Abstract
Most hematological malignancies occur in older patients. Until recently these patients and those with comorbidities were not candidates for treatment with allogeneic hematopoietic transplantation because they were unable to tolerate the heretofore used high-dose conditioning regimens. The finding that many of the cures achieved with allogeneic hematopoietic transplantation were due to graft-versus-tumor effects led to the development of less toxic and well-tolerated reduced intensity and nonmyeloablative regimens. These regimens enabled allogeneic engraftment, thereby setting the stage for graft-versus-tumor effects. This review summarizes the encouraging early results seen with the new regimens and discusses the two hurdles that need to be overcome for achieving even greater success, disease relapse and graft-versus-host disease.
Introduction
Conditioning for allogeneic hematopoietic cell transplantation (HCT) in the treatment of hematologic malignancies has traditionally involved high doses of total body irradiation (TBI) and/or chemotherapy. The dual purpose of conditioning has been to reduce the patients’ burden of malignant cells before HCT and suppress their immune system so that the allogeneic grafts are not rejected. The high intensity of the traditional regimens has precluded using allogeneic HCT in older patients or those with comorbidities because of unacceptable toxicities. This has been unfortunate, given that the median ages of patients at the time of diagnosis of most candidate malignancies, e.g. acute myelocytic leukemia (AML) or non-Hodgkin lymphoma (NHL), range from 65 to 75 years. The finding that the cure of hematologic malignancies not only results from intense conditioning but also in large part from the killing of tumor cells by transplanted donor immune cells, termed “graft-vs.-tumor” (GVT) effect, set the stage for the development of reduced-intensity conditioning (RIC) regimens. Such regimens need to be immunosuppressive enough to allow sustained engraftment, thereby enabling GVT effects. The markedly reduced toxicities associated with these novel regimens have allowed for the extension of allogeneic HCT to include older and medically infirm patients. The relative intensities of individual conditioning regimens vary considerably as far as their immunosuppressive and myelosuppressive properties are concerned (Figure 1). The choice of a given regimen may, in part, be dictated by the nature of the underlying malignancy and, in part, by comorbidities. The results of trials using RIC or nonmyeloablative (NMA) regimens have been surprisingly encouraging. However, all the trials share two major problems that have limited trial outcomes. These are non-relapse mortality (NRM), mainly related to concurrent or preceding graft-vs.-host disease (GVHD) and its treatment, and relapse mortality.
Figure 1.
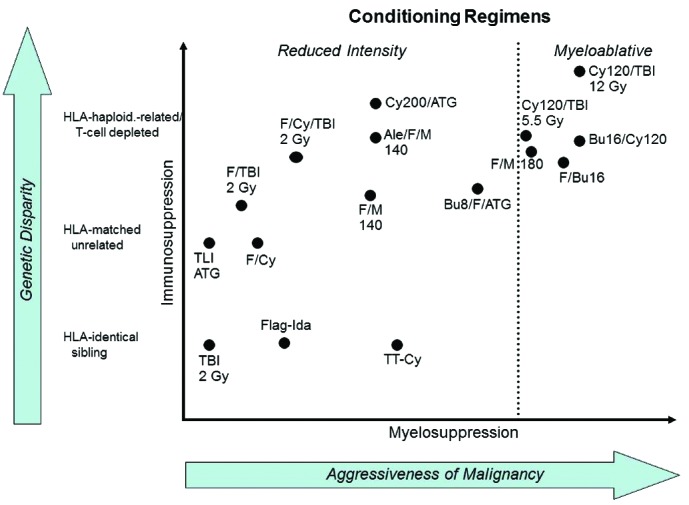
Reproduced from: Sandmaier BM, Storb R. Reduced-intensity allogeneic transplantation regimens, Chapter 21, In: Thomas’ Hematopoietic Cell Transplantation, 5th Edition. Forman SJ, Negrin RS, Antin JH, and Appelbaum FR, Eds., ©John Wiley & Sons, Ltd., in press.
This review will describe the preclinical basis for some of the RIC and NMA regimens, address GVT effects, summarize trial results with HLA-matched and mismatched grafts, address the use of older sibling donors, and explore ways to reduce the risks of GVHD and relapse.
Pre-clinical studies
We used a canine model of major histocompatibility complex (MHC=DLA)-matched marrow grafts to develop a minimal-intensity or NMA conditioning regimen. We found that 2 Gy TBI either without postgrafting immunosuppression or with monotherapy using cyclosporine (CSP) did not enable consistently sustained engraftment.1 However, when a short course of mycophenolate mofetil (MMF) was combined with CSP following 2 Gy TBI, synergism between the two drugs was noted, host T-cells were prevented from rejecting the donor marrow, and sustained engraftment was seen.2 Similar synergism was observed with rapamycin used in lieu of MMF.3 In other studies, which substituted 4.5 Gy irradiation targeted to the cervical, thoracic, and upper abdominal lymph node chain for 2 Gy TBI, we saw sustained engraftment in non-irradiated marrow and lymph node spaces, suggesting that the donor T-lymphocytes created space for grafts to home.4 The results of the canine studies were the basis for the successful clinical introduction of an NMA regimen of 2 Gy TBI combined with fludarabine (FLU) before and MMF/calcineurin inhibitor after HLA-matched related and unrelated HCT.
Further canine work focused on replacing or augmenting 2 Gy TBI with radiolabeled monoclonal antibodies (mAbs).5 Current clinical studies have already employed mAb to CD45 or CD20 coupled to beta-emitting radionuclides such as iodine-131 (131I)6 or yttrium-90 (90Y);7 however, the disadvantages of the beta-emitters became apparent, and included relatively long path lengths, long half-lives, and low energy. Therefore, we turned to alpha-emitting radionuclides, including bismuth-213 (213Bi)8 and astatine-211 (211At).9 211At coupled to an anti-CD45 mAb turned out to be more effective than 213Bi.10 Other advantages of 211At include that it is produced at the University of Washington Cyclotron Facility, has a short half-life of 7.2 hours, has high energy, and, importantly, a very short path length of approximately 0.04–0.06 mm, thereby reducing the risk of off-target effects. Dose-finding toxicity studies in dogs have been completed, and DLA-identical marrow grafts successfully established using a 211At-labeled anti-CD45 mAb.9 Clinical studies are in preparation that are aimed at increasing tumor cell kill in patients with hematologic malignancies and replacing systemic chemo/radiation therapy in those with nonmalignant diseases.
In 1991 Japanese investigators showed that treating MHC-mismatched murine recipients with high-dose cyclophosphamide (CY) after HCT induced tolerance of the grafted lymphocytes to host tissues, while not impairing hematopoietic engraftment.11 This has been possible since hematopoietic stem cells are protected against the toxic effect of CY metabolites by the presence within these cells of aldehyde dehydrogenase. These observations and those by investigators from Johns Hopkins Medical School12,13 set the stage for the development of an effective HLA-haploidentical transplant protocol. The protocol utilized the basic FLU/2 Gy TBI NMA regimen with two additional small doses of CY for conditioning.14 Patients were then given one or two high doses of CY on days 3 and/or 4 postgrafting, followed by MMF/calcineurin inhibitor.
Clinical results
HLA-matched related and unrelated HCT
The choice of conditioning regimen intensity depends in part on the underlying malignancy, disease burden, and comorbidities. The effects these variables can have on transplantation outcome are illustrated by results in 1,092 patients with advanced hematologic malignancies given a uniform NMA regimen of FLU/2 Gy TBI, which allowed for the purest assessment of GVT effects apart from conditioning and the best determination of GVHD not augmented by toxicities related to the regimen.15 Patients were either older or had serious comorbidities. Their median age was 56 (range 7 to 75) years. Thirty-five percent of patients were older than 60 years. Six hundred and eleven patients had HLA-matched related donors and 481 had unrelated donors (one HLA allele-level mismatch was permitted). Diseases and disease stages are shown in Table 1. Twenty percent of patients had failed high-dose autologous or allogeneic HCT or had developed a secondary, usually myeloid malignancy after autologous HCT for another malignancy. Forty-five percent of patients had HCT-Comorbidity Index (CI) scores of 3 or greater. Cumulative incidence rates of acute GVHD were 37% for grade 2, 9 % for grade 3, and 4% for grade 4, respectively; the rates were lower for related than for unrelated recipients. Table 1 divides patients based on low, standard, or high-risk of relapse as assessed by relapse rate per patient year. It is evident that disease and disease burden were major determinants for relapse risk. For example, patients with high-grade NHL in remission had a relapse rate of 0.16 per patient year in years 1–2, while those not in remission had a rate of 0.48. Similar findings were made for other diseases. These data suggested that reducing the tumor burden in certain diseases and disease stages before HCT might reduce the risk of relapse after HCT. Most relapses occurred in the first 2 years, and relapse rates in subsequent years were generally low. Five-year relapse mortality rates ranged from 18% to 50% depending on relapse risk (Figure 2). Of note, 5-year overall relapse mortality was the same among related and unrelated recipients, at 34.5% for both. Figure 2 also shows 5-year overall survivals which ranged from as low as 25% in patients with high relapse risk and high comorbidity scores to 60% in patients with low relapse risk and low comorbidity scores. Unrelated recipients had a significantly increased risk of GVHD-associated NRM compared to related recipients. Of note, a single HLA allele-level mismatch at class I did not adversely affect HCT outcome. Five-year overall NRM was 24% (20% related to preceding or concurrent GVHD), ranging from 14.7% (12% related to GVHD) among related recipients with low comorbidity scores to 36% (31.8% related to GVHD) among unrelated recipients with comorbidity scores of 3 and higher.
Table 1.
Relapse rates per patient year among 1,092 patients.15
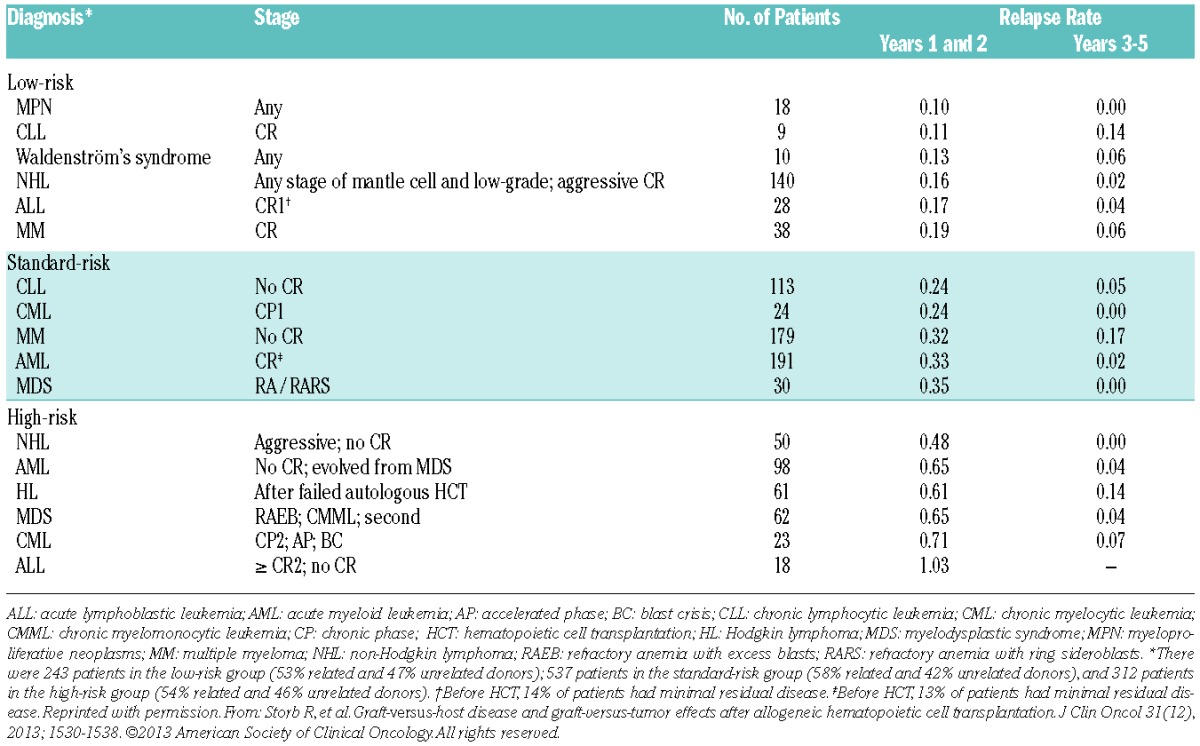
Figure 2.
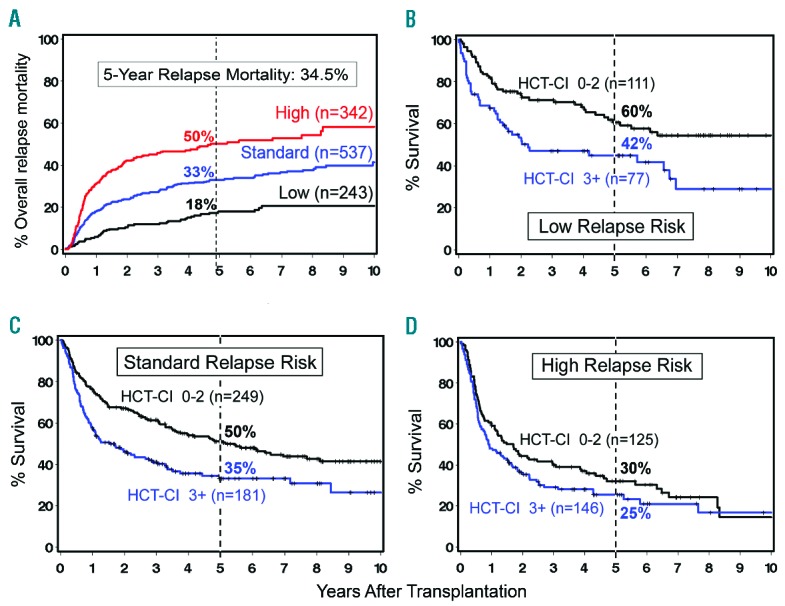
Five-year relapse mortality and overall survival of patients with advanced hematologic malignancies who were conditioned with FLU/2 Gy TBI before HLA-matched related or unrelated HCT and postgrafting immunosuppression with MMF/calcineurin inhibitor. Survival is shown depending on relapse risk and hematopoietic comorbidity scores (HCT-CI).
A phase II randomized clinical trial was carried out as part of an ongoing effort to optimize control of acute GVHD without reducing the GVT effect after unrelated HCT.16 Patients were randomized between three different post-HCT immunosuppressive regimens. In arm 1, tacrolimus was administered for 180 days and MMF for 95 days (n=69). In arms 2 (n=71) and 3 (n=68), tacrolimus and MMF were administered for 150 and 180 days, respectively, with the addition of 80 days of sirolimus in arm 3. Grade II–IV acute GVHD rates in the 3 arms were 64%, 48% and 47% at day 150. Steroid use was significantly lower at day 150 in arm 3 (32% vs. 55% in arm 1 and 49% in arm 2; and the day 150 incidence of cytomegalovirus reactivation was significantly lower in arm 3 (arm 1, 54%; arm 2, 47%; arm 3, 22%) (Figure 3). Currently a 2-arm phase III trial is ongoing using cyclosporine and MMF with and without sirolimus, in order to further evaluate the role of sirolimus.
Figure 3.
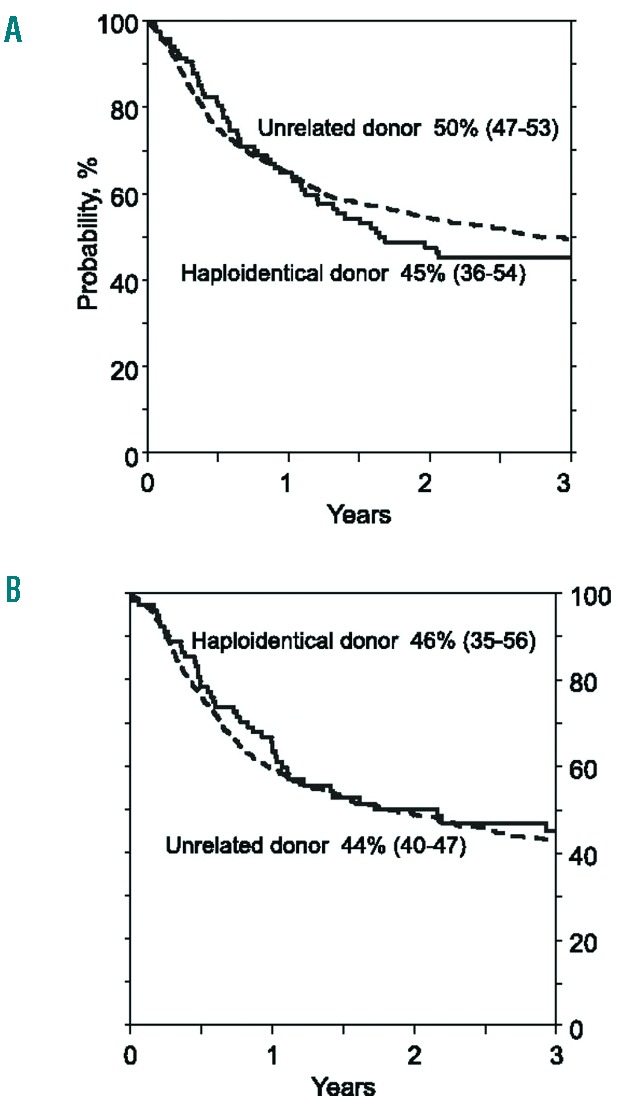
Overall survival. (A) The probability of OS by donor type after myeloablative conditioning regimen, adjusted for age and disease risk index. (B) The probability of OS by donor type after reduced intensity conditioning regimen, adjusted for disease risk index and secondary AML. (Originally published in Blood. Ciurea SO, Zhang MJ, Bacigalupo AA, et al. Haploidentical transplant with posttransplant cyclophosphamide vs. matched unrelated donor transplant for acute myeloid leukemia. Blood. 2015;126(8):1033–1040. ©The American Society of Hematology).
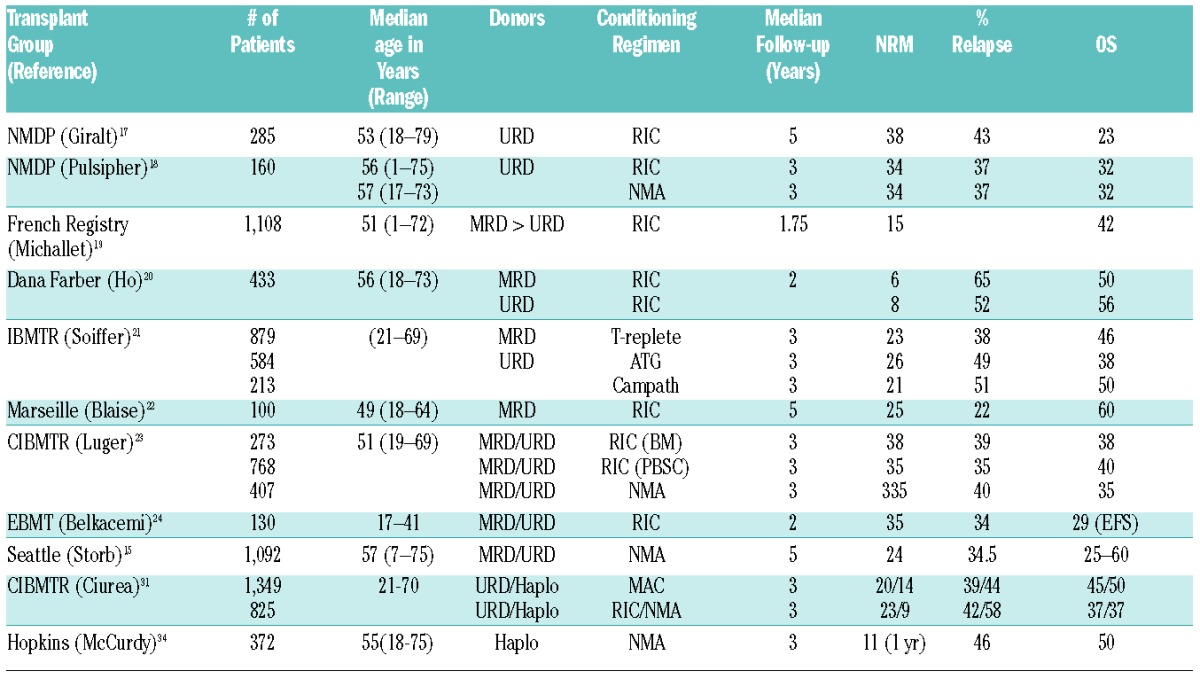
Table 2 shows results with RIC or NMA regimens reported by registries or individual transplant centers. Most regimens used were more intense and relied less on GVT effects than the NMA regimen used in studies shown in Table 1 and Figures 2 and 3. Information on comorbidity scores were generally not provided. The two NMDP studies focused on results with unrelated donors. Five-year outcomes in the former of the two included 38% NRM, 42% relapse, and 23% overall survival.17 The second study had a median follow-up of 3 years and showed that outcomes after RIC were comparable to those after NMA regimens, with approximately 34% NRM, 37% relapse, and 32% overall survival in both groups.18 A large French registry study included slightly younger patients receiving grafts from related or unrelated donors.19 Median follow-up was short at 1.75 years. Even though NRM was low at 15 %, overall survival was only 42%. A Dana-Farber report included 433 related and unrelated recipients given RIC.20 The median follow-up was 2 years. NRM rates were 6% for related and 8% for unrelated recipients, relapse rates were 65% and 52%, and overall survival rates were 50% and 56%, respectively. A large CIBMTR study of RIC and either T-replete or in vivo T-depleted (ATG or Campath) grafts from related or unrelated donors reported results with a median follow-up of 3 years.21 NRM ranged from 21% to 26%, relapse from 38% to 51%, and survival from 38% to 50%, respectively, with slightly better outcomes seen with T-replete grafts. A smaller single-center study from Marseille had a median follow-up of 5 years with grafts from related donors after RIC. NRM was 25%, relapse 22%, and survival 60%.22 Among other comparisons, a second large CIBMTR study compared results with marrow and PBSC grafts after RIC to grafts after NMA conditioning.23 Donors were either related or unrelated. With a median follow-up of 3 years, NRM ranged from 33.5% to 38%, relapse from 35% to 40%, and survival from 35% to 40%. An EBMT registry study in younger patients given either related or unrelated grafts after RIC, showed a 2-year NRM rate of 35%, relapse of 34% and event-free survival of 29%. In summary, the median follow-up in these studies was 3 (range, 1.75 to 5) years.24 Across the studies the median event rates were 43% (range, 22–65%) for relapse, 34% (range, 6–38%) for NRM and 38% (range, 22–65%) for overall survival.
Table 2.
Results of retrospective analyses of transplantation outcomes in patients with hematologic malignancies after reduced intensity (RIC) or nonmyeloablative (NMA) conditioning.
A phase III trial investigating conditioning intensity by the Blood and Marrow Transplant Clinical Trials Network (BMT CTN)25 randomized patients with MDS or AML to either a RIC regimen (FLU/BU2 or FLU/Mel) or a myeloablative conditioning (MAC) regimen (FLU/BU4, BU4Cy, or CyTBI). Inclusion criteria included <5% blasts, being between 18–65 years of age, an HCT-CI of < 4, both related and unrelated donors with 7/8 or 8/8 HLA loci matching, and either marrow or PBSC. The primary diagnosis was AML (80 %) and 92 % of patients received PBSC. The study was stratified by center. The primary endpoint was 18 months overall survival. The DSMB closed the study early at the second interim analysis after 272 patients were enrolled (MAC n=135; RIC n=137). Overall survival and progression-free survival at 18 months were 77.4% and 68.8% (MAC) and 67.7% and 47.3% (RIC), respectively (P=0.07; P<0.01). The incidences of both acute and chronic GVHD were significantly higher in MAC patients (P=0.024 and P=0.019, respectively). The primary causes of death were GVHD in the MAC arm (52%) and relapse in the RIC arm (82%). The conclusion was that MAC remains the treatment of choice for younger patients with MDS or AML.
HLA-mismatched unrelated HCT
While many patients who would benefit from HCT have a HLA-matched donor, a substantial number will not, particularly those who do not have white European ancestry. An analysis was performed on data from the NMDP unrelated donor and cord blood registries to predict the likelihood of identifying suitable donors for U.S. patients.26 The likelihood of finding an 8/8 HLA loci match ranged from 75% in white Europeans to 16–19% for Black/African racial groups. If one accepts a 7/8 HLA loci matched donor, the numbers increase to 97% and 66–76%, respectively. The CIBMTR compared outcomes in 563 recipients of a single HLA locus mismatch with 2,025 recipients of 8/8 HLA loci high-resolution matched unrelated RIC HCT.27 There were more grades II–IV acute GVHD, higher NRM and lower disease free survival and overall survival in recipients of 7/8 HLA loci matched URD. Interestingly, there was no difference in chronic GVHD or relapse. The decreases in overall and disease free survival using a 7/8 HLA loci matched donor were slightly less than those in the myeloablative setting, suggesting a role of tissue damage in mortality following higher dose regimens. The findings in this large registry study are consistent with another smaller prospective study.28
Taken together these studies show that relapse and NRM, mostly related to GVHD, represent the two major obstacles for patients given RIC or NMA regimens that need addressing in future trials.
HLA-haploidentical HCT
Many patients, particularly members of ethnic minorities, lack HLA-matched unrelated donors; however, most patients have a relative who is HLA-haploidentical. The development of low-toxicity regimens sufficient to overcome the immunologic barriers to engraftment is equally important for such patients. Johns Hopkins University and the Fred Hutchinson Cancer Research Center investigated a novel HLA-haploidentical marrow transplant trial using the fludarabine and 2 Gy TBI regimen and additional immunosuppression with CY both before and after HCT for the treatment of hematologic malignancies.14 This regimen was well tolerated and, considering the strong immunological barriers that needed to be overcome, the rejection incidence was low. In addition, the incidences of severe acute and chronic GVHD were encouragingly low. These results were confirmed in a multi-site trial conducted by the BMT CTN29 which also showed a relatively high relapse rate. A currently ongoing randomized study, BMT CTN Protocol 1101, compares HLA-haploidentical marrow vs. cord blood as a stem cell source.
A recent European publication noted a pronounced increase in the use of HLA-haploidentical family donors and a concurrent decrease in the use of cord blood donors.30 More than twice the number of HLA-haploidentical grafts have been reported since 2010 compared to cord blood transplants. CIBMTR is reporting similar trends in North America.
A recent CIBMTR study compared outcomes in 2,174 patients with AML given grafts from HLA-matched unrelated (n=1,982) or HLA-haploidentical related donors given regimens using post-HCT Cy (n=192).31 The study included patients with myeloablative (unrelated n=1,245; HLA-haploidentical n=104) and RIC/NMA conditioning (unrelated n=737; HLA-haploidentical n=88). There was no difference in overall and disease free survival between the different donor types in either the myeloablative or RIC/NMA recipients (Table 2 and Figure 4). There was significantly more acute and chronic GVHD in recipients of unrelated grafts but a lower risk of NRM (P=0.01), and a borderline increase risk of relapse (P=0.05) in RIC/NMA-conditioned recipients of HLA-haploidentical related grafts. A similar CIBMTR study compared outcomes in 917 patients with NHL receiving HLA-haploidentical related versus HLA-matched unrelated HCT, the latter either with or without ATG.31 There was no significant difference in overall survival between the 3 groups but there was inferior survival in those unrelated patients who received ATG. In a single center series of 372 patients, patients were stratified by the refined Disease Risk Index (DRI)32,33 and evaluated for outcomes. By refined DRI, 3-year progression-free survival in low, intermediate and high/very high-risk groups were 65%, 37% and 22%, respectively (Table 2).34 These results are similar to those historically seen with HLA-matched HCT, suggesting that prospective randomized trials are warranted to evaluate the use of alternative donors given the lower incidence of chronic GVHD seen after HLA-haploidentical HCT.
Figure 4.
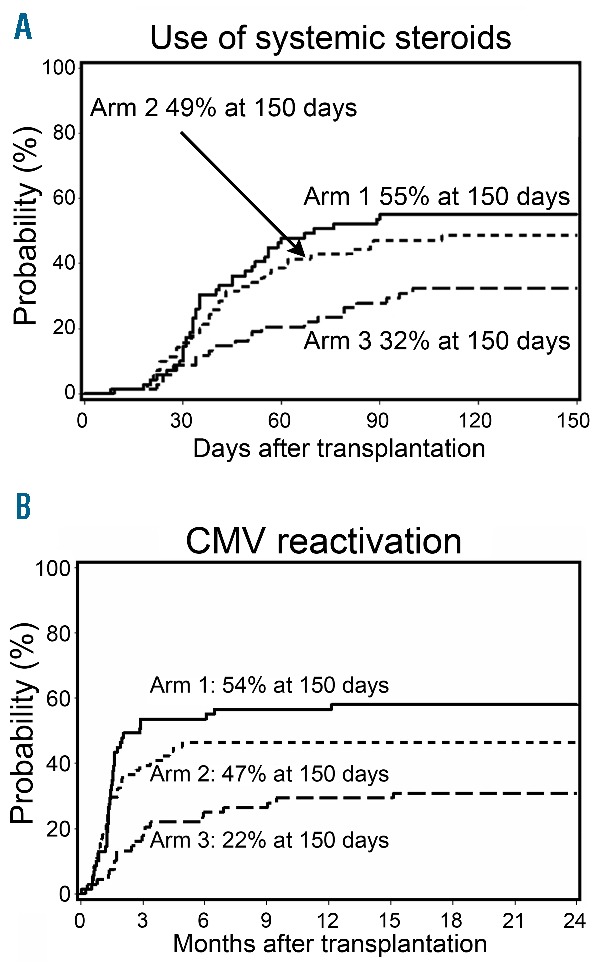
Graft-versus-host disease and use of systemic steroids. (A) Cumulative incidence of use of systemic steroids in arm 1 (n=69), arm 2 (n=71) and arm 3 (n=68). (B) Viral infections. Cumulative incidence of cytomegalovirus reactivation in arm 1 (n=69), arm 2 (n=71) and arm 3 (n=68). Originally published in Haematologica (Kornblit B, et al. A randomized phase II trial of tacrolimus, mycophenolate mofetil and sirolimus after non-myeloablative unrelated donor transplantation. Haematologica 2014; 99(10): 1624–1631. ©2014 Ferrata Storti Foundation).
It has been suggested that the use of PBSC may reduce the risk of relapse among HLA-haploidentical recipients without increasing the risk of GVHD. Concurrent studies using PBSC were carried out at 4 centers and analyzed together.35 Grades 2 and 3 acute GVHD developed in 53% and 8% of patients, respectively, and the 2 year incidence of chronic GVHD was 18%. The 2 year rates of NRM and relapse were 23% and 28%, respectively, suggesting that PBSC can be substituted for marrow in HLA-haploidentical HCT. Other strategies to prevent, preempt or treat relapse include planned donor lymphocyte infusions.36 A more novel approach includes preemptive infusions of donor NK cells. Thirty-six heavily pre-treated patients with hematologic malignancies, median age of 46 (range 8–75) years, were given donor NK cells on day 7 after HLA-haploidentical HCT.37 Patients had a median time from cancer diagnosis to transplant of 2.1 (0.3 – 9.9) years, including 7 patients with prior autologous HCT and 6 patients with 1 or more prior allogeneic HCT. Overall and relapse-free survivals at 1 year of 74% and 69%, and at 2 years of 63% and 51% were observed, respectively.
Engraftment kinetics and donor chimerism
The overall goal in malignant disorders is to achieve high levels of or even complete donor T-cell chimerism early after HCT, as this has been associated with lower risks of graft rejection and relapse.38–40 While complete donor chimerism develops rapidly following myeloablative allogeneic HCT, varying degrees of mixed donor host chimerism are seen initially following NMA conditioning, though the majority of patients will have full donor chimerism by day 100 after HCT. Many of the RIC regimens that are more myelosuppressive have kinetics of donor engraftment similar to those of myeloablative regimens. In addition to regimen intensity, other factors influence the kinetics of engraftment including the use of PBSC and in vivo T-cell depleting agents (such as ATG or alemtuzumab) and HLA disparity between donor and recipient. Patients who received myelosuppressive chemotherapy or a preceding autologous HCT had a more rapid engraftment of donor T-cells. An association between high levels of donor T-cell chimerism and GVHD has been observed using different conditioning regimens.39,40 When both NK and T-cell chimerism were modeled as continuous variables, only early donor T-cell chimerism was associated with acute GVHD, whereas high levels of NK chimerism were significantly associated with lower relapse rates but not with increased GVHD.41 A phase III trial among patients treated with 2 Gy TBI alone vs. TBI with fludarabine 90mg/m2 showed that adding fludarabine contributed to a more rapid T and NK cell chimerism and significantly less relapse (40 % vs. 55%), resulting in superior survival (60 % vs. 54% at 3 years).42 This supported the previous observations of higher donor chimerism being protective for relapse.
Toxicities and infections
High-dose conditioning is associated with higher NRM from organ toxicities and infectious complications. The former include hepatic sinusoidal obstruction syndrome/veno-occlusive disease (SOS / VOD) and idiopathic pneumonia syndrome (IPS). No cases of SOS were observed among 193 patients given NMA conditioning.43 Acute renal failure (ARF) (defined as a >50% decrease in glomerular filtration rate) occurred less often in patients given NMA HCT compared to myeloablative conditioning (43% vs. 73%), despite greater age and comorbidities among NMA recipients.44 A separate multivariate analysis revealed that ARF during the first 100 days was associated with the development of chronic kidney disease (CKD). CKD was defined as at least a 25% reduction in GFR from baseline. Previous autologous HCT, long-term calcineurin inhibitor use and extensive chronic GVHD were independently associated with CKD. CKD following NMA HCT appears to be a distinct clinical entity and likely not related to radiation nephritis.45 Pulmonary function was evaluated in patients before, at day 100, and 1 year after HCT.46 Results suggested that, despite having worse pre-transplant lung function, NMA patients experienced less pulmonary toxicity than myeloablative patients. The incidences and outcomes of IPS among NMA (n=183) versus myeloablative (n=917) patients were compared. The cumulative incidence of IPS was significantly lower at 120 days after NMA conditioning (2.2% vs. 8.4%). IPS occurred early after transplant, progressed rapidly, and had a high mortality rate (75%) despite aggressive support. These findings support the concept that lung damage from conditioning regimen plays a crucial role in IPS after HCT.
Following NMA conditioning, patients have less cytopenias including less neutropenia. Significantly fewer NMA recipients (n=503) required platelet transfusions (25% vs. 99%) and red blood cell transfusions (64% vs. 96%) than myeloablative (n=1,353) recipients.47 Among the NMA patients, platelet and RBC transfusions were less frequent among related compared to unrelated recipients. Major/bidirectionally ABO-mismatched recipients required more RBC transfusions than ABO-matched recipients, though ABO-mismatching did not affect other NMA HCT outcomes. It was also hypothesized that NMA conditioning would be associated with less neutropenia after day 28 following engraftment. However, while NMA conditioning had protective effects on anemia and thrombocytopenia after day 28 there was no significant reduction of neutropenia either overall or in the context of ganciclovir use.48 Elderly patients appear to be more prone to cumulative toxicities of post-HCT drug regimens, but NMA conditioning, optimized HLA matching, and higher doses of CD34+ cell infusions reduced the risk of cytopenia after day 28.
Multiple studies have shown that the incidence of infections early after HCT is reduced after RIC and NMA conditioning. There is less bacteremia in the first month presumably due to a lesser degree of neutropenias.49 While the incidence of CMV infection is the same in CMV positive recipients, NMA-HCT was associated with a lower risk of high-grade CMV infection.50
Older donors
As the age of HCT recipients has increased, the age of their sibling donors has increased as well. Concern has been raised that increasing donor age might adversely affect the functional fitness of hematopoietic cells and thereby impair the marrow recovery after transplantation. Hematopoietic cells are subject to aging mechanisms such as accumulated DNA damage, telomere shortening, and epigenetic modification. However, studies on the effect of donor age on the function of hematopoietic cells have yielded controversial results, especially the work on stem cell aging in murine model systems. Dutch investigators commented on the variable results seen: “the discrepant conclusions of these studies, however, could be partly caused by (the different) mouse strains used, because strain-dependent increases or decreases in primitive hematopoietic cell frequency and function have been reported.”51 Another concern is related to the longevity of hematopoietic stem cells which makes them ideal targets for mutagenic changes.52 The theoretical possibility was raised that recipients of aged stem cells might be at an increased risk of developing malignant clonal disorders.
Published clinical results on the effects of aging on stem cells also vary. An NMPD study from 2001 reported inferior survival among patients given grafts from donors older than 45 years.53 A French study initially saw no significant impact of donor age among MDS and AML patients undergoing transplantation.54 In contrast, a later analysis by the French group found that donor age ≥60 years had a significant adverse impact on overall recipient survival.55 A CIBMTR analysis from 2013 reported that outcomes were superior in recipients of grafts from HLA-identical sibling donors >50 years old compared to those with grafts from HLA-matched unrelated donors <50 years of age.56 We analyzed the effects of donor age on the speed of hematopoietic engraftment and donor chimerism, acute and chronic GVHD, and NRM among 1,174 patients undergoing myeloablative and 367 patients undergoing NMA conditioning before HLA-matched related or unrelated HCT.57 CD34 cell harvests were reduced in older (60–82 years) donors (median 5.6 × 106 cells/kg) compared to younger (<60 years) donors (median 7.7 × 106 cells/kg). However, sustained engraftment rates among recipients with older and younger donors were comparable. Sustained grafts were seen in 97% and 98% of patients given myeloablative and NMA conditioning, respectively, who had younger donors, and 90% and 100%, respectively, for those who had older donors. Also the tempo of neutrophil and platelet recoveries and donor chimerism did not show significant differences, except for an average 1.3-day delay in neutrophil recovery among myeloablative patients with older donors (P=0.04). Moreover, aged stem cells did not convey an increased risk of donor-derived clonal disorders after HCT since none were seen. Both myeloablative and NMA recipients with older sibling donors had significantly less grade 2–4 acute GVHD compared to recipients with grafts from younger unrelated donors. Rates of grade 3 and 4 acute GVHD, chronic GVHD, and NRM among recipients with older donors were not significantly different from those seen in recipients with younger donors. We concluded from this single-center study that grafts from donors ≥60 years of age did not adversely affect outcomes of HCT compared to grafts from younger donors <60 years of age.
Relapse
Relapse or progression of the underlying malignancy has remained the principal cause of failure of allogeneic HCT. This has been especially true in patients who for reasons of age or comorbidities have been conditioned with NMA regimens, where cure of malignancy depends almost entirely on GVT effects. The following sections will discuss outcomes with a minimal-intensity conditioning regimen and use the results as a basis for proposing ways to reduce relapse or progression.
We maintained the NMA FLU/low-dose TBI platform for patients with advanced hematologic malignancies because most of our patients either did not need or would not tolerate higher dose regimens, and because the regimen best defines the limits of GVT effects.15,58 Powerful GVT effects were seen across all disease stages except for ALL in CR2 and beyond, where all patients progressed. As shown in Figure 2, between 45% and 75% of patients experienced sustained remissions depending on the nature and stage of the underlying malignancy. Overall 5-year relapse mortality was 34.5%. Seventy percent of relapse or progression occurred in year 1 and much of the remainder in year 2 after HCT. We hypothesize that early disease relapse or progression was due to blunted GVT effects from early posttransplantation immune compromise. Later, as the donor immune system was being built up and immunosuppressive drugs tapered and then discontinued, the “brakes were taken off” the immune cells, enabling GVT effects. This hypothesis is indirectly supported by former extensive immune function studies showing recovery of antibody responses to neoantigens, such as bacteriophage ϕX174 and keyhole limpet hemocyanin, among others, as well as cellular immunity within 1–3 years after HCT.59 Consistent with this hypothesis, relapse rates in most diseases were markedly reduced in years 3–5.
The options for decreasing the still existing relapse or progression risk are limited. Increasing the intensity, and thereby the toxicity, of the conditioning regimen may be problematic for at least two reasons. One is that a majority of patients did not relapse and, therefore, would be exposed to unnecessary toxicity. The other is that most patients were elderly and/or had comorbidities which preempted dose escalation. Also, more than one-fifth of patients had failed preceding high-dose HCT and another one-fifth had planned autologous HCT, and receiving another high-dose HCT regimen might be too toxic. Given these limitations, we envision two principal approaches for reducing the risk of relapse or progression in elderly or medically infirm patients.
One approach is based on the hypothesis that delaying disease relapse or progression until the grafted immune system is recovered sufficiently to generate GVT effects would increase cure rates. Such a delay would be accomplished with well-tolerated drugs or antibody-drug conjugates which, even though not curative on their own, would pave the way for curative GVT effects. An example of such an approach has been the treatment of patients with Ph1+ ALL in first remission with a tyrosine kinase inhibitor for one year after HCT.60 The overall 5-year survival rate was 69% and 85% in the subgroup without MRD before HCT, which is impressively better than previous results without tyrosine kinase inhibitors. Candidate agents for patients with other malignancies include antibodies to CD20 (NHL) and CD30 (Hodgkin lymphoma), proteosome inhibitors (MM), and the FLT3 inhibitors (AML).
A second approach would be to reduce the tumor burden before HCT. One way to accomplish this is through the use of chimeric antigen receptor (CAR) T-cells in patients who have B cell lymphoid malignancies expressing CD19.61,62 Another way is to increase the pre-transplant tumor cell kill by low-toxicity, targeted radiation therapy using a mAb to CD45 coupled to radionuclides used in addition to the basic FLU/2 Gy TBI regimen. One preliminary study summarized early results in 58 patients with advanced AML or high-risk MDS who were older than 50 years and treated with the anti-CD45 mAb coupled to 131I.6 One-year survival was 41%. Another study added 90Y coupled to an anti-CD20 mAb to FLU/2 Gy TBI in 40 patients with persistent, high-risk NHL. The estimated 30-month progression-free survival was 51%.7 Several properties of the beta-emitting radionuclides 131I and 90Y limit their effectiveness including their long half-lives of 2.5 and 8 days, their relatively low energy of 0.7 and 2.3 MeV, and their long path lengths of 0.8–11.3 mm, respectively, which result in off-target effects. Additionally, 131I emits weak gamma radiation during its decay which necessitates placing patients in isolation rooms for several days. To get around these limitations, we have focused our attention on an alpha-emitting radionuclide, 211At, which has a half-life of 7.2 hours, high energy (5.9 MeV), and a path length of only 0.04–0.06 mm. This results in the unique ability of killing mAb-targeted cells while causing minimal damage to surrounding tissues. Moreover, the alpha particles cause multiple strand breaks, hence DNA repair mechanisms are inhibited, which reduces the risk of secondary cancer. An additional advantage of 211At is that it is relatively cheap compared to other alternatives. Our first clinical protocol has been firmly based on 15 years of experience with alpha-emitting radionuclides in a canine allogeneic HCT model.
Conclusions
Allogeneic HCT after RIC or MMA regimens to treat older or medically infirm patients with advanced hematological malignancies is feasible and effective. This is enabled in large part by GVT effects, and results in cures of appreciable numbers of malignancies. Increasing disease control and decreasing NRM, the latter mostly associated with or preceded by GVHD, will need to be addressed in future trials.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/101/5/521
Funding
The authors are grateful for research funding from the National Institutes of Health, Bethesda, MD, USA, grants CA078902, CA018029, CA015704 and HL122173. Further support came from the Laura Landro Salomon Endowment Fund. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health nor its subsidiary Institutes and Centers.
References
- 1.Yu C, Storb R, Mathey B, et al. DLA-identical bone marrow grafts after low-dose total body irradiation: Effects of high-dose corticosteroids and cyclosporine on engraftment. Blood. 1995;86(11):4376–4381. [PubMed] [Google Scholar]
- 2.Storb R, Yu C, Wagner JL, et al. Stable mixed hematopoietic chimerism in DLA-identical littermate dogs given sublethal total body irradiation before and pharmacological immunosuppression after marrow transplantation. Blood. 1997;89(8):3048–3054. [PubMed] [Google Scholar]
- 3.Hogan WJ, Little M-T, Zellmer E, et al. Postgrafting immunosuppression with sirolimus and cyclosporine facilitates stable mixed hematopoietic chimerism in dogs given sublethal total body irradiation before marrow transplantation from DLA-identical littermates. Biol Blood Marrow Transplant. 2003;9(8):489–495. [DOI] [PubMed] [Google Scholar]
- 4.Storb R, Yu C, Barnett T, et al. Stable mixed hematopoietic chimerism in dog leukocyte antigen-identical littermate dogs given lymph node irradiation before and pharmacologic immunosuppression after marrow transplantation. Blood. 1999;94(3):1131–1136. [PubMed] [Google Scholar]
- 5.Appelbaum FR, Brown P, Sandmaier B, et al. Antibody-radionuclide conjugates as part of a myeloblative preparative regimen for marrow transplantation. Blood. 1989;73(8): 2202–2208. [PubMed] [Google Scholar]
- 6.Pagel JM, Gooley TA, Rajendran J, et al. Allogeneic hematopoietic cell transplantation after conditioning with 131I-anti-CD45 antibody plus fludarabine and low-dose total body irradiation for elderly patients with advanced acute myeloid leukemia or high-risk myelodysplastic syndrome. Blood. 2009;114(27):5444–5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gopal AK, Guthrie KA, Rajendran J, et al. 90Y-Ibritumomab tiuxetan, fludarabine, and TBI-based nonmyeloablative allogeneic transplantation conditioning for patients with persistent high-risk B-cell lymphoma. Blood. 2011;118(4):1132–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sandmaier BM, Bethge WA, Wilbur DS, et al. Bismuth 213-labeled anti-CD45 radioimmunoconjugate to condition dogs for non-myeloablative allogeneic marrow grafts. Blood. 2002;100(1):318–326. [DOI] [PubMed] [Google Scholar]
- 9.Chen Y, Kornblit B, Hamlin DK, et al. Durable donor engraftment after radioimmunotherapy using -emitter astatine-211-labeled anti-CD45 antibody for conditioning in allogeneic hematopoietic cell transplantation. Blood. 2012;119(5):1130–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakamae H, Wilbur DS, Hamlin DK, et al. Biodistribution, myelosuppression, and toxicities in mice treated with an anti-CD45 antibody labeled with the -emitting radionuclides bismuth-213 or astatine-211. Cancer Res. 2009;69(6):2408–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eto M, Mayumi H, Tomita Y, et al. Specific destruction of host-reactive mature T cells of donor origin prevents graft-versus-host disease in cyclophosphamide-induced tolerant mice. J Immunol. 1991;146(5):1402–1409. [PubMed] [Google Scholar]
- 12.Luznik L, Engstrom LW, Iannone R, Fuchs EJ. Posttransplantation cyclophosphamide facilitates engraftment of major histocompatibility complex-identical allogeneic marrow in mice conditioned with low-dose total body irradiation. Biol Blood Marrow Transplant. 2002;8(3):131–138. [DOI] [PubMed] [Google Scholar]
- 13.Luznik L, O’Donnell PV, Fuchs EJ. Posttransplantation cyclophosphamide for tolerance induction in HLA-haploidentical bone marrow transplantation. Semin Oncol. 2012;39(6):683–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luznik L, O’Donnell PV, Symons HJ, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14(28):641–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Storb R, Gyurkocza B, Storer BE, et al. Graft-versus-host disease and graft-versus-tumor effects after allogeneic hematopoietic cell transplantation. J Clin Oncol. 2013;31(12):1530–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kornblit B, Maloney DG, Storer BE, et al. A randomized phase II trial of tacrolimus, mycophenolate mofetil and sirolimus after nonmyeloablative unrelated donor transplantation. Haematologica. 2014;99(10): 1624–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giralt S, Logan B, Rizzo D, et al. Reduced-intensity conditioning for unrelated donor progenitor cell transplantation: long-term follow-up of the first 285 reported to the National Marrow Donor Program. Biol Blood Marrow Transplant. 2007;13(7):844–852. [DOI] [PubMed] [Google Scholar]
- 18.Pulsipher MA, Chitphakdithai P, Logan BR, et al. Donor, recipient, and transplant characteristics as risk factors after unrelated donor PBSC transplantation: beneficial effects of higher CD34+ cell dose. Blood. 2009;114(13):2606–2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michallet M, Le QH, Mohty M, et al. Predictive factors for outcomes after reduced intensity conditioning hematopoietic stem cell transplantation for hematological malignancies: a 10-year retrospective analysis from the Societe Francaise de Greffe de Moelle et de Therapie Cellulaire. Exp Hematol. 2008;36(5):535–544. [DOI] [PubMed] [Google Scholar]
- 20.Ho VT, Kim HT, Aldridge J, et al. Use of matched unrelated donors compared with matched related donors is associated with lower relapse and superior progression-free survival after reduced-intensity conditioning hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2011;17(8): 1196–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soiffer RJ, Lerademacher J, Ho V, et al. Impact of immune modulation with anti-T-cell antibodies on the outcome of reduced-intensity allogeneic hematopoietic stem cell transplantation for hematologic malignancies. Blood. 2011;117(25):6963–6970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blaise D, Farnault L, Faucher C, et al. Reduced-intensity conditioning with Fludarabin, oral Busulfan, and thymoglobulin allows long-term disease control and low transplant-related mortality in patients with hematological malignancies. Exp Hematol. 2010;38(12):1241–1250. [DOI] [PubMed] [Google Scholar]
- 23.Luger SM, Ringdén O, Zhang M-J, et al. Similar outcomes using myeloablative vs reduced-intensity allogeneic transplant preparative regimens for AML or MDS. Bone Marrow Transplant. 2012;47(2):203–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Belkacemi Y, Labopin M, Hennequin C, et al. Reduced-intensity conditioning regimen using low-dose total body irradiation before allogeneic transplant for hematologic malignancies: Experience from the European Group for Blood and Marrow Transplantation. Int J Radiat Oncol Biol Phys. 2007;67(2):544–551. [DOI] [PubMed] [Google Scholar]
- 25.Scott BL, Pasquini MC, Logan B, et al. Results of a phase III randomized, multi-center study of allogeneic stem cell transplantation after high versus reduced intensity conditioning in patients with myelodysplastic syndrome (MDS) or acute myeloid leukemia (AML): Blood and Marrow Transplant Clinical Trials Network (BMT CTN) 0901. Blood. 2015;126(23):LBA-8. [Google Scholar]
- 26.Gragert L, Eapen M, Williams E, et al. HLA match likelihoods for hematopoietic stem-cell grafts in the U.S. registry. N Engl J Med. 2014;371(4):339–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verneris MR, Lee SJ, Ahn KW, et al. HLA mismatch Is associated with worse outcomes after unrelated donor reduced-Intensity conditioning hematopoietic cell transplantation: an analysis from the center for international blood and marrow transplant research. Biol Blood Marrow Transplant. 2015;21(10):1783–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakamae H, Storer BE, Storb R, et al. Low-dose total body irradiation and fludarabine conditioning for HLA class I-mismatched donor stem cell transplantation and immunologic recovery in patients with hematologic malignancies: a multicenter trial. Biol Blood Marrow Transplant. 2010;16(3):384–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brunstein CG, Fuchs EJ, Carter SL, et al. Alternative donor transplantation after reduced intensity conditioning: results of parallel phase 2 trials using partially HLA-mismatched related bone marrow or unrelated umbilical cord blood grafts. Blood. 2011;118(2):282–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Passweg JR, Baldomero H, Bader P, et al. Hematopoietic SCT in Europe 2013: recent trends in the use of alternative donors showing more haploidentical donors but fewer cord blood transplants. Bone Marrow Transplant. 2015;50(4):476–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ciurea SO, Zhang MJ, Bacigalupo AA, et al. Haploidentical transplant with posttransplant cyclophosphamide vs matched unrelated donor transplant for acute myeloid leukemia. Blood. 2015;126(8):1033–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sayer HG, Kröger M, Beyer J, et al. Reduced intensity conditioning for allogeneic hematopoietic stem cell transplantation in patients with acute myeloid leukemia: disease status by marrow blasts is the strongest prognostic factor. Bone Marrow Transplant. 2003;31(12):1089–1095. [DOI] [PubMed] [Google Scholar]
- 33.Armand P, Kim HT, Logan BR, et al. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood. 2014;123(23):3664–3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCurdy SR, Kanakry JA, Showel MM, et al. Risk-stratified outcomes of nonmyeloablative HLA-haploidentical BMT with high-dose posttransplantation cyclophosphamide. Blood. 2015;125(19):3024–3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jaiswal SR, Chakrabarti A, Chatterjee S, et al. Haploidentical peripheral blood stem cell transplantation with posttransplantation cyclophosphamide in children with advanced acute leukemia with a fludarabine, busulfan and melphalan based conditioning. Biol Blood Marrow Transplant. 2015. pii:S1083-8791(15)00737-5. [DOI] [PubMed] [Google Scholar]
- 36.Ghiso A, Raiola AM, Gualandi F, et al. DLI after haploidentical BMT with posttransplant CY. Bone Marrow Transplant. 2015;50(1):56–61. [DOI] [PubMed] [Google Scholar]
- 37.Thakar M, Hari PN, Keever-Taylor CA, et al. Donor natural killer (NK) cell immunotherapy following non-myeloablative HLA-haploidentical hematopoietic cell transplantation (HCT) improves relapse and progression free survival (PFS) in patients with hematologic malignancies [abstract]. Biol Blood Marrow Transplant. 2016. (epub). [Google Scholar]
- 38.McSweeney PA, Niederwieser D, Shizuru JA, et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood. 2001;97(11):3390–3400. [DOI] [PubMed] [Google Scholar]
- 39.Childs R, Clave E, Contentin N, et al. Engraftment kinetics after nonmyeloablative allogeneic peripheral blood stem cell transplantation: full donor T-cell chimerism precedes alloimmune responses. Blood. 1999;94(9):3234–3241. [PubMed] [Google Scholar]
- 40.Baron F, Baker JE, Storb R, et al. Kinetics of engraftment in patients with hematologic malignancies given allogeneic hematopoietic cell transplantation after nonmyeloablative conditioning. Blood. 2004;104(8):2254–2262. [DOI] [PubMed] [Google Scholar]
- 41.Baron F, Petersdorf EW, Gooley T, et al. What is the role for donor natural killer cells after nonmyeolablative conditioning? Biol Blood Marrow Transplant. 2009;15(5):580–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kornblit B, Maloney DG, Storb R, et al. Fludarabine and 2 Gy TBI is superior to 2 Gy TBI as conditioning for HLA-matched related hematopoietic cell transplantation: a phase III randomized trial. Biol Blood Marrow Transplant. 2013;19(9):1340–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hogan WJ, Maris M, Storer B, et al. Hepatic injury after nonmyeloablative conditioning followed by allogeneic hematopoietic cell transplantation: a study of 193 patients. Blood. 2004;103(1):78–84. [DOI] [PubMed] [Google Scholar]
- 44.Parikh CR, Schrier RW, Storer B, et al. Comparison of ARF after myeloablative and nonmyeloablative hematopoietic cell transplantation. American Journal of Kidney Diseases. 2005;45(3):502–509. [DOI] [PubMed] [Google Scholar]
- 45.Weiss AS, Sandmaier BM, Storer B, Storb R, McSweeney PA, Parikh CR. Chronic kidney disease following nonmyeloablative hematopoietic cell transplantation. Am J Transplant. 2006;6(1):89–94. [DOI] [PubMed] [Google Scholar]
- 46.Fukuda T, Hackman RC, Guthrie KA, et al. Risks and outcomes of idiopathic pneumonia syndrome after nonmyeloablative and conventional conditioning regimens for allogeneic hematopoietic stem cell transplantation. Blood. 2003;102(8):2777–2785. [DOI] [PubMed] [Google Scholar]
- 47.Wang Z, Sorror ML, Leisenring W, et al. The impact of donor type and ABO incompatibility on transfusion requirements after non-myeloablative hematopoietic cell transplantation. Br J Haematol. 2010;149(1):101–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nakamae H, Storer B, Sandmaier BM, et al. Cytopenias after day 28 in allogeneic hematopoietic cell transplantation: impact of recipient/donor factors, transplant conditions and myelotoxic drugs. Haematologica. 2011;96(12):1838–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Junghanss C, Marr KA, Carter RA, et al. Incidence and outcome of bacterial and fungal infections following nonmyeloablative compared with myeloablative allogeneic hematopoietic stem cell transplantation: a matched control study. Biol Blood Marrow Transplant. 2002;8(9):512–520. [DOI] [PubMed] [Google Scholar]
- 50.Nakamae H, Kirby KA, Sandmaier BM, et al. Effect of conditioning regimen intensity on CMV infection in allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2009;15(6):694–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.de Haan G, Nijhof W, Van Zant G. Mouse strain-dependent changes in frequency and proliferation of hematopoietic stem cells during aging: correlation between lifespan and cycling activity. Blood. 1997;89(5):1543–1550. [PubMed] [Google Scholar]
- 52.Rossi DJ, Jamieson CH, Weissman IL. Stems cells and the pathways to aging and cancer (Review). Cell. 2008;132(4):681–696. [DOI] [PubMed] [Google Scholar]
- 53.Kollman C, Howe CWS, Anasetti C, et al. Donor characteristics as risk factors in recipients after transplantation of bone marrow from unrelated donors: the effect of donor age. Blood. 2001;98(7):2043–2051. [DOI] [PubMed] [Google Scholar]
- 54.Robin M, Porcher R, Ades L, et al. Matched unrelated or matched sibling donors result in comparable outcomes after non-myeloablative HSCT in patients with AML or MDS. Bone Marrow Transplant. 2013; 48(10): 1296–1301. [DOI] [PubMed] [Google Scholar]
- 55.Peffault de Latour R, Brunstein CG, Porcher R, et al. Similar overall survival using sibling, unrelated donor, and cord blood grafts after reduced-intensity conditioning for older patients with acute myelogenous leukemia. Biol Blood Marrow Transplant. 2013;19(9): 1355–1360. [DOI] [PubMed] [Google Scholar]
- 56.Alousi AM, Le-Rademacher J, Saliba RM, et al. Who is the better donor for older hematopoietic transplant recipients: an older-aged sibling or a young, matched unrelated volunteer? Blood. 2013;121(13):2567–2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rezvani AR, Storer BE, Guthrie KA, et al. Impact of donor age on outcome after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2015; 21(1):105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Storb R, Gyurkocza B, Storer BE, et al. Allogeneic hematopoietic cell transplantation following minimal intensity conditioning: predicting acute graft-versus-host disease and graft-versus-tumor effects. Biol Blood Marrow Transplant. 2013;19(5):792–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Witherspoon RP, Storb R, Ochs HD, et al. Recovery of antibody production in human allogeneic marrow graft recipients: Influence of time posttransplantation, the presence or absence of chronic graft-versus-host disease, and antithymocyte globulin treatment. Blood. 1981;58(2):360–368. [PubMed] [Google Scholar]
- 60.Ram R, Storb R, Sandmaier BM, et al. Non-myeloablative conditioning with allogeneic hematopoietic cell transplantation for the treatment of high-risk acute lymphoblastic leukemia. Haematologica. 2011;96(8):1113–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Davila ML, Riviere I, Wang X, et al. Efficacy and toxicity management of 19–28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Science Transl Med. 2014;6(224): 224ra25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Turtle CJ, Berger C, Sommermeyer D, et al. Anti-CD19 chimeric antigen receptor-modified T cell therapy for B cell non-Hodgkin lymphoma and chronic lymphocytic leukemia: Fludarabine and cyclophosphamide lymphodepletion improves in vivo expansion and persistence of CAR-T cells and clinical outcomes [abstract]. Blood. 2015;126(23):184. [Google Scholar]


