Anecdotal clinical cases previously suggested the potential of cytokine-induced killer (CIK) cells as an anti-leukemic immunotherapy (IT). Here, we report the aggregate experience from three hematology-oncology centers regarding the feasibility, safety and efficacy of interleukin (IL)-15-activated CIK cell treatment for the immunological control of relapse in 13 patients with high-risk leukemia after allogeneic hematopoietic stem cell transplantation (HSCT). In 10 of 13 patients, CIK cell therapy was administered upon molecular relapse, and in 3 of 13 patients, it was offered upon relapsed chemotherapy-resistant progressive disease at HSCT. Overall, 3 of 13 (23%) patients, all of whom had received CIK cells from haploidentical donors, developed transient grade 3 acute graft-versus-host disease (aGvHD), whereas continued complete molecular remission (CMR) was sustained in 9 of 13 patients (70%), and 4 of 13 patients (30%) experienced delayed hematologic relapse at a median of five months after the detection of molecular disease, demonstrating the feasibility, safety and efficacy of CIK cell treatment for high-risk leukemia after HSCT.
Among allogeneic HSCT, haploidentical HSCT is an established treatment for high-risk leukemia in patients lacking a human leukocyte antigen (HLA)-identical donor.1 The major cause for post-transplant mortality is relapse. Those patients who do relapse often acquire refractory disease, while at the same time being particularly susceptible to chemotherapy-related adverse effects because of cumulative treatment toxicity.
Impending relapse can be detected by minimal residual disease (MRD)2 or chimerism3 monitoring and can, at this stage in principle, be treated by rapid tapering of immunosuppressive medication or infusions of donor lymphocytes (DLI). In general, infused T cells are associated with risk of GvHD induction, especially in the haploidentical transplantation setting. Therefore, novel cellular therapies manipulated to minimize GvHD while enhancing the graft-versus-leukemia efficacy are currently being tested in early phase clinical trials. Immune cells genetically modified by non-HLA-restricted chimeric antigen receptors can overcome tumor immune evasion but require the presence of specific, targetable tumor antigens on malignant cells.4
Cytokine-induced killer cells represent a heterogeneous predominantly polyclonal T-cell population with phenotypical and functional properties of natural killer cells acquired during in vitro expansion.5 Due to their heterogeneity, CIK cells mediate both specific T-cell receptor (TCR)/MHC-restricted recognition and non-MHC-restricted cytotoxicity against malignant cells while sparing nonmalignant cells.6 Therefore, CIK cell therapy seems to be an obvious treatment option for patients with high-risk leukemia not eligible or not receptive to novel specific/targeted therapies for leukemia after allogeneic HSCT. However, data on the toxicity, feasibility and efficacy associated with the use of CIK cell treatment are still limited specifically in the haploidentical setting for prevention of relapse post transplant.
IL-15-activated CIK cells are an approved advanced therapy medicinal product (ATMP) for patients with high-risk leukemia and myelodsyplastic syndrome in Germany, and the authors hold a manufacturing license (ATMP § 4b Abs. 3 AMG, license number: PEI.A.11630.01.1) and marketing authorization for CIK cells.
Between September 2012 and April 2015, 13 patients, 11 pediatric (aged 1–17, median 9 years) and two adult patients (aged 42 and 69 years) with leukemia (ALL n=7, AML n=5, CML n=1) without immunosuppression and aGvHD not exceeding grade 1 who showed chemotherapy-resistant disease at the time of HSCT or molecular relapse [mixed chimerism ≥1% of recipient (autologous) signals; detectable MRD ≥10−6 or BCR-ABL/ABL ≥10−4] but not overt clinical relapse in the post-transplant period were included in this analysis after obtaining written informed consent (Table 1). CIK cell treatment was initiated at a median of 5.7 months (range 0.7–31.8) in the MRD-based (10 of 13 patients) and at a median of 1.4 months (range 1.1–1.8) post transplant in the prophylactic setting (3 of 13 patients). The prescribing directions for CIK cells considered titration of T cells because of the inherent risk for aGvHD; accordingly doses were escalated from starting doses of 1×106 in adults and 5×106 CD3+CD56− CIK cells/kg in pediatric patients, irrespective of the degree of HLA-matching between recipient and donor, to a maximum of 1×108 CD3+CD56- CIK cells/kg if aGvHD did not exceed grade 1.
Table 1.
Patients’ characteristics and outcome.
A median number of 3 (range 1–9) matched or of 2.5 (range 1–6) haploidentical CIK cell doses were given per patient, for a total of 43 infusions. Median T-cell numbers infused were 22.2 (range 1–100) ×106/kg in the matched setting, and due to appearance of aGvHD 5.0 (range 1–20.1) ×106/kg in the haploidentical setting (P=0.006). aGvHD, irrespective of grade, developed 2–4 weeks after infusion. In total, aGvHD occurred in 6 of 13 patients (46%) after CIK cell transfusion: 3 each with grade 1 and grade 3 (Table 1). Two of 3 grade 3 aGvHD-patients went on to develop limited chronic GvHD.
Three of 13 patients (23%) died of complications of the HSCT (Figure 1A), apparently unrelated to CIK cell treatment: one after second and one after fourth HSCT in the context of cumulative transplant-related toxicity developed impairment of cardiac, renal or liver function. One patient died due to cardiac insufficiency, one patient died due to invasive fungal infection and one patient died due to pulmonary infection several months after the last CIK cell treatment.
Figure 1.
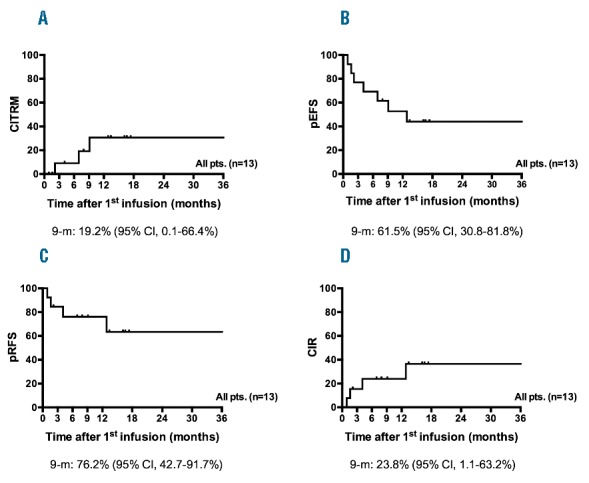
Outcome after first cytokine-induced killer (CIK) cell infusion. With the first CIK cell infusion patients were followed for a median of 9.1 months (range 0.9–36.3 months). Three out of 13 patients succumbed to transplant-related complications (TRM) apparently unrelated to CIK cell therapy (9-month CITRM 19.2%, 95%CI: 0.1%–66.4%. (A) Given historically high rates of relapse for this high-risk population within six months, a 9-month follow-up period was chosen for estimates of survival; 9-month probabilities of event-free survival (EFS) and relapse-free survival (RFS) for the 13 patients were estimated to be 61.5% (95%CI: 30.8%–81.8%) and 76.2% (95%Cl: 42.7%–91.7%), respectively (B and C). Four of 13 patients relapsed after transplantation (9-month CIR, 23.8%, 95%CI, 1.1%–63.2%. (D) All of these patients had shown molecular relapse at a median of 3.2 months (range, 2.3–4.1 months) after hematopoietic stem cell transplantation (HSCT). Hematologic relapses occurred at a median of 8.2 months (range 4–17.5 months) after transplantation suggesting that CIK cell treatment may have delayed recurrence of disease.
Regarding toxicity, some studies of pre-emptive IT including withdrawal of immunosuppression and DLI report GvHD rates as high as 70% in adult patients,7 but these were less frequent in pediatric patients:8 GvHD developed in 19% of pediatric patients, and toxic deaths due to GvHD occurred in 4%. The limited published data on haploidentical DLI report that 25% of the 40 patients who received haploidentical DLI developed aGvHD, of whom 15% had severe aGvHD and 2 severe aGvHD-patients died.9 In this report, a dose of 1×106 T cells/kg was associated with grade 2–4 GvHD in 16.7% of patients. In our analysis, severe GvHD occurred after doses of 5, 5, and 20×106 CD3+CD56− CIK cells/kg in 3 of 13 patients after infusions of haploidentical CIK cells, which had been administered in the early post-transplant period. Of these patients, all of whom could be successfully treated are alive, in very good clinical condition and CMR. Immune reconstitution monitoring showed that T-cell expansion had occurred in parallel (Figure 2F, H and J). Therefore, T-cell recovery that occurred at the same time could have also been responsible for induction of severe aGvHD in these patients. Even without lymphodepletion before infusion, our immune monitoring data suggest that infused CD3+CD56− CIK cells may persist in vivo. Therefore, we suggest prospectively monitoring T-cell recovery before CIK cell treatment. Care should be taken when administering haploidentical CIK cell infusions to enhance T-cell expansion in order to avoid additive T-cell toxicity.
Figure 2.
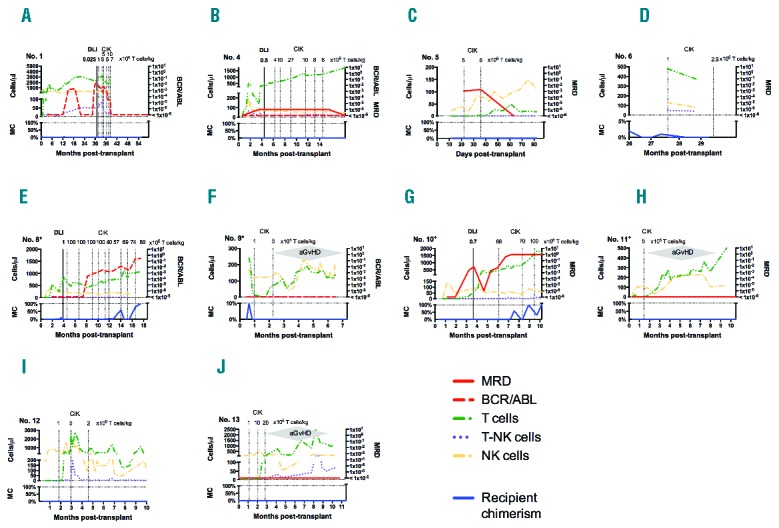
Immune reconstitution. After cytokine-induced killer (CIK) cell treatment patients were sequentially screened for relapse, for occurrence of acute graft-versus-host-disease (aGvHD) and for T, natural killer (NK), and T-NK cell counts. Patients with treatment responses for whom immune monitoring data were available are shown. Immune monitoring demonstrated that T-cell numbers, followed by NK and to a lesser extent T-NK cell counts constantly increased after receiving more than 1×106 CD3+CD56− CIK cells/kg, which correlated with appearance of aGvHD especially in the haploidentical transplantation setting (F, H, J). In most cases T-cell counts slightly decreased 2–4 weeks after infusions, but increased thereafter above levels before infusion. During increase of immune effector cells, short-term anti-leukemic effects were observed in the matched transplantation setting (B, D, E, G) whereas in the haploidentical setting (A, C, F, H, I, J), increase of immune cells resulted in clearance of minimal residual disease (MRD) and BCR-ABL/ABL or conversion to full donor chimerism.
Interestingly, no signs of aGvHD occurred in one patient amongst others with a maximum dose of 10×106 CD3+CD56− haploidentical CIK cells/kg, which was administrated 3.1 years after HSCT, suggesting that timing clearly matters. Accordingly, irrespective of the number of T cells infused, low risk for GvHD has also been described in DLI-patients more than three months after transplantation.10
Ten of 13 patients with molecular relapse provided first insights on efficacy of IL-15-activated CIK cell treatment. Treatment responses were monitored weekly in peripheral blood and monthly, if applicable, in bone marrow samples. Treatment was continued until either clearance of molecular disease or disease progression: 6, 3, 6, 2, 2 and 2 CIK cell infusions sustained CMR for 2.6 years, 4.6 months, 6.9 months, 1.7 months, 5.7 months and 1.1 years in 6 of 10 patients with molecular relapse (Table 1). Treatment responses lasted 7.3 months in another patient with molecular relapse until reappearance of recipient chimerism. Despite continuing with CIK cell treatment, this patient finally relapsed.
Altogether, 4 of 13 patients relapsed (30%), one of whom died. Three were offered subsequent transplantation. All 3 prophylactically treated patients remained in CMR 1.5 years, 9.1 months and 1.6 years after first CIK cell infusion. Accordingly, a few recent reports with heterogeneous groups of patients and treatment strategies indicated that DLI may also be able to overcome MRD. Studies with post-transplantation evidence of MRD or increasing host chimerism and DLI reported response rates of 14% in the matched11 and 30% in the haploidentical setting,9 respectively. However, patients with evidence of pre-transplantation disease remained at increased risk of relapse (63%), despite the IT approach.12 Therefore, according to the desperate clinical situations of some of our patients, one dose of DLIs was also given to 5 of 10 patients with evidence of molecular relapse in order to bridge the period of generation of CIK cells. Nevertheless, levels of MRD increased in 3 of these patients during DLI treatment, potentially worsening the initial situation for subsequent CIK cell therapy. However, 3 of the 5 patients with DLI prior to CIK cell treatment achieved continuous complete remission.
Importantly, 4 of our 10 patients who were MRD positive (which represents a nearly 100% probability of relapse) and who had not received classical DLI achieved sustained molecular remissions, providing strong support for the therapeutic potential of CIK cells. Furthermore, continuous CMR was sustained in each of our 6 patients after haploidentical CIK cell treatment, suggesting that CIK cells may work better in haploidentical than in matched settings. However, efficacy of matched CIK cell treatment has also been reported by others.13–15 The frequency with which such outcomes can be expected must now be established in the controlled setting of a formal clinical trial enrolling exclusively patients with molecular relapse. Based on the incidence of aGvHD in our analysis, CIK cell treatment should be applied with an interval of 4–6 weeks according to a dose escalation schedule, not exceeding a maximal dose of 1×107 CD3+CD56− CIK cells/kg. In the presence of at least grade 1 aGvHD, or in case of T-cell recovery post transplant, the next scheduled infusion should not be administered, or if administration is considered, this should be performed with great care. Assessment of efficacy implemented by frequent monitoring for relapse might include reduction or clearance of molecular disease, rate of and time to hematologic relapse, and rate and duration of complete or partial molecular response.
In conclusion, our findings demonstrate the feasibility and safety of CIK cell treatment, and suggest it is active in a heterogeneous cohort of pediatric and adult HSCT recipients. Further analysis of efficacy will focus on optimal cell dosage and timing of upfront CIK cell interventions in relapsing leukemia patients who are not receptive to or eligible for specific or targeted cellular therapies after allogeneic HSCT.
Footnotes
Funding: the authors thank the LOEWE Center for Cell and Gene Therapy Frankfurt, funded by: Hessian Ministry of Higher Education, Research and the Arts, funding reference number: III L 4- 518/17.004 (2013), and the Else Kröner-Fresenius-Stiftung (P75/08//A62/08 and 2014_A305) for funding of this study.
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Kanda J, Chao NJ, Rizzieri DA. Haploidentical transplantation for leukemia. Curr Oncol Rep. 2010;12(5):292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bader P, Kreyenberg H, von Stackelberg A, et al. Monitoring of Minimal Residual Disease After Allogeneic Stem-Cell Transplantation in Relapsed Childhood Acute Lymphoblastic Leukemia Allows for the Identification of Impending Relapse: Results of the ALL-BFM-SCT 2003 Trial. J Clin Oncol. 2015;33(11):1275–1284. [DOI] [PubMed] [Google Scholar]
- 3.Bader P, Kreyenberg H, Hoelle W, et al. Increasing mixed chimerism is an important prognostic factor for unfavorable outcome in children with acute lymphoblastic leukemia after allogeneic stem-cell transplantation: possible role for pre-emptive immunotherapy¿ J Clin Oncol. 2004;22(9):1696–1705. [DOI] [PubMed] [Google Scholar]
- 4.Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmidt-Wolf IG, Negrin RS, Kiem HP, Blume KG, Weissman IL. Use of a SCID mouse/human lymphoma model to evaluate cytokine-induced killer cells with potent antitumor cell activity. J Exp Med. 1991;174(1):139–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pievani A, Borleri G, Pende D, et al. Dual-functional capability of CD3+CD56+ CIK cells, a T-cell subset that acquires NK function and retains TCR-mediated specific cytotoxicity. Blood. 2011;118(12):3301–3310. [DOI] [PubMed] [Google Scholar]
- 7.Lutz C, Massenkeil G, Nagy M, et al. A pilot study of prophylactic donor lymphocyte infusions to prevent relapse in adult acute lymphoblastic leukemias after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2008;41(9):805–812. [DOI] [PubMed] [Google Scholar]
- 8.Haines HL, Bleesing JJ, Davies SM, et al. Outcomes of donor lymphocyte infusion for treatment of mixed donor chimerism after a reduced-intensity preparative regimen for pediatric patients with nonmalignant diseases. Biol Blood Marrow Transplant. 2015;21(2):288–292. [DOI] [PubMed] [Google Scholar]
- 9.Zeidan AM, Forde PM, Symons H, et al. HLA-haploidentical donor lymphocyte infusions for patients with relapsed hematologic malignancies after related HLA-haploidentical bone marrow transplantation. Biol Blood Marrow Transplant. 2014;20(3):314–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collins RH, Jr, Shpilberg O, Drobyski WR, et al. Donor leukocyte infusions in 140 patients with relapsed malignancy after allogeneic bone marrow transplantation. J Clin Oncol. 1997;15(2):433–444. [DOI] [PubMed] [Google Scholar]
- 11.Balduzzi A, Di Maio L, Silvestri D, et al. Minimal residual disease before and after transplantation for childhood acute lymphoblastic leukaemia: is there any room for intervention¿ Br J Haematol. 2014;164(3):396–408. [DOI] [PubMed] [Google Scholar]
- 12.Lankester AC, Bierings MB, van Wering ER, et al. Preemptive alloimmune intervention in high-risk pediatric acute lymphoblastic leukemia patients guided by minimal residual disease level before stem cell transplantation. Leukemia. 2010;24(8):1462–1469. [DOI] [PubMed] [Google Scholar]
- 13.Linn YC, Niam M, Chu S, et al. The anti-tumour activity of allogeneic cytokine-induced killer cells in patients who relapse after allogeneic transplant for haematological malignancies. Bone Marrow Transplant. 2012;47(7):957–966. [DOI] [PubMed] [Google Scholar]
- 14.Introna M, Borleri G, Conti E, et al. Repeated infusions of donor-derived cytokine-induced killer cells in patients relapsing after allogeneic stem cell transplantation: a phase I study. Haematologica. 2007;92(7):952–959. [DOI] [PubMed] [Google Scholar]
- 15.Laport GG, Sheehan K, Baker J, et al. Adoptive immunotherapy with cytokine-induced killer cells for patients with relapsed hematologic malignancies after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2011;17(11):1679–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]


