Abstract
Background
Acetylcholinesterase (AChE) inhibitors have been shown to be effective in treating cognitive impairment in animal models and in human subjects with major depressive disorder (MDD). Huperzine A (HupA), a Traditional Chinese Medicine derived from a genus of clubmosses known as Huperzineserrata, is a powerful AChE inhibitor that has been used as an adjunctive treatment for MDD, but no meta-analysis on HupA augmentation for MDD has yet been reported.
Aim
Conduct a systematic review and meta-analysis of randomized controlled trials (RCTS) about HupA augmentation in the treatment of MDD to evaluate its efficacy and safety.
Methods
Two evaluators independently searched nine English-language and Chinese-language databases, selected relevant studies that met pre-determined inclusion criteria, extracted data about outcome and safety, and conducted quality assessments and data synthesis.
Results
Three low-quality RCTs (pooled n=238) from China were identified that compared monotherapy antidepressant treatment for depression versus combined treatment with antidepressants and HupA. Participants in the studies ranged from 16 to 60 years of age. The average duration of adjunctive antidepressant and HupA treatment in the studies was only 6.7 weeks. All three studies were open label and non-blinded, so their overall quality was judged as poor. Meta-analysis of the pooled sample found no significant difference in the improvement in depressive symptoms between the two groups (weighted mean difference: -1.90 (95%CI: -4.23, 0.44), p=0.11). However, the adjunctive HupA group did have significantly greater improvement than the antidepressant only group in cognitive functioning (as assessed by the Wisconsin Card Sorting Test and the Wechsler Memory Scale-Revised) and in quality of life. There was no significant difference in the incidence of adverse drug reactions between groups.
Conclusions
The data available on the effectiveness and safety of adjunctive treatment using HupA in patients with MDD who are receiving antidepressants is insufficient to arrive at a definitive conclusion about its efficacy and safety. Pooling of the data from three low-quality RCTs from China found no advantage of adjunctive HupA in the treatment of depressive symptoms, but adjunctive treatment with HupA was associated with a faster resolution of the cognitive symptoms that frequently accompany MDD.
Keywords: depression, meta-analysis, cognitive function, huperzine A, adjunctive treatment
Abstract
背景
乙酰胆碱酯酶(Acetylcholinesterase, AChE)抑制剂在重性抑郁障碍(Major Depressive Disorder, MDD)的动物模型和人类患者中已被证实可以有效地治疗认知障碍。石杉碱甲(Huperzine A, HupA)是一种来自于被称为蛇足石杉(Huperzineserrata)的石松属传统中医药,是一种强有力的AChE抑制剂,已被用于抑郁症的辅助治疗,但有尚无关石杉碱甲对MDD的强化治疗作用的meta分析。
目标
对有关石杉碱甲强化治疗抑郁症的随机对照试验进行系统综述和meta分析,评估其疗效及安全性。
方法
两位评估者独立检索9个英文和中文数据库,选择符合预先确定的纳入标准的相关研究,提取有关疗效和安全性的数据,并进行质量评估和数据拟合合成。
结果
纳入了三项中国低质量的随机对照试验(总共n=238),这些试验比较了单用抗抑郁药治疗抑郁症与抗抑郁药和石杉碱甲的联合治疗,试验中的被试从16岁到60岁。研究中石杉碱甲辅助抗抑郁药治疗的平均时间仅为6.7周。这三项研究都是公开标签未使用盲法,所以他们的总体质量评定为差。总体样本的Meta分析发现两组抑郁症状的改善没有显著性差异(差异加权差为-1.90,95%CI可信区间为-4.23至0.44,p=0.11)。然而,石杉碱甲辅助治疗组比单用抗抑郁药治疗组在认知功能和生活质量方面有显著改善(如威斯康星卡片分类测验、韦氏记忆量表修订的评估)。组间药物不良反应的发生率无显著性差异。
结论
有关在接受抗抑郁药的MDD患者使用HupA辅助治疗的疗效和安全性的可获取数据不足,难以得出有关其疗效和安全性的明确结论。汇集国内3项低质量的RCT数据没有发现采用辅助使用HupA治疗抑郁症状的优势,但辅助使用HupA与更快改善经常伴随MDD出现的认知症状相关。
中文全文
本文全文中文版从2016年8月25日起在http://dx.doi.org/10.11919/j.issn.10020829.216003可供免费阅览下载
1. Introduction
Major depressive disorder (MDD) is a common psychiatric illness that is often associated with cognitive dysfunction.[1] One hypothesis about the mechanism of cognitive decline in MDD links it to decreasing acetylcholinesterase (AChE) activity of the cholinergic system in the hippocampus, frontal cortex, and septum.[2] Some studies suggest that AChE inhibitors (e.g., donepezil,[3] rivastigmine,[4] and galantamine[5]) can ameliorate cognitive impairment in animal models of depression and in humans with MDD.[5, 6, 7] Huperzine A (HupA) is a Traditional Chinese Medicine (TCM) isolated from Huperzineserrata (a genus of clubmosses), also known as ground pines or creeping cedar, in the family Lycopeodiaceae (a family of fern-allies). It is a powerful, highly specific, and reversible inhibitor of AChE.[8, 9, 10] Because of its popularity as a TCM medication in mainland China, extensive clinical experience and research about HupA in China may help clarify the mechanism of action for its potential efficacy in the treatment of MDD. However, to date no systematic review or meta-analysis on HupA augmentation for MDD has been published. The primary aim of this study was to conduct a systematic review and meta-analysis about the efficacy and safety of HupA in the treatment of MDD based on published RCTs identified by searching international and Chinese databases.
2. Methods
2.1. Types of studies
All publications of randomized controlled trials (RCTs) which reported on the efficacy and/or safety of antidepressants combined with HupA in the treatment of MDD were eligible for inclusion. Case reports/series, observational trials, meta-analyses, and systematic reviews were excluded.
2.2. Outcome measures
The primary outcome measure of interest was cognitive function measured by the Wisconsin Card Sorting Test (WCST)[11] or the Wechsler Memory Scale-Revised, Chinese version (WMS-RC).[12] Key secondary outcomes were improvement in depressive and anxiety symptoms assessed by the Hamilton Depression Rating Scale (HAMD)[13] and the Hamilton Anxiety Rating Scale (HAMA),[14] self-reported quality of life assessed by the General Quality of Life Inventory of the World Health Organization (WHOQOL-100),[15] causes for discontinuation of treatment, and adverse drug reactions measured by the Dosage Record Treatment Emergent Symptom Scale (DOTES).[16] Clinical outcomes were based on intent-to-treat (ITT) analysis.
2.3. Selection of studies
PubMed, PsycINFO, Embase, Cochrane Library databases, the Cochrane Controlled Trials Register, ClinicalTrials.gov (https://www.clinicaltrials.gov/), and Chinese databases (WanFang Database, Chinese Biomedical database, and China Journal Net) were searched from the inception of the databases through March 12, 2016 using the following search terms: (Depressive Disorders OR Disorder, Depressive OR Disorders, Depressive OR Neurosis, Depressive OR Depressive Neuroses OR Depressive Neurosis OR Neuroses, Depressive OR Depression, Endogenous OR Depressions, Endogenous OR Endogenous Depression OR Endogenous Depressions OR Depressive Syndrome OR Depressive Syndromes OR Syndrome, Depressive OR Syndromes, Depressive OR Depression, Neurotic OR Depressions, Neurotic OR Neurotic Depression OR Neurotic Depressions OR Melancholia OR Melancholias OR Unipolar Depression OR Depression, Unipolar OR Depressions, Unipolar OR Unipolar Depressions) AND (Huperzine A OR Huperzine OR HupA) AND (randomized controlled trial OR controlled clinical trial OR randomized OR placebo OR drug therapy OR randomly OR trial OR groups). We also hand-searched reference lists from identified and relevant review articles for additional studies and contacted authors for unpublished data.
2.4. Data extraction
Two authors (ZW and XYQ) independently conducted the literature search and extracted the data. Any disagreement was resolved by a third author (XYT). Data presented only in graphs and figures were extracted whenever possible. Authors were contacted to obtain missing information or clarification if possible. If cases were from multicenter studies, whenever possible, data were extracted separately for each center.
2.5. Statistical methods
We used RevMan (version 5.1.7.0) in this meta-analysis according to the recommendations of the Cochrane Collaboration. For continuous data, weighted mean difference (WMD) with 95% CI was used to compare groups, and for dichotomous data, risk ratio (RR) with 95% confidence intervals (Cis) were computed to compare groups. The I2 statistic assessed statistical heterogeneity between the three studies: when I2≥50%, a random effects model was used;[17] otherwise, a fixed effect model was employed.[18] All analyses were two-tailed with alpha set at 0.05.
2.6. Risk of bias assessment
The methods of random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other biases were assessed using the Risk of Bias (ROB) scale developed to assess RCTs by the Cochrane Collaboration.[19]
3. Results
3.1. Results of the literature search
The search yielded 54 potentially relevant articles, of which four articles were published in English and 50 in Chinese. Of the 54 studies, 3 RCTs met the inclusion criteria.[20, 21, 22] As shown in Figure 1, the total number of subjects included in the three studies was 238, with 119 receiving an antidepressant augmentated with HupA and 119 only receiving an antidepressant.
Figure 1. Identification of included studies.
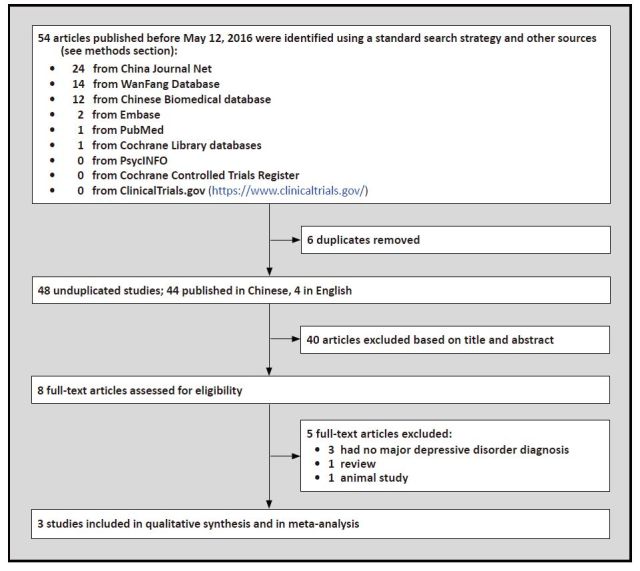
3.2. The characteristics of included studies
As shown in Table 1, all three RCTs [20, 21, 22] were conducted in China and used the criteria of the Chinese Classification of Mental Disorders, 3rd edition (CCMD-3)[23] to diagnose depression. Males accounted for 45.4% of the sample (range 30% to 58% in the three studies), the weighted mean age of participants was 29.6 (range 16-60) years; and the weighted mean duration of illness was 3.3 (range 1.2 to 5.2) years. The weighted mean duration of the treatment trial reported in the studies was 6.7 (range 6-8) weeks. None of the studies were supported by pharmaceutical companies.
Table 1. Characteristics of included studies.
| Study | N | Design Settings | Trial duration (weeks) | Country | Diagnosis Diagnostic criteria Mean illness duration |
Weighted mean age in years (range) | Male n (%) | Interventions: [M] mean dose (mg/day) [R] range (mg/day) [n] number of patients |
Outcome assessments |
|---|---|---|---|---|---|---|---|---|---|
| Gao 2007[20] | 100 | Open-label inpatients and outpatients | 6 | China | MDD CCMD-3 5.2 years | 30.4 (18-50) | 30 (30%) | 1. FLU(M=NR; R=20-40) + HupA (fixed dose at 0.3); n=50 2. FLU(M=NR; R=20-40); n=50 |
HAMD; WCST; WHOQOL-100 |
| Yang 2010[21] | 78 | Open-label Inpatients and outpatients | 8 | China | MDD CCMD-3 2.5 years | 29.9 (18-60) | 45 (58%) | 1. FLU(M=NR; R=20-40) + HupA (fixed dose at 0.3); n=39 2. FLU(M=NR; R=20-40); n=39 |
HAMD; WMS-RC |
| Liu 2010[22] | 60 | Open-label Inpatients and outpatients | 6 | China | MDD CCMD-3 1.2 years | 27.9 (16-48) | 33 (55%) | 1. VEN(M=107; R=50-150) + HupA (M=NR; R=0.1-0.2); n=30 2. VEN(M=105; R=50-150); n=30 |
HAMD; HAMA; DOTES |
aMDD, Major Depressive Disorder
CCMD-3, Chinese Mental Disorders Classification and Diagnostic Criteria, Third Edition[23]
FLU, fluoxetine
NR, not recorded
HupA, huperzine A
HAMD, Hamilton Depression Rating Scale[13]
WCST, Wisconsin Card Sorting Test[11]
WHOQOL-100, General Quality of Life Inventory of World Health Organization[15]
WMS-RC, Wechsler Memory Scale-Revised, Chinese version[12]
VEN, venlafaxine
HAMA, Hamilton Anxiety Rating Scale[14]
DOTES, Dosage Record Treatment Emergent Symptom Scale[16]
3.3. Assessment of risk of bias
The risk of different types of biases of the three studies is shown in Table 2. Two studies[21, 22] mentioned "random" assignment without a description of the method of randomizing, and one RCT[20] was rated as high risk of selection bias because patients were classified into two groups according to the order of admission. None of the studies were blinded so the risk of allocation bias, performance bias, and detection bias were high. The studies reported the outcomes of all enrolled subjects, so the risk of attrition bias was low; but in the absence of study registration materials it was impossible to determine whether or not there was selective reporting (i.e., reporting bias). There was no evidence of other types of biases (e.g., drug company sponsorship of the study). Overall, all three studies were considered at high risk of bias and, thus, relatively low-quality studies.
Table 2.
Evaluation of risk of bias in the three included studies
| study | sequence generation | allocation sequence concealment | blinding of participants and personnel | blinding of outcome assessment | incomplete outcome data | selective outcome reporting | other potential threats to validity |
|---|---|---|---|---|---|---|---|
| Gao 2007[20] | high | high | high | high | low | N/A | low |
| Yang 2010[21] | N/A | high | high | high | low | N/A | low |
| Liu 2010[22] | N/A | high | high | high | low | N/A | low |
N/A=no information available
Because there were only three RCTs included in the meta-analysis, publication bias could not be tested.[24]
3.4. Changes in severity of depressive symptoms
In all three studies there were differences between groups in changes of the total HAMD score over the study period. As shown in Figure 2, one of the studies[22] reported a significantly greater reduction of depressive symptoms (based on the HAMD) when adjunctive HupA was provided to patients with MDD being treated with antidepressants, but the other two studies did not find a significant advantage of adjunctive treatment with HupA. When pooling the three studies in a random effects meta-analysis, there was no statistically significant difference in the improvement in depressive symptoms between MDD patients who only received antidepressants and those who received antidepressants and adjunctive HupA.
Figure 2.
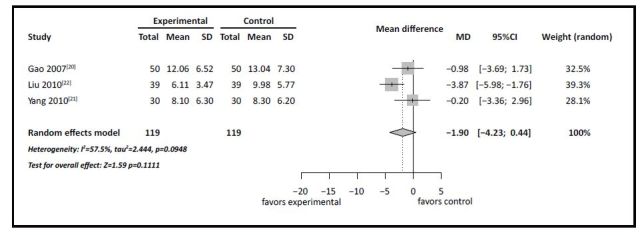
Adjunctive Huperzine A for MDD: forest plot for improvement in depressive symptoms assessed by change in total score of the Hamilton Depression Scale
3.5. Cognitive results
The other results from the three studies are shown in Table 3. Only two studies[20, 21] assessed the cognitive effects of the treatment. Both studies reported a significant advantage of using adjunctive HupA. In one study,[21] memory functioning at the end of the 8-week trial was better in patients taking antidepressants with adjunctive HupA than in those who were only taking antidepressants. In another study,[20] several measures of executive functioning derived from the WCST were significantly better at the end of the 6-week trial in depressed patients taking antidepressants with adjunctive HupA. These cognitive outcome measures were quite different so it was not possible to pool the results of the two studies into a meta-analysis.
Table 3. Comparison of cognitive function, anxiety, and quality of life in patients with depression at end of course of treatment with either antidepressants and adjunctive HupA (experimental group) or with antidepressants alone (control group).
| measure | study | control group | experimental group | t-test (p) | |||
|---|---|---|---|---|---|---|---|
| n | mean (sd) | n | mean (sd) | ||||
| Cognitive measures | |||||||
| WMS-RC | Yang[21] | 39 | 92.1 (16.7) | 39 | 103.0 (15.0) | 3.04 (0.003) | |
| WCST (non-perseverative errors) | Gao[20] | 50 | 35.7 (5.4) | 50 | 27.5 (8.5) | 5.71 (<0.001) | |
| WCST (perseverative errors) | Gao[20] | 50 | 37.7 (7.4) | 50 | 26.4 (9.7) | 6.60 (<0.001) | |
| WCST (correct responses) | Gao[20] | 50 | 24.3 (6.2) | 50 | 31.9 (11.3) | 4.17 (<0.001) | |
| WCST (categories completed) | Gao[20] | 50 | 3.96 (0.83) | 50 | 4.52 (1.07) | 2.92 (0.004) | |
| Anxiety (HAMA total score) | Liu[22] | 30 | 8.1 (7.3) | 30 | 8.3 (7.3) | 0.11 (0.909) | |
| WHOQOL-100 total score | Gao[20] | 50 | 12.9 (3.9) | 50 | 18.6 (12.5) | 3.08 (0.003) | |
3.6. Other results
The level of anxiety was only assessed in one of the studies.[22] Based on the total score of the HAMA at the end of the 6-week trial, there was no significant difference in the severity of anxiety symptoms between the two groups (Table 3).
Only one study[20] assessed quality of life. As measured by WHOQOL-100,[15] quality of life was significantly better at the end of the trial in individuals who received combined treatment with antidepressants and HupA (Table 3).
Only one study [22] assessed adverse reactions. The study assessed adverse events using the DOTES[16] which considers tachycardia, dysuria, electrocardiographic abnormality, dry mouth, drowsiness, nausea, constipation, blurred vision, and insomnia. It found no difference in the prevalence of adverse events between the two treatment groups
None of the included RCTs reported the rate or causes of treatment discontinuation.
4. Discussion
4.1. Main finding
Despite an extensive review of both English-language and Chinese-language literature, we only identified three RCTs that assessed the potential benefit of adjunctive HupA when treating individuals with depression who are currently using antidepressants. All three studies were open label and the outcome evaluation in the trials was not blinded, so the overall strength of the studies was rated as 'poor'. The pooled sample from the three studies, all of which were published in Chinese, was 238 individuals, but it was only possible to conduct a meta-analysis for the results related to changes in depressive symptoms because other outcomes of interest (e.g., cognitive changes, quality of life changes, etc.) were only considered in one or two of the studies. Overall, the results suggest that adjunctive treatment with HupA over 6 to 8 weeks in patients with depression who are currently taking antidepressants does not result in a better reduction of depressive symptoms, but it does appear to lead to less cognitive impairment in depressed individuals and, possibly, to a better self-reported quality of life for depressed individuals.
4.2. Limitations
The small number of studies identified and the limited measures employed in the identified studies made it impossible to conduct a full meta-analysis, so we could not do a sensitivity analysis or subgroup analyses, and we could not construct a funnel plot to assess potential publication bias. Specifically, there were not enough studies with data on cognitive functioning to conduct a meta-analysis of this important outcome. Moreover, the relatively low quality of the available studies (open label, non-blinded) and the relatively short duration of the studies (from 6 to 8 weeks) means that the findings that were significant - the benefit of HupA augmentation for cognitive functioning and quality of life in depressed patients - are not robust; they need to be replicated in larger, methodologically more rigorous RCTs that follow participants for much longer.
4.3. Importance
Despite the limited number of RCTs identified and the methodological limitations of the identified studies,[25] this review does provide some support for the suggestion that AChE inhibitors such as HupA can ameliorate the cognitive decline that is often associated with depression and, possibly, improve the quality of life of individuals being treated for depression with antidepressant medications. Similar to our findings, a recent meta-analyses[26] found that adjunctive HupA is an effective choice for improving cognitive function in individuals with schizophrenia. The mechanism of action of HupA in improving cognitive functioning (or preventing cognitive decline) remains unknown, but given the importance of cognitive impairment in a wide range of mental disorders, further work in this promising area is merited.
Biography

Dr. Wei Zheng obtained a bachelor's degree from Hebei Medical University in 2012 and a master's degree of psychiatry from Capital Medical University in Beijing in 2015. He is currently a resident psychiatrist in the Department of Psychiatry at the Affiliated Brain Hospital of Guangzhou Medical University (Guangzhou Huiai Hospital) in Guangdong Province, China.
Funding Statement
The study was supported by the Start-up Research Grant (SRG2014-00019-FHS) and the Multi-Year Research Grant (MYRG2015-00230-FHS) from the University of Macau. Trial registration number: CRD42015024796 (http://www.crd.york.ac.uk/prospero/)
Footnotes
Conflict of interest statement: The authors report no conflict of interest in conducting this study and preparing the manuscript.
Authors' contribution: WZ designed the study and was assisted by YQX and YTX in the search for papers, data extraction, and analysis. WZ and YTZ drafted the manuscript. GSU, HFKC, CHN, and YW made critical revisions to the manuscript. All authors approved the final version for publication.
References
- 1.Bhagya V, Srikumar BN, Raju TR, Shankaranarayana Rao BS. The selective noradrenergic reuptake inhibitor reboxetine restores spatial learning deficits, biochemical changes, and hippocampal synaptic plasticity in an animal model of depression. J Neurosci Res. 2015;93(1):104–120. doi: 10.1002/jnr.23473. [DOI] [PubMed] [Google Scholar]
- 2.Srikumar BN, Raju TR, Shankaranarayana Rao BS. The involvement of cholinergic and noradrenergic systems in behavioral recovery following oxotremorine treatment to chronically stressed rats. Neuroscience. 2006;143(3):679–688. doi: 10.1016/j.neuroscience.2006.08.041. [DOI] [PubMed] [Google Scholar]
- 3.Pelton GH, Andrews H, Roose SP, Marcus SM, D'Antonio K, Husn H, et al. Donepezil treatment of older adults with cognitive impairment and depression (DOTCODE study): clinical rationale and design. Contemp Clin Trials. 2014;37(2):200–208. doi: 10.1016/j.cct.2013.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Islam MR, Moriguchi S, Tagashira H, Fukunaga K. Rivastigmine improves hippocampal neurogenesis and depression-like behaviors via 5-HT1A receptor stimulation in olfactory bulbectomized mice. Neuroscience. 2014;272:116–130. doi: 10.1016/j.neuroscience.2014.04.046. [DOI] [PubMed] [Google Scholar]
- 5.Ago Y, Koda K, Takuma K, Matsuda T. Pharmacological aspects of the acetylcholinesterase inhibitor galantamine. J Pharmacol Sci. 2011;116(1):6–17. doi: 10.1254/jphs.11r01cr. [DOI] [PubMed] [Google Scholar]
- 6.McDermott CL, Gray SL. Cholinesterase inhibitor adjunctive therapy for cognitive impairment and depressive symptoms in older adults with depression. The Annals of pharmacotherapy. 2012;46(4):599–605. doi: 10.1345/aph.1Q445. [DOI] [PubMed] [Google Scholar]
- 7.Matsuda T, Ago Y, Takuma K. [Pharmacological profiles of galantamine: the involvement of muscarinic receptor] Nihon shinkei seishin yakurigaku zasshi (Japanese Journal of Psychopharmacology) 2012;32(1):1–8. Japanese. [PubMed] [Google Scholar]
- 8.Ma X, Tan C, Zhu D, Gang DR, Xiao P. Huperzine A from Huperzia species — an ethnopharmacolgical review. J Ethnopharmacol. 2007;113(1):15–34. doi: 10.1016/j.jep.2007.05.030. [DOI] [PubMed] [Google Scholar]
- 9.Zhang HY, Tang XC. Neuroprotective effects of huperzine A: new therapeutic targets for neurodegenerative disease. Trends Pharmacol Sci. 2006;27(12):619–625. doi: 10.1016/j.tips.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Xing SH, Zhu CX, Zhang R, An L. Huperzine A in the treatment of Alzheimer's disease and vascular dementia: a metaanalysis. Evid Based Complement Alternat Med. 2014;2014:363985. doi: 10.1155/2014/363985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kongs SK, Thompson LL, Iverson GL, Heaton RK. Wisconsin Card Sorting Test-64 Card Version (WCST-64). Odessa, FL: Psychological Assessment Resources; 2000. [Google Scholar]
- 12.Chelune GJ, Bornstein RA, Prifitera A. The Wechsler Memory Scale-Revised. Springer: Advances in Psychological Assessment; 1990. pp. 65–99. [Google Scholar]
- 13.Hamilton M. A rating scale for depression. Journal of Neurology. Neurosurgery and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shear MK, Vander Bilt J, Rucci P, Endicott J, Lydiard B, Otto MW, et al. Reliability and validity of a structured interview guide for the Hamilton Anxiety Rating Scale (SIGH-A) Depression & Anxiety. 2001;13(4):166–178. doi: 10.1002/da.1033.abs. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization . WHOQOL: Measuring Quality of Life. Division of Mental Health and Prevention of Substance Abuse. World Health Organization; 1997. [Google Scholar]
- 16.Guy, W. ECDEU assessment manual. In: US Department of Health. Education and Welfare, Alcohol. Drug Abuse and Mental Health Administration. Rochville, MD: National Institute of Mental Health; 1976. [Google Scholar]
- 17.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 18.Der Simonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 19.Higgins JPT, Green S (eds) Cochrane Handbook for Systematic Reviews of Interventions. UK, Chichester: John Wiley & Sons; 2008. [Google Scholar]
- 20.Gao YF, Li J, Meng HQ, Luo QH, Hu H, Du L. [Effects of huperzine on cognition function and life quality of patients with depression] Chongqing Yi Xue. 2007;36(6):483–485. doi: 10.3969/j.issn.1671-8348.2007.06.001. [DOI] [Google Scholar]
- 21.Yang ZB, Deng XM, Zhang GX, Yu XR. [The study of huperzine combined with fluoxetine on cognition function of patients with depression] Lin Chuang Jing Shen Yi Xue Za Zhi. 2010;20(6):418–419. [Google Scholar]
- 22.Liu SZ, Wang PJ, Yin A J, Dang XJ, Guang H. [Effects of huperzine A combined with venlafaxine for patients with depression] Zhongguo Shi Yong Yi Yao. 2010;5(11):151–152. doi: 10.3969/j.issn.1673-7555.2010.11.116. [DOI] [Google Scholar]
- 23.Chinese Medical Association . [Chinese Mental Disorders Classification and Diagnostic Criteria, Third Edition (CCMD-3)]. Jinan: Shandong Science and Technology Press; 2001. Chinese. [Google Scholar]
- 24.Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel ploy asymmetry in meta-analyses of randomized controlled trials. BMJ. 2011;343:d4002. doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- 25.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng W, Xiang YQ, Li XB, Ungvari GS, Chiu HFK, Sun F, et al. Adjunctive huperzine A for cognitive deficits in schizophrenia: a systematic review and meta-analysis. Hum Psychopharmacol: Clinical and Experimental. 2016 doi: 10.1002/hup.2537. [DOI] [PubMed] [Google Scholar]


