Abstract
Allogeneic bone marrow transplantation is an essential therapy for acquired aplastic anemia and prognosis has recently improved. However, engraftment failure and graft-versus-host disease are potential fatal complications. Various risk factors for poor prognosis have been identified, such as patient age and human-leukocyte antigen disparity, but the relationship between donor age and prognosis is still unknown. Therefore, we performed a cohort study to compare the prognosis of unrelated bone marrow transplantation from younger and older donors using the registry database in Japan. We evaluated 427 patients (age 16–72 years) with aplastic anemia who underwent bone marrow transplantation from younger (≤39 years, n=281) or older (≥40 years, n=146) unrelated donors. Overall survival of the older donor group was significantly inferior to that of the younger donor group (adjusted hazard ratio 1.64; 95% confidence interval 1.15–2.35; P<0.01). The incidence of fatal infection was significantly higher in the older donor group (13.7% vs. 7.5%; P=0.03). Primary engraftment failure and acute graft-versus-host disease were significantly more frequent in the older donor group (9.7% vs. 5.0%; adjusted hazard ratio 1.30; P=0.01, and 27.1% vs. 19.7%; adjusted hazard ratio 1.56; P=0.03, respectively). Acute graft-versus-host disease was related to a worse prognosis in the whole cohort. This study showed the inferiority of older donors in aplastic anemia; thus, donor age should be considered when multiple donors are available. A large-scale prospective study is warranted to establish a better donor selection algorithm for bone marrow transplantation in aplastic anemia.
Introduction
Allogeneic hematopoietic cell transplantation is an effective and, therefore, indispensable therapy for acquired aplastic anemia (AA) in adults.1 Patients with AA are eligible for transplant if they are under 40 years of age or when they are refractory to immunosuppressive therapy;1,2 bone marrow transplantation (BMT) from a human leukocyte antigen (HLA)-matched sibling donor or an unrelated donor is selected according to the donor availability.2 The prognosis of BMT for AA has recently improved and 5-year overall survival (OS) is as high as 72% for younger patients (≤40 years old) and 53% for older patients (>40 years).3
However, severe complications, such as engraftment failure, infection, and graft-versus-host disease (GvHD), are problems that need to be addressed in order to improve the overall prognosis of AA, especially for unrelated BMT.2,3 Various risk factors are reportedly associated with these complications and poor prognosis, such as older patient age, longer periods from diagnosis to transplantation, HLA-mismatched donors, and female donors.2–4 In addition to these, biological speculation from previous published studies regarding hematopoietic stem cell repopulation and donor-derived T-cell function have suggested that transplantation from older donors may result in a higher incidence of engraftment failure and acute GvHD (aGvHD), and, as a result, increase transplant-related death and lead to inferior OS. According to murine studies, hematopoietic stem cells from older donors do not re-populate as efficiently,5,6 and grafts from older donors have a higher ratio of memory T cells to naïve T cells;7 an increase in peripheral blood memory T cells has been shown to be related to the occurrence of aGvHD in humans.8,9
The influence of donor age in unrelated hematopoietic cell transplantation has long been discussed in various studies, and some have shown a relationship between older donor and worse prognosis.10–15 Most of these cohorts, however, were mainly composed of hematologic malignancies, and AA cases were not included,11–15 or, if they were, they made up only a small proportion of the cohort.10 AA should be analyzed independently from malignant diseases, especially with regard to engraftment and GvHD, because the incidence of graft failure is more often documented in AA, and GvHD more directly impacts OS.2 Moreover, engraftment and GvHD are closely related to pre- or post-transplant tumor load in hematologic malignancies, which is irrelevant to AA patients.16–18 As far as we know, however, no studies have investigated donor age as a candidate risk factor for poor prognosis in transplantation for AA.
Therefore, we performed a cohort study to compare the prognosis of patients with AA who underwent BMT from younger donors versus older donors using the Japanese transplant registry database, in particular on engraftment and GvHD. We focused on BMT from unrelated donors in order to avoid the correlation between patient and donor age; thus, BMT from related sibling donors were excluded because siblings tend to be born only a few years apart.10 Our study should provide important insights into donor selection algorithms for BMT in patients with AA.
Methods
Inclusion criteria and clinical procedures in BMT
Data for adult patients (age >16 years) with AA who underwent a first allogeneic BMT from unrelated donors between January 1 1993 and December 31 2013 were obtained from the Transplant Registry Unified Management Program (TRUMP) in Japan.19 The eligibility criteria for transplantation was in accordance with international guidelines and recommendations;1,2 BMT is the first-line treatment for young patients with severe AA with a sibling donor, and the second-line treatment following immunosuppressive treatment in older patients or in those to be grafted from an unrelated donor.2
The unrelated donor selection was based primarily on HLA disparity, and candidates were nominated among 8/8 or, if not available, 7/8 (or lower) HLA-A, B, C, and DR allele matched volunteers (age 20–55 years) registered in the Japanese Marrow Donor Program. Data on 10 alleles including HLA-DQ were not available. The donor was finally determined after consideration of various factors, such as ABO blood type, sex, and body weight; donor age usually has little significance on donor selection in Japan.20
Selection of conditioning regimens and GvHD prophylaxis is at the discretion of attending physicians in each institute, considering disease status, number of transfusions and amounts transfused, patients’ age and performance status, the risk of infections, etc.; donor age is not usually considered. Donor-derived serum and/or erythrocytes were depleted from grafts in cases of mismatched ABO blood types, and grafts were transplanted without ex vivo T-cell depletion. Our protocol complied with the Declaration of Helsinki and was approved by the TRUMP Data Management Committee and by the Ethics Committee of Kyoto University where the study was performed. Patient information was anonymized, so consent was not required.
Data collection and definition of each covariate
From the registry database, we extracted data on basic pre-transplant characteristics and post-transplant clinical courses. Donors were categorized into two groups with respect to age (younger vs. older than the 75th percentile; the closest value which is the multiple of 5 was adopted as the cut-off point). Donor age was considered a continuous variable and its influence was analyzed. Conditioning regimens were summarized according to the definitions of myeloablative conditioning (MAC) and reduced-intensity conditioning (RIC), which were consistent with those established in the RIC regimen workshop.21 Data on the use of anti-thymocyte or anti-T-cell globulin (ATG) before and after BMT were also collected. Periods between diagnosis and BMT were calculated from the day of initial diagnosis of AA.
With respect to the post-transplant clinical course, engraftment of neutrophils and platelets was defined as the first of three consecutive days during which neutrophil and platelet counts were at least 500/μL and 5.0×104/μL without transfusion support, respectively. Diagnosis and classification of GvHD were defined according to traditional criteria.22,23 A protective environment, prophylactic administration of antibiotics, and intravenous immunoglobulin replacement were adopted as standard prevention strategies for infection in accordance with the guideline from the Japanese Society for Hematopoietic Cell Transplantation.
Analytical methods are shown in the Online Supplementary Appendix.
Results
Patients’ characteristics
We evaluated 427 patients aged 16–72 years (median 30 years) who underwent unrelated BMT for AA. Donor age ranged from 21 to 55 years old (median 35 years; 75th percentile 42 years); therefore, the cut-off point for age was set at 40 years (the multiple of 5 which is the closest to 75th percentile), and younger donors were defined as 39 years old or under (n=281) and older donors as 40 years or over (n=146) (Table 1). There was no significant correlation between patient and donor age in the whole cohort (Pearson correlation coefficient 0.09) (Online Supplementary Figure S1) or in any subgroups regarding patients’ pre-transplant characteristics (data not shown). There were a median 355.5 days between diagnosis and BMT (range 138–827 days), and no significant differences were seen between the donor age groups (P=0.40). During these periods, all the patients underwent at least one course of immunosuppressive therapy, such as rabbit anti-human thymocyte immunoglobulin (ATG) (81.0%) and/or cyclosporine (88.8%) with or without granulocyte colony-stimulating factor (53.0%); type of previous therapies showed no correlation with donor age. MAC regimens were mainly composed of cyclophosphamide plus total body irradiation (TBI) (CY/TBI; CY 120 mg/kg; TBI 10–12 Gy) with or without ATG (Online Supplementary Table S1). High-dose TBI (10–12 Gy) was selected in cases transplanted before 2006, but not in more recent cases (after 2006); this regimen is strongly discouraged due to higher adverse events.24 On the other hand, RIC consisted of CY (200 mg/kg), TBI (2–4 Gy), and/or fludarabine (100–120 mg/m2) with or without ATG (Thymoglobulin), 2.5–10 mg/kg, or rabbit anti-human T-cell immunoglobulin (ATG-F), 10–25 mg/kg. GvHD prophylaxis was composed of cyclosporine- and tacrolimus-based regimens, and both were usually coupled with short-term methotrexate (98.6% and 94.2%, respectively). There was no significant difference between distribution of donor age according to year of BMT (before vs. after 2006).
Table 1.
Patients’ characteristics.
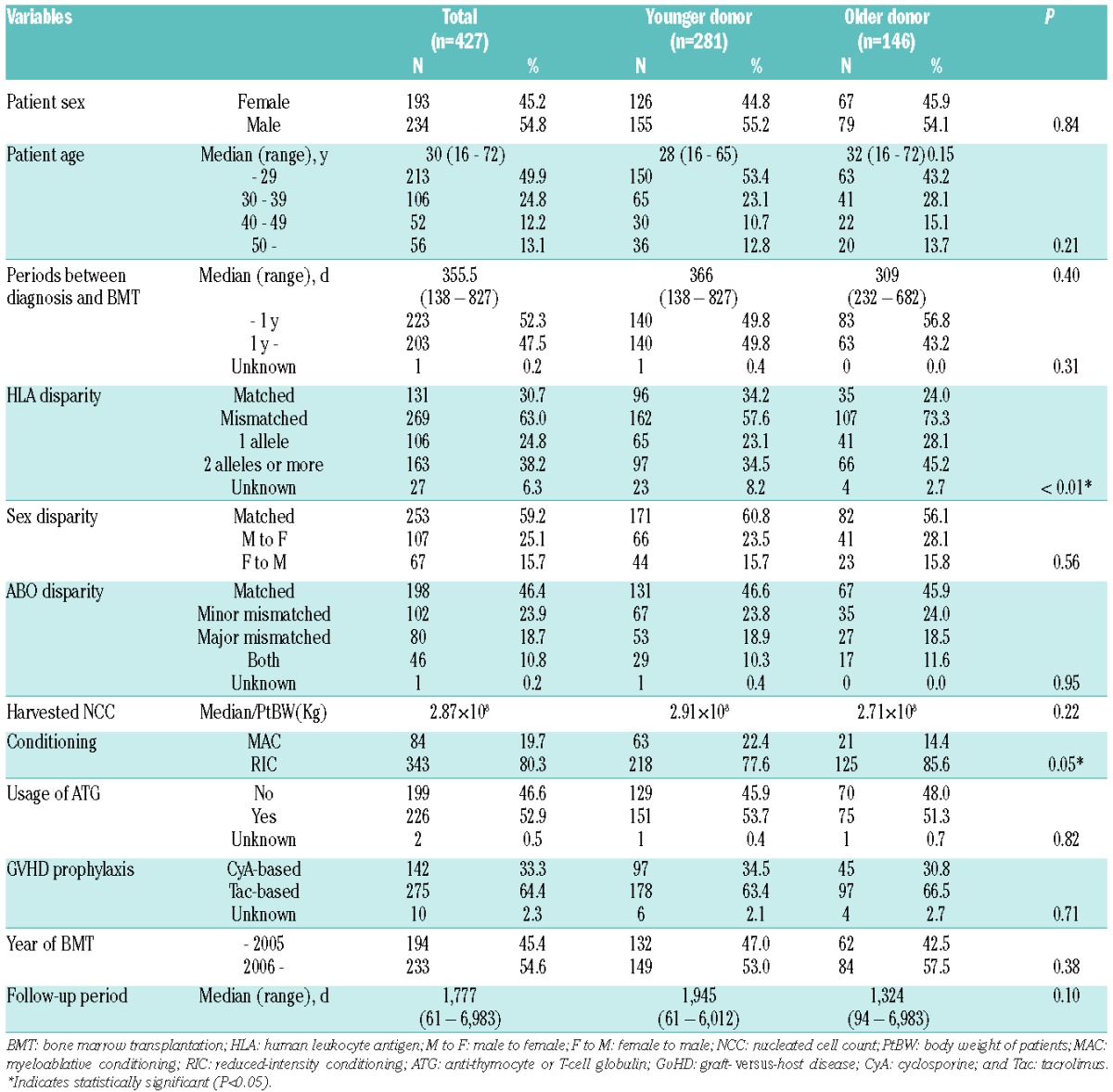
Overall survival was significantly worse following BMT from older donors
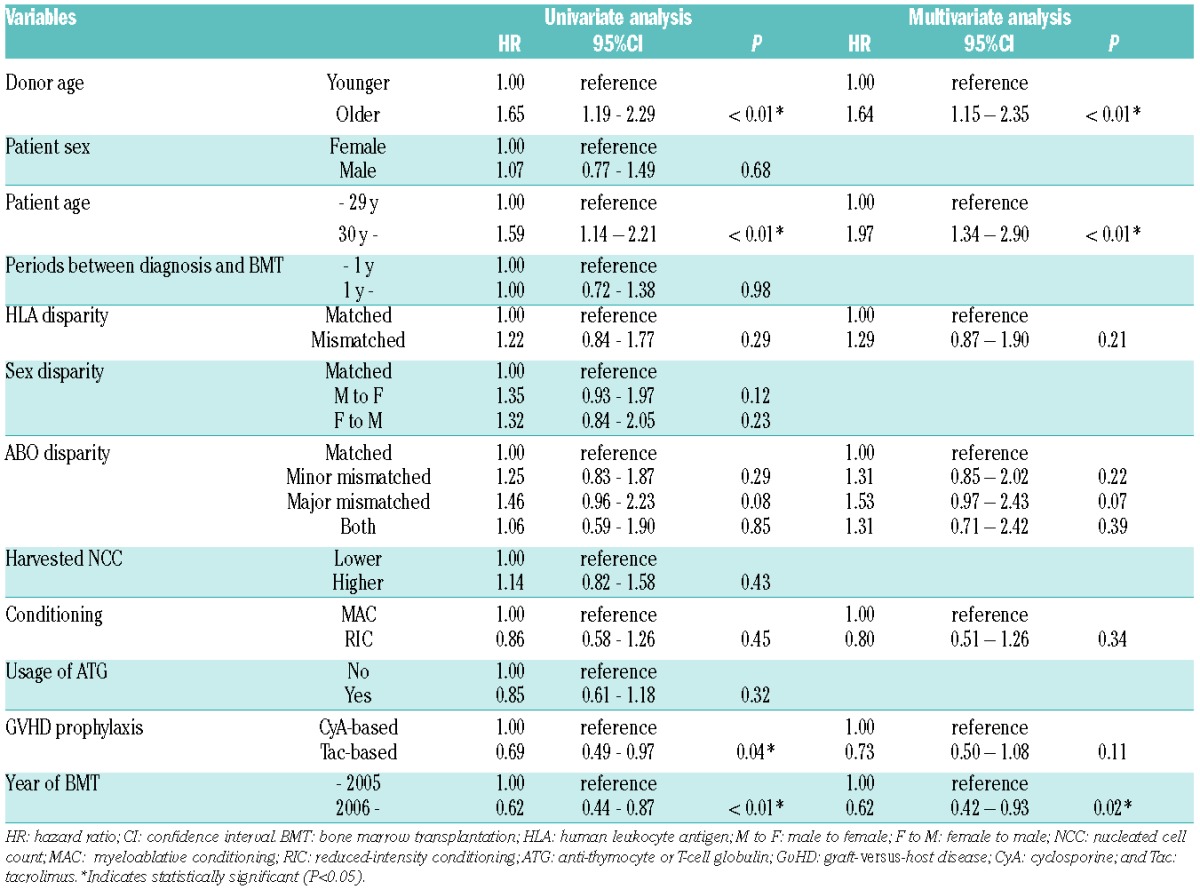
Overall survival of the older donor group was inferior to that of the younger donor group (65.9% vs. 77.7% at 1 year, 54.3% vs. 71.7% at 5 years after BMT) (Figure 1). This difference was significant in the univariate analysis [hazard ratio (HR)] of overall mortality in the older donor group compared to the younger donor group, 1.65; 95% confidence interval (CI) 1.19–2.29; P<0.01] (Table 2). Among other variables, older age of patients (≥30 years) (Online Supplementary Figure S2), ABO blood type major mismatch, GvHD prophylaxis with cyclosporine, and BMT before 2006 were associated with a worse survival (P<0.1). In the multivariate analysis, including these factors and the other known confounders (HLA disparity and conditioning regimens), the older donor group showed significantly higher overall mortality (HR 1.64; 95%CI: 1.15–2.25; P<0.01) (Table 2).
Figure 1.
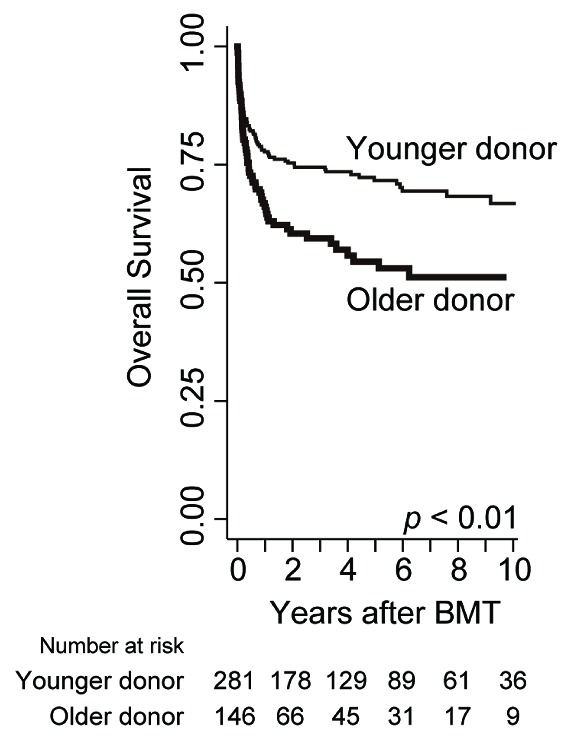
Prognosis after BMT in each donor age group. Overall survival (OS) is calculated with the Kaplan-Meier method in each donor age group, and compared with Cox proportional hazards model after being adjusted for confounding factors (see Table 2).
Table 2.
Overall mortality according to each variable before BMT.
This inferiority of OS in the older donor group (i.e. superiority in the younger donor group) was observed in each subgroup according to patient characteristics, with adjusted HRs being more than 1 in almost all subgroups (Figure 2). This tendency was also confirmed when we confined the analysis to only more recent cases (BMT after 2006) transplanted within one year after diagnosis using RIC regimen including ATG (n=128; adjusted HR 2.03; 95%CI: 0.94–4.39; P=0.07). Moreover, we compared OS between each donor group using Kaplan-Meier curves stratified by subgroups of patient age, HLA disparity, and conditioning regimens (Online Supplementary Figure S3), because patient age is a known strong prognostic factor,2 and HLA and conditioning regimens were statistically related to donor age in this cohort (Table 1). Differences in survival according to donor age were also apparent in each subgroup.
Figure 2.
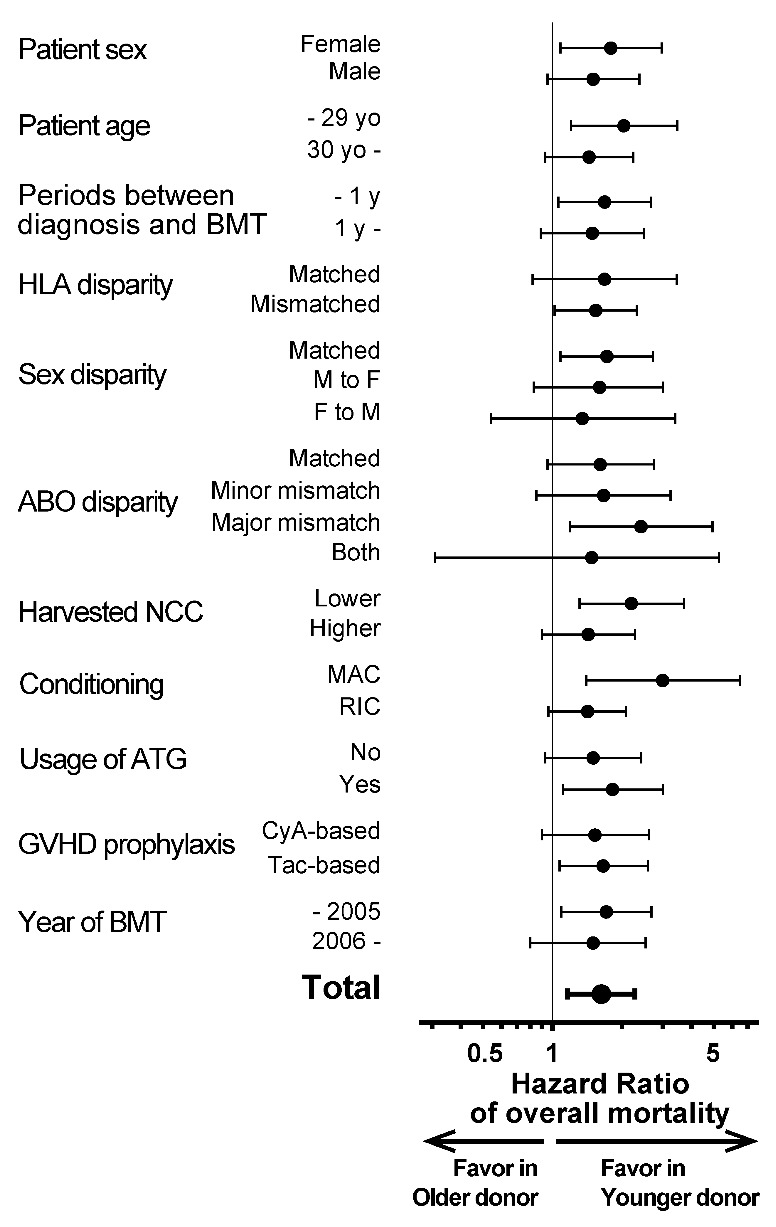
Subgroup analyses of overall survival (OS) with respect to patients’ pre-transplant characteristics. OS is compared in each subgroup with respect to pre-transplant patients’ characteristics. Hazard ratios (HRs) of overall mortality in the older donor group are shown in comparison with the younger donor group (i.e. HR >1 indicates better OS in the younger donor group). Black dots: HRs. Black bars: 95%CI ranges. CyA: cyclosporine; Tac: tacrolimus.
When treating donor age as a continuous variable (supposing that the increase of one year in donor age has the same impact on OS), it is significantly related to poorer OS in multivariate analyses adjusted by confounding factors (HR 1.03; 95%CI: 1.01–1.05 per one year increase in age, P<0.01; HR 1.36; 95%CI: 1.08–1.70 per 10 years increase in age) (Online Supplementary Table S2), supporting our findings obtained by analyses treating donor age as the binary variable, and indicating that donor age is the independent risk factor.
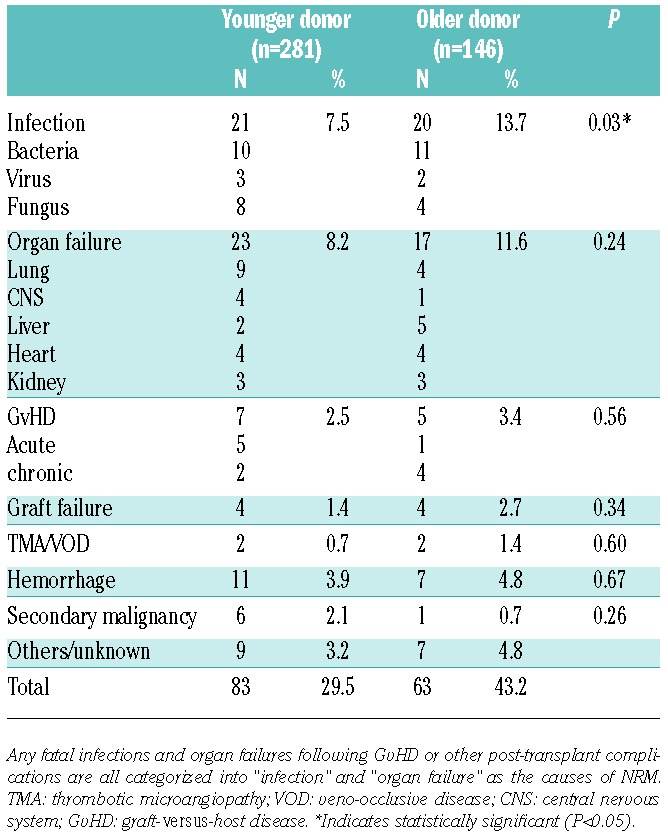
The causes of mortality were summarized and compared between the two donor groups (Table 3). The major causes included infection and organ failure in both groups, and the incidence of fatal infections, especially bacterial infections, was significantly higher in the older donor group (13.7% vs. 7.5%; P=0.03). The reasons for mortality beyond one year after BMT were also summarized, because OS decreased during this period, especially in the older donor group. GvHD, infections, and organ failures were more often documented in patients transplanted from older donors, though no significant differences were detected because of the relatively smaller number of patients (Online Supplementary Table S3).
Table 3.
Comparisons of the causes of mortality in each age group of donors.
Poorer engraftment and higher incidence of aGvHD were associated with older donors
In order to address the causes underlying the differences in OS and mortality between the younger and the older donor groups, we compared clinical courses between donor age groups, with a particular focus on engraftment and GvHD because they are critical parameters that may determine the prognosis of patients with AA after BMT.2
As for engraftment, the older donor group showed a significantly lower proportion of neutrophil and platelet engraftment following BMT (Table 4 and Figure 3A). Primary engraftment failure was more frequently observed in the older donor group than in the younger donor group (9.7% vs. 5.0%, HR 1.15; P<0.01). Neutrophil engraftment or engraftment failure was still significantly higher in the older donor group after multivariate analyses adjusted for confounding factors such as patient age, HLA disparity, ABO disparity, harvested NCC, conditioning regimens, and GvHD prophylaxis (adjusted P=0.01) (Table 4). When treating donor age as the continuous variable, adjusted HR of engraftment failure per 1-year increase in age is 1.01 (95%CI: 1.003–1.03; P<0.01) and this is 1.16 per 10-year increase (95%CI: 1.03–1.32).
Table 4.
Comparisons of clinical courses after BMT in each group of donors.
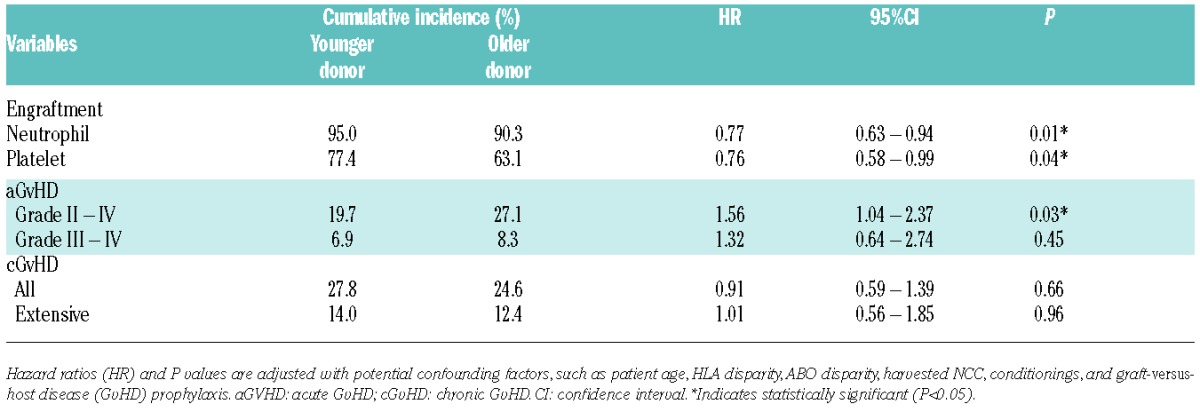
Figure 3.
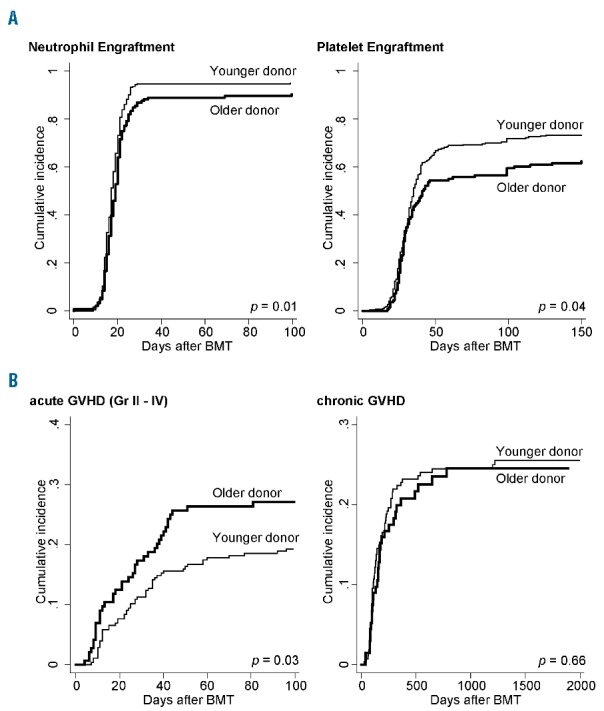
Cumulative incidence of engraftment and graft-versus-host disease (GvHD) in each donor age group. Incidence of (A) neutrophil and platelet engraftment and (B) acute GvHD (aGvHD) (grade II-IV) and chronic GvHD (cGvHD) (all grades) are calculated with Gray’s method considering death or salvage transplantation (after graft failure) as competing risks. Fine-Gray proportional hazard models are used to compare these incidences; P values are adjusted according to confounding factors, such as patient age, HLA disparity, ABO disparity, harvested NCC, conditionings, and GvHD prophylaxis.
With regard to GvHD, grade II–IV aGvHD was significantly more frequent in the older donor group (27.1% vs. 19.7%; adjusted HR 1.56; P=0.03) (Table 4 and Figure 3B), while there was no significant difference in grade III-IV aGvHD between groups (8.3% vs. 6.9%; adjusted HR 1.32; P=0.45); in addition, the incidence of cGvHD was almost the same in both groups (24.6% vs. 27.8%; adjusted HR 0.91; P=0.66) (Table 4 and Figure 3B). The incidence of grade II–IV aGvHD in the older donor groups compared with the younger donor groups was analyzed in various subgroups; the older donor group showed a tendency to have higher incidence of aGvHD in many subgroups, with higher HRs in older patients and HLA-mismatched transplantation (adjusted HR 2.07 and 1.61, respectively). Among patients diagnosed with grade II aGvHD, 33.5% of them were refractory to the primary corticosteroid administration, requiring the stronger immunosuppressive therapies for longer periods, while 66.6% in grade III-IV aGvHD patients underwent secondary therapies.
When treating donor age as the continuous variable, adjusted HR of grade II-IV aGvHD per 1-year increase in age is 1.03 (95%CI: 1.01–1.05; P=0.01) and per 10-year increase is 1.34 (95%CI: 1.05–1.72).
Impact of aGvHD on overall survival and its relationship to mortality
It has been thought that complications with aGvHD may directly result in poor OS in patients with AA because the graft-versus-host effect does not have the same merit as the graft-versus-leukemia effects observed in transplant for leukemia.17 To confirm this hypothesis in our cohort, we determined OS regarding aGvHD as a time-dependent covariate;25 as a result, aGvHD (grade II–IV) showed a tendency towards a worse prognosis in the whole cohort (adjusted HR 1.42; 95%CI: 0.95–2.11; P=0.08) and in both donor age groups. Landmark analysis (on day 30 or day 60 after BMT) also showed a trend towards a worse survival in patients with aGvHD (data not shown). Poor response to immunosuppressive therapies even in grade II aGvHD can support these data, and the higher incidence of aGvHD in the older donor group may partially account for the worse prognosis in this group due to cases of infection and organ failure (Table 3 and Online Supplementary Table S3).
Discussion
This cohort study regarding donor age and prognosis of unrelated BMT for AA revealed three major findings: 1) OS in transplantation from older donors (>40 years old) was significantly worse than that from younger donors; 2) neutrophil and platelet engraftment was suppressed and engraftment failure was more often observed following transplant from older donors; and 3) the older donor group had a higher incidence of aGvHD.
First, we clearly showed an inferior prognosis in the older donor group compared to the younger donor group. This result was confirmed by multivariate and various subgroup analyses, in order to exclude the influence of confounding factors, such as patient age, HLA disparity, conditionings, etc. Our data indicate that older donor age can be considered an independent risk factor for poor prognosis after unrelated BMT for AA irrespective of whether it is treated as the binary covariate or the continuous covariate. It should be emphasized that donor age was not correlated with patient age (which is the strongest prognostic factor2) either in our whole cohort (Online Supplementary Figure S1) or in any subgroup of patients’ characteristics, such as sex, HLA disparity, conditioning regimens, GvHD prophylaxis, and year of BMT. As far as we know, so far there have been no reports of a relationship between donor age and prognosis in AA patients.
This difference in prognosis can be explained in part by the significantly higher incidence of fatal infection (especially bacterial infection) in the older donor group (Table 3), which may have been due to insufficiency or dysfunction of immune cells derived from older donor grafts. Actually, this speculation is supported by previous studies in mice indicating that recovery of the absolute number of lymphocytes in the early post-transplant period was delayed in recipients transplanted from older donors even after bone marrow engraftment, suggesting the delayed recovery of cytotoxic T cells and immunoglobulin-secreting B cells (leading to hypogammaglobulinemia).26,27 Moreover, suppression of neutrophil function was shown in neutrophils from aged donors due to the decrease in secondary messenger generation, such as diacylglycerol and inositol-triphosphate, and the defect in superoxide generation which is essential for bacterial killing.28 Unfortunately, there were no data on lymphocyte characteristics and neutrophil function in our dataset, but our epidemiological data and biological studies in mice suggest that controlling severe infection, especially bacterial infection, might be a key issue in improving prognosis following transplantation from older donors.
Next, we observed a relationship between older donor grafts and a higher incidence of primary graft failure in both analyses, whether treating donor age as the binary or as the continuous variables. Engraftment of donor grafts is an essential factor in transplantation in AA.2 The inferiority in engraftment with older donors in combination with poor recovery of CD4+ naïve T cells and B cells mentioned above may increase opportunistic infections and account for the worse OS. In addition, higher transplant-related mortality following salvage secondary transplant after engraftment failure generally results in an even worse prognosis.
Poor engraftment with older donors has also been shown in murine transplant models,5,6 and this kind of “aging” in grafts from older donors may be related to age-associated modifications in DNA methylation patterns29 and/or shorter length of telomeres in hematopoietic stem or progenitor cells from older donors.30
Finally, we showed a higher incidence of aGvHD in older donors in both analyses, whether treating donor age as the binary or the continuous variable. This may be explained by the higher ratio of memory T-cell to naïve T-cell subsets in older people;7 recent clinical studies have shown that peripheral blood CD8+ effector memory T cells are closely associated with aGvHD in humans,8,9 in contrast to previous findings in a murine model.31 Different gene expression profiles regarding GvHD, such as transforming growth factor-β in CD4+ and CD8+ T cells, were also shown in older donors.32 In our cohort, all the grafts were injected without ex vivo T-cell depletion; therefore, it may be speculated that massive amounts of antigen-experienced memory T cells (including those which can recognize and attack the recipient-specific major and/or minor histocompatibility antigens) were injected, especially in cases with older donors, which initiated an allo-reaction leading to aGvHD. At the same time, a hyper-acute phase of aGvHD targeting the bone marrow niche may induce engraftment failure in BMT from an older donor.33 These speculations suggest that appropriate use of ATG could be helpful in overcoming this disadvantage in choosing older donor-derived bone marrow grafts.
The impact of aGvHD on OS is another important point that needs to be discussed. In transplantation for hematologic malignancies, aGvHD, if not severe and beyond control, can be an indicator for better survival because GvHD may guarantee graft-versus-tumor effects that can suppress post-transplant relapse.17 In AA, however, we confirmed that GvHD, regardless of the severity, does not have any beneficial effects on patients, and worsens prognosis; grade II-IV aGvHD was related to inferior OS in both donor age groups, and grade III-IV aGvHD increased mortality to an even greater extent (HR 3.19; P<0.01). One of the explanations for this inferior survival is the refractoriness of aGvHD in our cohort; more than 30% of patients were refractory to the initial steroid therapy even in grade II aGvHD, and more than 60% of those with grade III-IV required secondary immunosuppressive therapies. Therefore, the higher incidence of aGvHD following BMT from older donors may also explain the worse prognosis in this group.
Graft-versus-host disease was selected as the main cause of mortality in only a small number of patients, and there was no difference between donor age groups (Table 3). It is suspected that most of the patients who experienced long-term episodes of GvHD acquired fatal infection or organ dysfunction after continuous immunosuppressive status due to the nature of the GvHD itself or its treatment.34,35 Among these patients, the main cause of mortality was recorded as infection or organ failure in our database.
In summary, we found the inferiority of older donors in unrelated BMT for AA compared to younger donors (treated as the binary covariate; >40 years vs. >39 years or older, or the continuous covariate), mainly because of the higher incidence of engraftment failure and aGvHD in the former group; these complications can induce fatal infections. This analysis suggests that donor age should receive a special focus as criterion when multiple unrelated donors are available for AA, and there should be a concerted effort to recruit younger voluntary candidate BMT donors. Our study, however, was retrospective in design and was conducted in only one country. In addition, due to the long period of patient recruitment, protocols were not necessarily compatible with the current guidelines in some patients; the widely recommended protocol is to transplant as soon as possible after diagnosis with a conditioning regimen including cyclophosphamide, ATG, and low-dose TBI.24 We confirm that our main results can be reproduced in the subgroup analyses of patients who were treated according to the current guidelines. Moreover, it is difficult to carry forward a discussion regarding the choice between a younger unrelated donor and an older matched sibling donor in a retrospective study; therefore, large-scale international prospective studies are needed to validate these results and to revise the donor selection algorithm for the future.
Acknowledgments
The authors would like to thank all the physicians and data managers at the centers who contributed valuable data on transplantation to the Japan Society for Hematopoietic Cell Transplantation (JSHCT), Japan Marrow Donor Program (JMDP), and TRUMP.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/101/5/644
Funding
This study was supported by research funding from the Ministry of Education, Science, Sports, and Culture in Japan to T. Kondo.
References
- 1.Marsh JC, Ball SE, Cavenagh J, et al. Guidelines for the diagnosis and management of aplastic anaemia. Br J Haematol. 2009;147(1):43–70. [DOI] [PubMed] [Google Scholar]
- 2.Marotta S, Pagliuca S, Risitano AM. Hematopoietic stem cell transplantation for aplastic anemia and paroxysmal nocturnal hemoglobinuria: current evidence and recommendations. Expert Rev Hematol. 2014;7(6):775–789. [DOI] [PubMed] [Google Scholar]
- 3.Gupta V, Eapen M, Brazauskas R, et al. Impact of age on outcomes after bone marrow transplantation for acquired aplastic anemia using HLA-matched sibling donors. Haematologica. 2010;95(12):2119–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stern M, Passweg JR, Locasciulli A, et al. Influence of donor/recipient sex matching on outcome of allogeneic hematopoietic stem cell transplantation for aplastic anemia. Transplantation. 2006;82(2):218–226. [DOI] [PubMed] [Google Scholar]
- 5.Liang Y, Van Zant G, Szilvassy SJ. Effects of aging on the homing and engraftment of murine hematopoietic stem and progenitor cells. Blood. 2005;106(4):1479–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kamminga LM, van Os R, Ausema A, et al. Impaired hematopoietic stem cell functioning after serial transplantation and during normal aging. Stem Cells. 2005;23(1):82–92. [DOI] [PubMed] [Google Scholar]
- 7.Miller RA. The aging immune system: primer and prospectus. Science. 1996;273(5271):70–74. [DOI] [PubMed] [Google Scholar]
- 8.Khandelwal P, Lane A, Chaturvedi V, et al. Peripheral Blood CD38 Bright CD8+ Effector Memory T Cells Predict Acute Graft-versus-Host Disease. Biol Blood Marrow Transplant. 2015;21(7):1215–1222. [DOI] [PubMed] [Google Scholar]
- 9.Loschi M, Porcher R, Peffault de Latour R, et al. High number of memory t cells is associated with higher risk of acute graft-versus-host disease after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2015;21(3):569–574. [DOI] [PubMed] [Google Scholar]
- 10.Kollman C, Howe CW, Anasetti C, et al. Donor characteristics as risk factors in recipients after transplantation of bone marrow from unrelated donors: the effect of donor age. Blood. 2001;98(7):2043–2051. [DOI] [PubMed] [Google Scholar]
- 11.Carreras E, Jimenez M, Gomez-Garcia V, et al. Donor age and degree of HLA matching have a major impact on the outcome of unrelated donor haematopoietic cell transplantation for chronic myeloid leukaemia. Bone Marrow Transplant. 2006;37(1):33–40. [DOI] [PubMed] [Google Scholar]
- 12.Mehta J, Gordon LI, Tallman MS, et al. Does younger donor age affect the outcome of reduced-intensity allogeneic hematopoietic stem cell transplantation for hematologic malignancies beneficially? Bone Marrow Transplant. 2006;38(2):95–100. [DOI] [PubMed] [Google Scholar]
- 13.Fabre C, Koscielny S, Mohty M, et al. Younger donor’s age and upfront tandem are two independent prognostic factors for survival in multiple myeloma patients treated by tandem autologous-allogeneic stem cell transplantation: a retrospective study from the Societe Francaise de Greffe de Moelle et de Therapie Cellulaire (SFGM-TC). Haematologica. 2012;97(4):482–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finke J, Schmoor C, Bethge WA, et al. Prognostic factors affecting outcome after allogeneic transplantation for hematologi-cal malignancies from unrelated donors: results from a randomized trial. Biol Blood Marrow Transplant. 2012;18(11):1716–1726. [DOI] [PubMed] [Google Scholar]
- 15.Servais S, Porcher R, Xhaard A, et al. Pre-transplant prognostic factors of long-term survival after allogeneic peripheral blood stem cell transplantation with matched related/unrelated donors. Haematologica. 2014;99(3):519–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arai Y, Aoki K, Takeda J, et al. Clinical significance of high-dose cytarabine added to cyclophosphamide/total-body irradiation in bone marrow or peripheral blood stem cell transplantation for myeloid malignancy. J Hematol Oncol. 2015;8(102). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arai Y, Takeda J, Aoki K, et al. Efficiency of high-dose cytarabine added to CY/TBI in cord blood transplantation for myeloid malignancy. Blood. 2015;126(3):415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olsson R, Remberger M, Schaffer M, et al. Graft failure in the modern era of allogeneic hematopoietic SCT. Bone Marrow Transplant. 2013;48(4):537–543. [DOI] [PubMed] [Google Scholar]
- 19.Atsuta Y, Suzuki R, Yoshimi A, et al. Unification of hematopoietic stem cell transplantation registries in Japan and establishment of the TRUMP System. Int J Hematol. 2007;86(3):269–274. [DOI] [PubMed] [Google Scholar]
- 20.Kanda J, Fuji S, Kato S, et al. Decision analysis for donor selection in stem cell transplantation-HLA-8/8 allele-matched unrelated donor vs HLA-1 AG mismatched related donor. Blood Cancer J. 2014;4:e263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giralt S, Ballen K, Rizzo D, et al. Reduced-intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the center for international blood and marrow transplant research. Biol Blood Marrow Transplant. 2009;15(3):367–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glucksberg H, Storb R, Fefer A, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18(4):295–304. [DOI] [PubMed] [Google Scholar]
- 23.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11(12):945–956. [DOI] [PubMed] [Google Scholar]
- 24.Socie G. Allogeneic BM transplantation for the treatment of aplastic anemia: current results and expanding donor possibilities. Hematology Am Soc Hematol Educ Program. 2013;2013:82–86. [DOI] [PubMed] [Google Scholar]
- 25.Iacobelli S, Committee ES. Suggestions on the use of statistical methodologies in studies of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2013;48Suppl 1:S1–37. [DOI] [PubMed] [Google Scholar]
- 26.Hirayama M, Azuma E, Jiang Q, et al. The reconstitution of CD45RBhiCD4+ naive T cells is inversely correlated with donor age in murine allogeneic haematopoietic stem cell transplantation. Br J Haematol. 2000;111(2):700–707. [DOI] [PubMed] [Google Scholar]
- 27.Azuma E, Hirayama M, Yamamoto H, Komada Y. The role of donor age in naive T-cell recovery following allogeneic hematopoietic stem cell transplantation: the younger the better. Leuk Lymphoma. 2002;43(4):735–739. [DOI] [PubMed] [Google Scholar]
- 28.Lipschitz DA, Udupa KB, Indelicato SR, Das M. Effect of age on second messenger generation in neutrophils. Blood. 1991;78(5):1347–1354. [PubMed] [Google Scholar]
- 29.Poglio S, Cahu X, Uzan B, et al. Rapid childhood T-ALL growth in xenograft models correlates with mature phenotype and NF-kappaB pathway activation but not with poor prognosis. Leukemia. 2015;29(4):977–980. [DOI] [PubMed] [Google Scholar]
- 30.Gadalla SM, Wang T, Haagenson M, et al. Association between donor leukocyte telomere length and survival after unrelated allogeneic hematopoietic cell transplantation for severe aplastic anemia. JAMA. 2015;313(6):594–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moncrieffe H, Coles M, Stockinger B. The influence of CD4 T-cell subsets on control of CD4 T-cell-mediated graft-versus-host disease. Immunology. 2008;125(4):459–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baron C, Somogyi R, Greller LD, et al. Prediction of graft-versus-host disease in humans by donor gene-expression profiling. PLoS Med. 2007;4(1):e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shono Y, Ueha S, Wang Y, et al. Bone marrow graft-versus-host disease: early destruction of hematopoietic niche after MHC-mismatched hematopoietic stem cell transplantation. Blood. 2010;115(26):5401–5411. [DOI] [PubMed] [Google Scholar]
- 34.Arai Y, Yamashita K, Mizugishi K, et al. Risk factors for hypogammaglobulinemia after allo-SCT. Bone Marrow Transplant. 2014;49(6):859–861. [DOI] [PubMed] [Google Scholar]
- 35.Arai Y, Yamashita K, Mizugishi K, et al. Serum neutrophil extracellular trap levels predict thrombotic microangiopathy after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2013; 19(12): 1683–1689. [DOI] [PubMed] [Google Scholar]


