerla FM. Alzheimer’s disease. N Engl J Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- Ravaglia G, et al. Homocysteine and folate as risk factors for dementia and Alzheimer disease. Am J Clin Nutr. 2005;82:636–643. doi: 10.1093/ajcn.82.3.636. [DOI] [PubMed] [Google Scholar]
- Rothman SM, et al. 3xTgAD mice exhibit altered behavior and elevated Abeta after chronic mild social stress. Neurobiol Aging. 2012;33:830. doi: 10.1016/j.neurobiolaging.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman SM, Mattson MP. Adverse stress, hippocampal networks, and Alzheimer’s disease. Neruomol Med. 2010;12:56–70. doi: 10.1007/s12017-009-8107-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandi C. Stress, cognitive impairment and cell adhesion molecules. Nat Rev Neurosci. 2004;5:917–930. doi: 10.1038/nrn1555. [DOI] [PubMed] [Google Scholar]
- Seo JS, Lee KW, Kim TK, Baek IS, Im JY, Han PL. Behavioral stress causes mitochondrial dysfunction via ABAD up-regulation and aggravates plaque pathology in the brain of a mouse model of Alzheimer disease. Free Radic Biol Med. 2011;50:1526–1535. doi: 10.1016/j.freeradbiomed.2011.02.035. [DOI] [PubMed] [Google Scholar]
- Seshadri S. Elevated plasma homocysteine levels: risk factor or risk marker for the development of dementia and Alzheimer’s disease? J Alzheimers Dis JAD. 2006;9:393–398. doi: 10.3233/jad-2006-9404. [DOI] [PubMed] [Google Scholar]
- Wang HX, Wahlberg M, Karp A, Winblad B, Fratiglioni L. Psychosocial stress at work is associated with increased dementia risk in late life. Alzheimers Dement J Alzheimers Assoc. 2012;8:114–120. doi: 10.1016/j.jalz.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Wang Y, et al. Glucocorticoids facilitate astrocytic amyloid-beta peptide deposition by increasing the expression of APP and BACE1 and decreasing the expression of amyloid-beta-degrading proteases. Endocrinology. 2011;152:2704–2715. doi: 10.1210/en.2011-0145. [DOI] [PubMed] [Google Scholar]
- Williams JH, Pereira EA, Budge MM, Bradley KM. Minimal hippocampal width relates to plasma homocysteine in community-dwelling older people. Age Ageing. 2002;31:440–444. doi: 10.1093/ageing/31.6.440. [DOI] [PubMed] [Google Scholar]
- Xie F, Zhang JC, Fu H, Chen J. Age-related decline of myelin proteins is highly correlated with activation of astrocytes and microglia in the rat CNS. Int J Mol Med. 2013;32:1021–1028. doi: 10.3892/ijmm.2013.1486. [DOI] [PubMed] [Google Scholar]
- Xinxing W, Wei L, Lei W, Rui Z, Baoying J, Lingjia Q. A neuroendocrine mechanism of co-morbidity of depression-like behavior and myocardial injury in rats. PLoS One. 2014;9:e88427. doi: 10.1371/journal.pone.0088427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang CE, et al. Hyperhomocysteinemia increases beta-amyloid by enhancing expression of gamma-secretase and phosphorylation of amyloid precursor protein in rat brain. Am J Pathol. 2009;174:1481–1491. doi: 10.2353/ajpath.2009.081036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZH, LN W, Song JG, Li WQ. Correlations between cognitive impairment and brainderived neurotrophic factor expression in the hippocampus of post-stroke depression rats. Mol Med Rep. 2012;6:889–893. doi: 10.3892/mmr.2012.1009. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Qian L. Homocysteine-mediated intestinal epithelial barrier dysfunction in the rat model of irritable bowel syndrome caused by maternal separation. Acta Biochim Biophys Sin. 2014;46:917–919. doi: 10.1093/abbs/gmu076. [DOI] [PubMed] [Google Scholar]
- Zhao Y, et al. Inhibition of cystathionine beta-synthase is associated with glucocorticoids over-secretion in psychological stress-induced hyperhomocystinemia rat liver. Cell Stress Chaperones. 2013;18:631–641. doi: 10.1007/s12192-013-0416-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo JM, Pratico D. Acceleration of brain amyloidosis in an Alzheimer’s disease mouse model by a folate, vitamin B6 and B12-deficient diet. Exp Gerontol. 2010;45:195–201. doi: 10.1016/j.exger.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]