Abstract
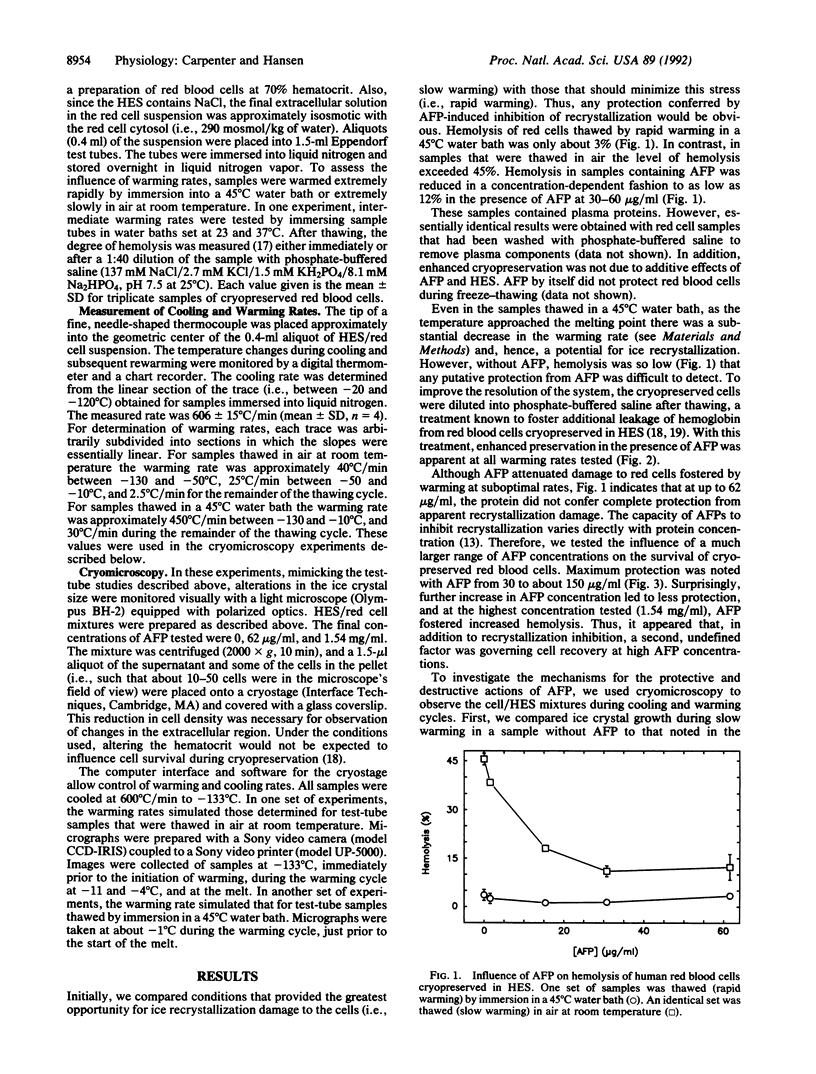
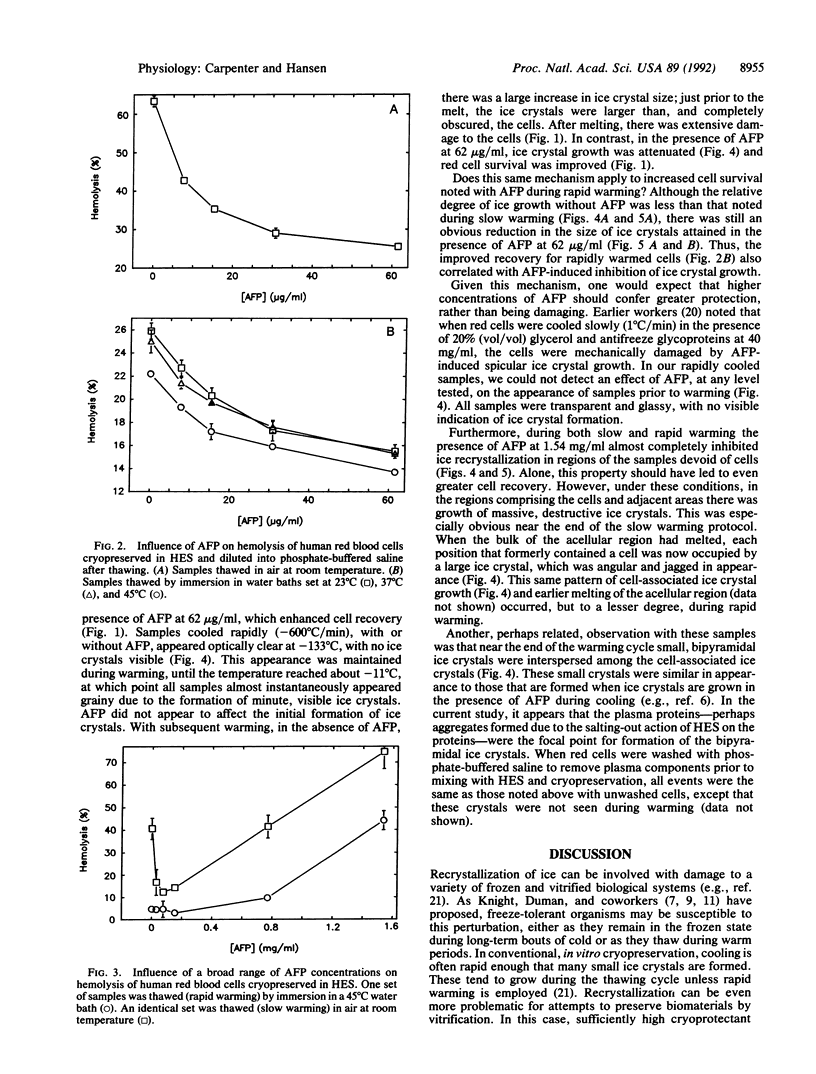
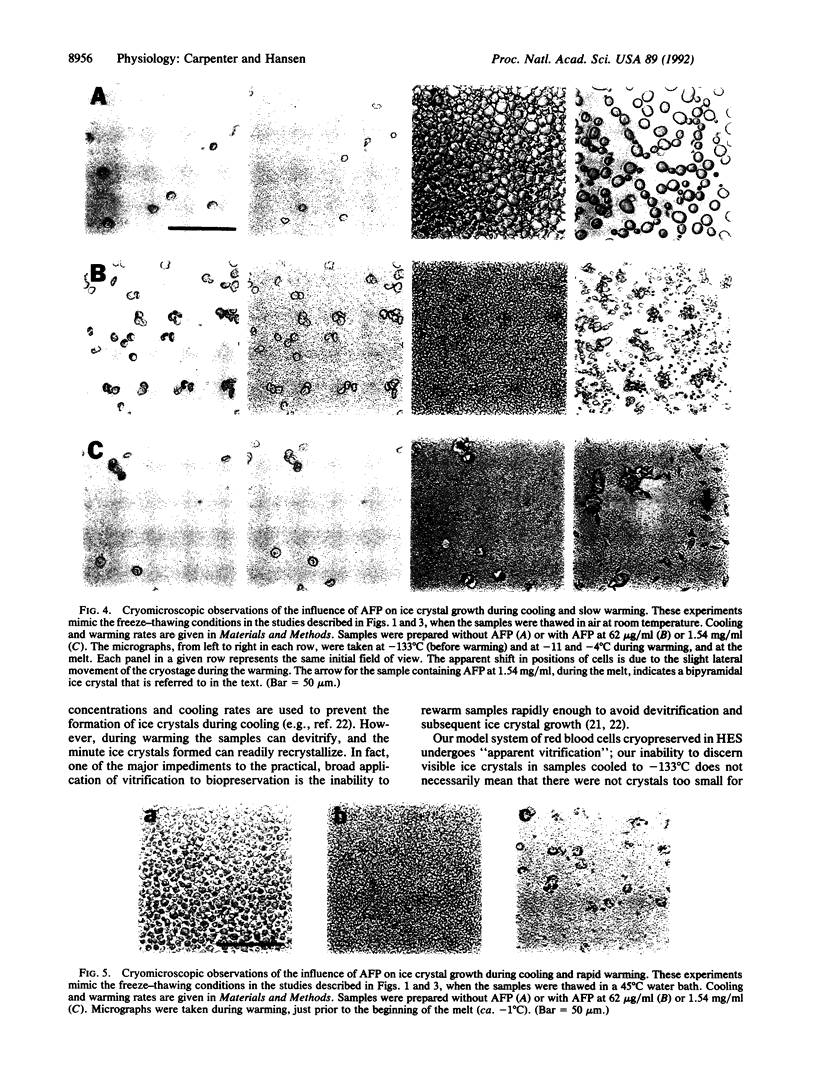
Antifreeze proteins (AFPs) are extremely efficient at inhibiting ice recrystallization in frozen solutions. Knight and Duman [Knight, C. A. & Duman, J. G. (1986) Cryobiology 23, 256-263] have proposed that this may be an important function of the proteins in freeze-tolerant organisms. We have tested this proposal in vitro by characterizing the influence of AFP on the recovery of cryopreserved cells, which often can survive cooling and yet subsequently be damaged by ice crystal growth during warming. Relatively low concentrations (e.g., 5-150 micrograms/ml) of winter flounder (Pseudopleuronectes americanus) AFP enhance survival of red blood cells cryopreserved in hydroxyethyl starch solutions. This effect is most apparent in samples warmed at suboptimal rates, i.e., where ice recrystallization would be exaggerated. Cryomicroscopy demonstrates that AFP inhibits ice recrystallization in the extracellular regions during the latter stages of the warming cycle. AFP concentrations that enhance survival of red cells confer partial inhibition of recrystallization. Relatively high concentrations of AFP (e.g., 1.54 mg/ml) are much more effective at inhibiting extracellular recrystallization. However, extensive growth of ice around the cell, and concomitant cell damage, is noted. The mechanism for this AFP-induced ice growth is unknown. We propose that there is a delicate balance between AFP-induced enhancement of cell preservation and AFP-induced enhancement of cell preservation and AFP-induced enhancement of cell damage and that this balance hinges on the degrees of inhibition of ice recrystallization and of preferential growth of ice around the cells. We conclude that, under appropriate conditions, one of the proposed functions of AFPs in nature can be emulated, and perhaps have application, in cryopreservation of materials of biomedical interest.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Davies P. L., Hew C. L. Biochemistry of fish antifreeze proteins. FASEB J. 1990 May;4(8):2460–2468. doi: 10.1096/fasebj.4.8.2185972. [DOI] [PubMed] [Google Scholar]
- Duman J., Horwath K. The role of hemolymph proteins in the cold tolerance of insects. Annu Rev Physiol. 1983;45:261–270. doi: 10.1146/annurev.ph.45.030183.001401. [DOI] [PubMed] [Google Scholar]
- Fahy G. M., Levy D. I., Ali S. E. Some emerging principles underlying the physical properties, biological actions, and utility of vitrification solutions. Cryobiology. 1987 Jun;24(3):196–213. doi: 10.1016/0011-2240(87)90023-x. [DOI] [PubMed] [Google Scholar]
- Knight C. A., DeVries A. L., Oolman L. D. Fish antifreeze protein and the freezing and recrystallization of ice. Nature. 1984 Mar 15;308(5956):295–296. doi: 10.1038/308295a0. [DOI] [PubMed] [Google Scholar]
- Knight C. A., Hallett J., DeVries A. L. Solute effects on ice recrystallization: an assessment technique. Cryobiology. 1988 Feb;25(1):55–60. doi: 10.1016/0011-2240(88)90020-x. [DOI] [PubMed] [Google Scholar]
- Lionetti F. J., Hunt S. M., Lin P. S. Improved method for the cryopreservation of human red cells in liquid nitrogen with hydroxyethyl starch. Cryobiology. 1976 Oct;13(5):489–499. doi: 10.1016/0011-2240(76)90142-5. [DOI] [PubMed] [Google Scholar]
- Mazur P. Cryobiology: the freezing of biological systems. Science. 1970 May 22;168(3934):939–949. doi: 10.1126/science.168.3934.939. [DOI] [PubMed] [Google Scholar]
- Mazur P. Freezing of living cells: mechanisms and implications. Am J Physiol. 1984 Sep;247(3 Pt 1):C125–C142. doi: 10.1152/ajpcell.1984.247.3.C125. [DOI] [PubMed] [Google Scholar]
- Pegg D. E., Diaper M. P., Skaer H. L., Hunt C. J. The effect of cooling rate and warming rate on the packing effect in human erythrocytes frozen and thawed in the presence of 2 M glycerol. Cryobiology. 1984 Oct;21(5):491–502. doi: 10.1016/0011-2240(84)90047-6. [DOI] [PubMed] [Google Scholar]
- Rubinsky B., Arav A., Devries A. L. The cryoprotective effect of antifreeze glycopeptides from antarctic fishes. Cryobiology. 1992 Feb;29(1):69–79. doi: 10.1016/0011-2240(92)90006-n. [DOI] [PubMed] [Google Scholar]
- Urrutia M. E., Duman J. G., Knight C. A. Plant thermal hysteresis proteins. Biochim Biophys Acta. 1992 May 22;1121(1-2):199–206. doi: 10.1016/0167-4838(92)90355-h. [DOI] [PubMed] [Google Scholar]
- Williams R. J. The surface activity of PVP and other polymers and their antihemolytic capacity. Cryobiology. 1983 Oct;20(5):521–526. doi: 10.1016/0011-2240(83)90040-8. [DOI] [PubMed] [Google Scholar]




