Research efforts to establish therapeutic modalities for treating sickle cell disease (SCD) and β-thalassemia have been hindered by our incomplete understanding of the developmental regulation of human β-like globin gene expression. It has been demonstrated that increased embryonic globin (ε-globin) gene expression, similar to fetal globin (γ-globin), can ameliorate the severity of SCD and β-thalassemia in mice. Therefore, elucidating the molecular mechanism of ε-globin gene silencing is of biologic and clinical importance. Herein, we show that a histone lysine methyltransferase, SUV4-20h1, plays a critical role in silencing ε-globin gene expression in both K562 cells and primary erythroid progenitor cells. We found that ε-globin gene expression could be modulated by coordinate SUV4-20h1-dependent histone modification and DNA methylation. We also found that SUV4-20h1 repressed embryonic globin gene expression in mice, suggesting a conserved role for SUV4-20h1 during mammalian evolution. These results identify SUV4-20h1 as a novel epigenetic suppressor of ε-globin gene expression implying a potential alternative therapeutic approach for the treatment of β-thalassemia and SCD.
The human β-like globin genes, located on chromosome 11, comprise 5 homologous genes (5′-ε−Gγ-Aγ-δ-β−3′) arranged in order of their expression during development.1 Although the mechanisms that fine tune the expression of these globin genes are largely unknown, it is believed that developmental stage-specific factors, including transcription factors and epigenetic modulators, are involved.2,3 The β-hemoglobinopathies, such as sickle cell disease (SCD) and β-thalassemia, are the most common single-gene inherited red blood cell disorders.2 Increased fetal hemoglobin (γ-globin) expression significantly ameliorates the symptoms of both diseases, providing a rational basis for molecular therapeutic strategies. As is the case for γ-globin, the embryonic hemoglobin (ε-globin) gene remains intact, but its expression is autonomously silenced after birth.4 If reactivated in adults, ε-globin can also function in a similar manner as γ-globin to ameliorate symptoms of SCD and β-thalassemia.5,6 Expression of human ε-globin has been shown to restore the normal phenotype in mouse models of SCD and β-thalassemia.5,6 Various transcription factors, including GATA1, YY1, EKLF, BCL11A, SOX6, and TR2/TR4, have been shown to directly regulate ε-globin gene expression.7–11
In previous studies, we showed that PRMT5 induces coordinated repressive epigenetic marks in erythroid cells at the γ-promoter via the assembly of a multiprotein repressor complex containing, among other components, the histone modifying enzyme SUV4-20h1.12,13 To investigate the role of SUV4-20h1 in ε−globin gene expression, we examined both mRNA and protein levels of ε−globin in SUV4-20h1 knockdown K562 cells compared to control cells expressing scrambled shRNAs. In the knockdown cell line, the level of SUV4-20h1 mRNA was reduced by approximately 90% (Figure 1A). Concomitantly, we detected a dramatic increase (over 400-fold) in ε-globin gene expression as well as a significant increase in γ-globin gene expression (Figure 1B) and a substantial induction of ε−globin protein in SUV4-20h1 knockdown cells (Figure 1C). Since antibodies suitable for ChIP analysis of human SUV4-20h1 are not currently available, we used K562 cell line stably overexpressing hemagglutinin (HA)-tagged SUV4-20h1, and performed ChIP analysis using anti-HA antibody. SUV4-20h1 was found bind to the ε-promoter (Figure 1D). In addition, both PRMT5 and DNMT3A also bound to the ε-promoter (Figure 1E). As expected, the enrichment of histone mark H4K20me3 on the ε-promoter was decreased in SUV4-20h1 knockdown cells compared to the scramble control cells (Figure 1F, left panel). Similar to the scenario on the γ-promoter, the enrichment of histone mark H4R3me2s triggered by PRMT5 on the ε-promoter was substantially decreased in SUV4-20h1 knockdown cells compared to the scramble control cells (Figure 1F, right panel). These results indicate that ε-globin gene silencing induced by SUV4-20h1 is associated with coordinated histone modification changes, and suggest that SUV4-20h1 plays a critical role in ε-globin gene silencing.
Figure 1.
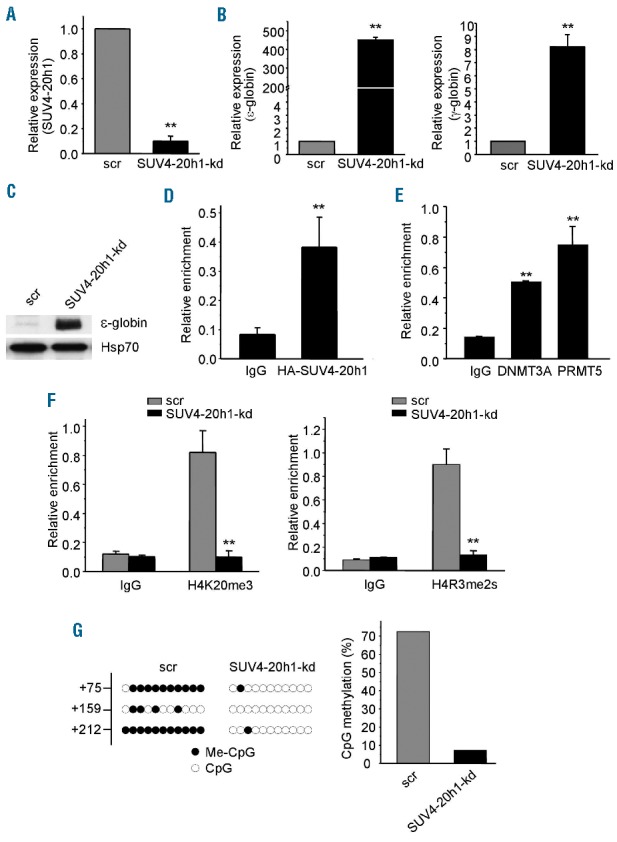
SUV4-20h1 represses ε-globin expression in K562 cells. (A) SUV4-20h1 gene expression analysis by Q-RT-PCR of RNA extracted from SUV4-20h1 knockdown (SUV4-20h1-kd) and scrambled control (scr) K562 cells. Data are normalized to GAPDH mRNA. Results are shown as mean ± SD from three independent experiments; **P<0.01 compared to the scrambled control. (B) ε-globin and γ-globin gene expression analysis by Q-RT-PCR of RNA from SUV4-20h1-kd and scrambled control (scr) K562 cells. Data are normalized to GAPDH mRNA. Results are shown as mean ± SD from three independent experiments; **P<0.01 compared to the scrambled control. (C) Western blot analysis with indicated antibodies of cellular extracts from SUV4-20h1-kd and scrambled (scr) control K562 cells. Hsp70 served as a loading control. (D) ChIP analysis of SUV4-20h1 binding to the ε-globin proximal promoter. HA-tagged SUV4-20h1 detected with anti-HA antibody; mouse IgG served as a control. Results are shown as mean ± SD from three independent experiments; **P<0.01 compared to control. (E) ChIP analysis of DNMT3A and PRMT5 binding to the ε-globin proximal promoter. IgG from mouse served as a control. Results are shown as mean ± SD from three independent experiments; **P<0.01 compared to control. (F) ChIP analysis of histone mark H4K20me3 (left panel) and H4R3me2s (right panel) on the ε-globin promoter of SUV4-20h1-kd cells and scrambled control cells. Results are shown as mean ± SD from three independent experiments; **P<0.01 compared to the scrambled control. (G) DNA methylation at the human ε-gene in SUV4-20h1 knockdown and scrambled control cells. Each line shows the methylation status of individual CpG dinucleotides derived from sequence analysis of 10 representative (of at least 25) individual cloned polymerase chain reaction (PCR) products of the ε-gene after bisulfite modification of the DNA from SUV4-20h1 knockdown and control cells. The differences between the knockdown lines and the scrambled controls are significant (Fisher’s exact test, P<0.0001). The numbers on the left represent the positions of CpG dinucleotides relative to the transcriptional start site of the ε-gene. The results are quantitated in the bar chart (right panel).
Gene silencing is associated with histone modification and methylation of CpG dinucleotides in the proximal promoter region.14 We have previously demonstrated that SUV4-20h1 knockdown led to a distinct reduction in methylation of 4 CpG dinucleotides in the proximal promoter region of the γ-gene resulting in activation of γ-globin expression.12 Similarly, the levels of DNA methylation of three CpG dinucleotides in the first exon of the ε-globin gene were also greatly reduced in SUV4-20h1 knockdown cells compared to the scrambled control cells (Figure 1G). These data are consistent with the induction of ε-globin gene expression in SUV4-20h1 knockdown cells, and are in line with our previous findings that SUV4-20h1, PRMT5, and DNMT3A exert coordinate functions in γ-gene silencing.12,13
Next, we examined the role of SUV4-20h1 in human adult primary erythroid progenitor cells. As previously reported, to mimic normal erythroid cell differentiation from undifferentiated blasts to orthochromatic normoblasts, purified CD34+ hematopoietic progenitors were cultured through an expansion stage and differentiation period in different conditioned media.10 To examine the effect of SUV4-20h1 knockdown in adult bone marrow cells, we stably knocked down SUV4-20h1 via lentivirus-mediated shRNA infection. SUV4-20h1 expression was reduced to ~50% of scrambled control in the knockdown cells (Figure 2A). In addition to our previous observation that γ-globin was significantly reactivated (~10-fold induction), absolute quantitative analysis of globin gene mRNA showed that expression of the ε-gene was also increased by a robust ~70-fold in SUV4-20h1 knockdown cells (Figure 2B). Expression of the β-globin gene in these cells was not elevated (Figure 2B). This effect was specific as expression of other erythroid transcription factors, such as GATA1, NF-E2, BCL11A, and KLF1, remained unchanged (Figure 2C). Consistent with these findings, flow cytometric analysis of the erythroid surface markers transferrin receptor (CD71) and glycophorin A (CD235a) demonstrated a similar percentage of double positive (CD71+/CD235a+) mature erythroid cells (Figure 2D). No morphological differences were observed in these cells after SUV4-20h1 knockdown, indicating that SUV4-20h1 may not perturb the overall process of erythroid differentiation (Figure 2D). Interestingly, the bisulfite DNA methylation sequencing analysis revealed that the levels of DNA methylation of three CpG dinucleotides in the first exon of the ε-globin gene were also significantly reduced in SUV4-20h1 knockdown bone marrow cells compared to the scrambled control cells (Figure 2E). These results further indicated that expression of ε-globin is controlled by a coordinated epigenetic program associated with SUV4-20h1.
Figure 2.
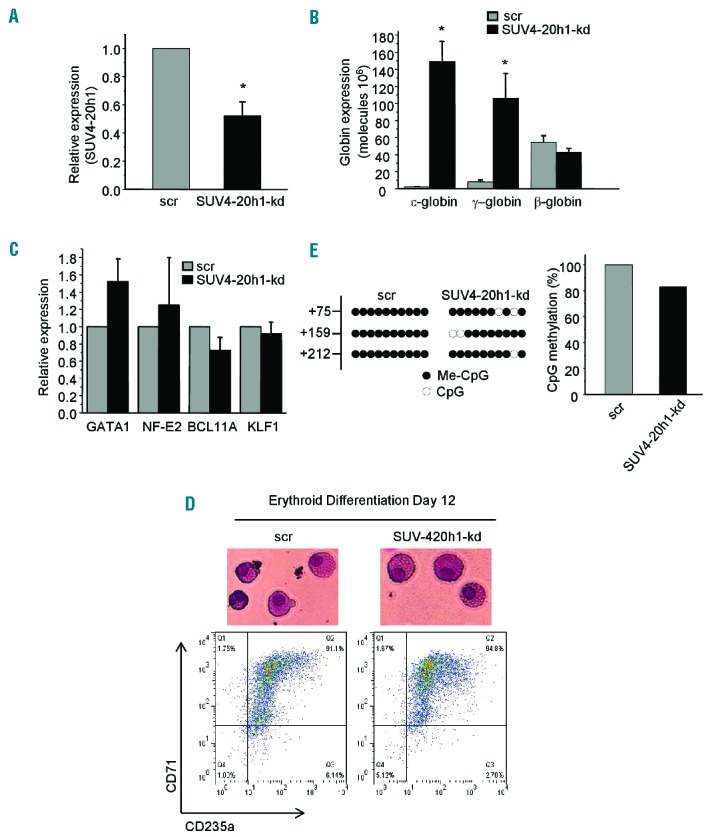
SUV4-20h1 represses ε-globin expression in primary erythroid progenitor cells. (A) Q-RT-PCR analysis of SUV4-20h1 gene expression in primary erythroid progenitor cells of bone marrow (PEBM) from SUV4-20h1 knockdown and scrambled control. Data are normalized to GAPDH mRNA. Results are shown as mean ± SD from three independent experiments; *P<0.05 compared to the scrambled control. (B) Q-RT-PCR analysis of ε-globin, γ-globin, and β-globin gene expression in SUV4-20h1 knockdown and scrambled control PEBM cells. Plasmid DNA encoding ε-globin, γ-globin or β-globin was used to generate the standard curve for determination of copy number. Results are shown as mean ± SD from three independent experiments; *P<0.05 compared to the scrambled control. (C) Q-RT-PCR analysis of GATA1, NF-E2, BCL11A, and KLF1 gene expression in SUV4-20h1-kd and scrambled control PEBM cells. Data are normalized to GAPDH mRNA. Results are shown as mean ± SD from three independent experiments. (D) Wright-Giemsa-stained PEBM cells from day 12 of differentiation (top panel). Flow cytometric analysis of CD71 and CD235 expression of SUV4-20h1-kd and scrambled PEBM cells on day 12 of differentiation (bottom panel). (E) Effect of perturbed SUV4-20h1 expression on DNA methylation at the human ε-gene of PEBM cells. Each lane shows the methylation status of individual CpG dinucleotides derived from sequence analysis of 10 representative individual cloned PCR products of the ε-gene following bisulfite modification of the DNA from SUV4-20h1 knockdown and scrambled control PEBM cells. The differences between the two lines were significant (Fisher’s exact test, P<0.001). The numbers on the left represent the position of the CpG dinucleotides relative to the transcriptional start site of the ε-gene. The results are quantitated in the bar chart (right panel).
The mouse and human β-like loci are highly conserved and share many functional elements.10 To determine if SUV4-20h1-regulated silencing of the embryonic globin gene is conserved between mouse and human, we examined the expression levels of murine globins, including embryonic εy- and βh1-globin, from embryos of SUV4-20h1 knockout mice which display perinatal lethality.15 SUV4-20h1 mRNA expression was almost undetectable in embryos at both day 11.5 and 13.5, confirming knockout of the SUV4-20h1 gene in these mice (Figure 3A). Expression levels of εy-, βh1-, βmin- and βmaj-globin genes in knockout and wild-type mice were examined at two developmental stages. At day 10.5, no induction of murine embryonic globin was observed in the yolk sac of SUV4-20h1 knockout mice (Figure 3B, left panel). Interestingly, at day 13.5, embryonic εy- and βh1-gene expression in fetal liver had increased 3- to 5-fold in the SUV4-20h1 knockout mice compared to the wild-type control mice (Figure 3B, right panel). βmaj- and βmin-globin gene expression in fetal liver of these mice did not change significantly (Figure 3B, right panel). These results suggest that SUV4-20h1 modulates embryonic globin gene silencing in mouse, and are consistent with the role of SUV4-20h1 in regulating ε- and γ-globin gene expression in human. Therefore, SUV4-20h1 seems to be a potential alternative therapeutic target for treatment of SCD and β-thalassemia. It would be very informative to breed the SUV4-20h1 conditional knockout mice into one of the model mice of SCD or β-thalassemia and test whether the increase in embryonic and fetal globin gene expression is sufficient to ameliorate the disease.
Figure 3.
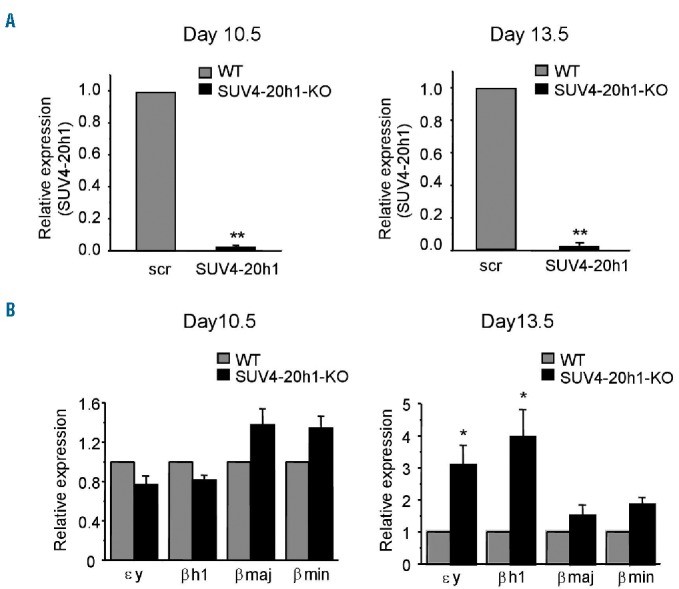
Effect of SUV4-20h1 on murine globin gene expression. (A) Relative mRNA expression of SUV4-20h1 is examined by Q-RT-PCR in SUV4-20h1−/− and wildtype (WT) mice at two developmental stages. Data are normalized to GAPDH mRNA. Total RNA was extracted from the yolk sac at day 10.5 and from fetal liver at day 13.5. Results are shown as mean ± SD from three SUV4-20h1−/− or WT mice; **P<0.01 compared to WT mice. (B) Q-RT-PCR analysis of εy-globin, βh1-globin, βmaj-globin, and βmin-globin gene expression in mRNA from the yolk sac at day 10.5 and from fetal liver at day 13.5 from SUV4-20h1−/− and WT mice. Data are normalized to GAPDH mRNA. Results are shown as mean ± SD from three SUV4-20h1−/− and three WT mice; *P<0.05 compared to WT mice.
In summary, we have identified SUV4-20h1 as a novel epigenetic regulator of embryonic globin, although the precise mechanism by which ε-globin gene expression is regulated remains elusive. Our results suggest that therapeutic intervention with SUV4-20h1 might be used to treat SCD and β-thalassemia by simultaneously elevating both ε- and γ-globin.
Acknowledgments
We are grateful to members of the Jane and Zhao laboratories for helpful discussions.
Footnotes
Funding: this work was supported by National Natural Science Foundation of China NSFC31170716, 31470750, 81421091, 31270811, 2015M571737 and SQJ Biotechnologies Limited.
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Stamatoyannopoulos JA, Nienhuis AW. Therapeutic approaches to hemoglobin switching in treatment of hemoglobinopathies. Annu Rev Med. 1992;43:497–521. [DOI] [PubMed] [Google Scholar]
- 2.Higgs DR, Engel JD, Stamatoyannopoulos G. Thalassaemia. Lancet. 2012;379(9813):373–383. [DOI] [PubMed] [Google Scholar]
- 3.Ginder GD. Epigenetic regulation of fetal globin gene expression in adult erythroid cells. Transl Res. 2015;165(1):115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gumucio DL, Shelton DA, Zhu W, et al. Evolutionary strategies for the elucidation of cis and trans factors that regulate the developmental switching programs of the beta-like globin genes. Mol Phylogenet Evol. 1996;5(1):18–32. [DOI] [PubMed] [Google Scholar]
- 5.He Z, Russell JE. A human embryonic hemoglobin inhibits Hb S polymerization in vitro and restores a normal phenotype to mouse models of sickle cell disease. Proc Natl Acad Sci USA. 2002; 99(16):10635–10640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Russell JE, Liebhaber SA. Reversal of lethal alpha- and beta-thalassemias in mice by expression of human embryonic globins. Blood. 1998;92(9):3057–3063. [PubMed] [Google Scholar]
- 7.Raich N, Clegg CH, Grofti J, Romeo PH, Stamatoyannopoulos G. GATA1 and YY1 are developmental repressors of the human epsilon-globin gene. EMBO J. 1995;14(4):801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanimoto K, Liu Q, Grosveld F, Bungert J, Engel JD. Context-dependent EKLF responsiveness defines the developmental specificity of the human epsilon-globin gene in erythroid cells of YAC transgenic mice. Genes Dev. 2000;14(21):2778–2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alhashem YN, Vinjamur DS, Basu M, Klingmuller U, Gaensler KM, Lloyd JA. Transcription factors KLF1 and KLF2 positively regulate embryonic and fetal beta-globin genes through direct promoter binding. J Biol Chem. 2011;286(28):24819–24827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu J, Sankaran VG, Ni M, et al. Transcriptional silencing of {gamma}-globin by BCL11A involves long-range interactions and cooperation with SOX6. Genes Dev. 2010;24(8):783–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cui S, Tanabe O, Sierant M, et al. Compound loss of function of nuclear receptors Tr2 and Tr4 leads to induction of murine embryonic beta-type globin genes. Blood. 2015;125(9):1477–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rank G, Cerruti L, Simpson RJ, Moritz RL, Jane SM, Zhao Q. Identification of a PRMT5-dependent repressor complex linked to silencing of human fetal globin gene expression. Blood. 2010;116(9):1585–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao Q, Rank G, Tan YT, et al. PRMT5-mediated methylation of histone H4R3 recruits DNMT3A, coupling histone and DNA methylation in gene silencing. Nat Struct Mol Biol. 2009;16(3):304–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lavelle D, Vaitkus K, Hankewych M, Singh M, DeSimone J. Developmental changes in DNA methylation and covalent histone modifications of chromatin associated with the epsilon-, gamma-, and beta-globin gene promoters in Papio anubis. Blood Cells Mol Dis. 2006;36(2):269–278. [DOI] [PubMed] [Google Scholar]
- 15.Schotta G, Sengupta R, Kubicek S, et al. A chromatin-wide transition to H4K20 monomethylation impairs genome integrity and programmed DNA rearrangements in the mouse. Genes Dev. 2008;22(15):2048–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]


