Abstract
Regulation of blood pH—critical for virtually every facet of life—requires that the renal proximal tubule (PT) adjust its rate of H+ secretion (nearly the same as the rate of HCO3− reabsorption, JHCO3) in response to changes in blood [CO2] and [HCO3−]. Yet CO2/HCO3− sensing mechanisms remain poorly characterized. Because receptor tyrosine kinase inhibitors render JHCO3 in the PT insensitive to changes in CO2 concentration, we hypothesized that the structural features of receptor protein tyrosine phosphatase-γ (RPTPγ) that are consistent with binding of extracellular CO2 or HCO3− facilitate monitoring of blood CO2/HCO3− concentrations. We now report that PTs express RPTPγ on blood-facing membranes. Moreover, RPTPγ deletion in mice eliminated the CO2 and HCO3− sensitivities of JHCO3 as well as the normal defense of blood pH during whole-body acidosis. Thus, RPTPγ appears to be a novel extracellular CO2/HCO3− sensor critical for pH homeostasis.
Keywords: metabolic acidosis, pH, bicarbonate transport, proximal tubule, receptor protein tyrosine phosphatase, kidney
The regulation of pH is critically important because nearly all biologic processes, both inside cells and in the extracellular fluid, are pH sensitive.1–3 Intracellular pH (pHi) depends on extracellular fluid pH (pHo); the latter, in turn, depends on arterial pH (pHa), which reflects the ratio of arterial carbon dioxide concentration to arterial bicarbonate concentration [CO2]a/[HCO3−]a. Pulmonary ventilation controls [CO2]a, and the kidneys—particularly the proximal tubules (PTs)—control [HCO3−]a by adjusting the rate of HCO3− transfer (JHCO3) from the epithelial cell, across the basolateral (BL) membrane, into the blood. Curiously, JHCO3 is acutely independent of pH at the BL surface (pHBL) of PTs yet rises steeply with increases in [CO2]BL or decreases in [HCO3−]BL.4 Nevertheless, how PTs, or other cells, sense changes in [CO2]BL or [HCO3−]BL has remained elusive. However, two key insights on how this sensing occurs are that (1) drugs blocking the ErbB receptor protein tyrosine kinases eliminate PT CO2 sensitivity5 and (2) the receptor protein tyrosine phosphatases RPTPγ (which are approximately ubiquitous) and RPTPζ (mainly in the brain) have extracellular ligand-binding domains that are 35%–40% identical to the catalytic domains of human carbonic anhydrases (CAs).6 Our goal was to determine whether RPTPγ, with no previously established function in ligand sensing, plays a role in CO2/HCO3− sensing.
In the early 1950s, three groups demonstrated in dogs that respiratory acidosis—a rise in [CO2] that produces a fall in pH and a rise in [HCO3−]—causes a rapid increase in renal acid secretion.7–9 Later investigators showed that alkalosis inhibits acid secretion at the level of renal tubules.10,11 However, with classic acid-base disturbances at least two of the three chemical parameters ([CO2], [HCO3−], and pH) must change under equilibrium conditions, making it impossible to define the key underlying parameters. The invention of out-of-equilibrium (OOE) CO2/HCO3− solutions12 made it possible to alter each of the three parameters independently, leading to the discovery that PTs sense [CO2]BL and [HCO3−]BL per se.4
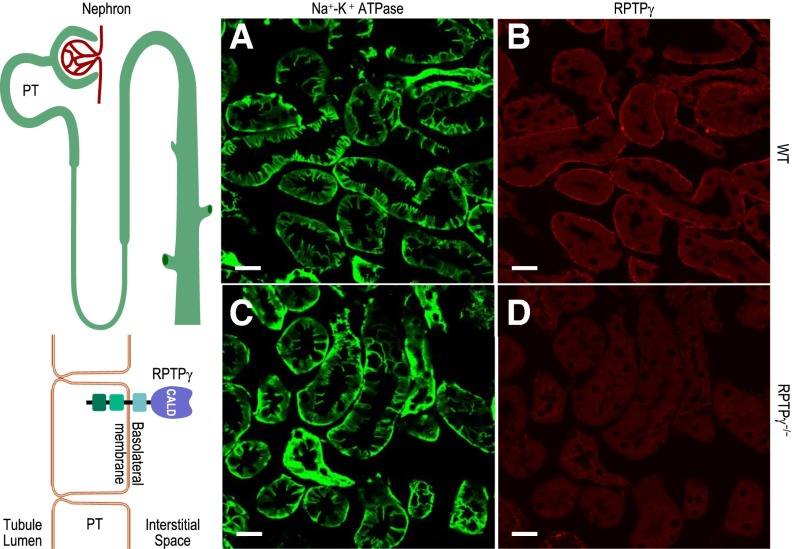
PCR data show that mouse kidney expresses two splice variants (designated A and B) of the Ptprg gene (Supplemental Figure 1). We developed a polyclonal antibody to a unique 17-residue sequence in the carbonic-anhydrase–like domain (CALD) of RPTPγ. Western blotting of mouse whole-kidney lysates shows reactivity at approximately 200 kDa (predicted, unglycosylated molecular mass: approximately 160 kDa) for wild-type (WT) and RPTPζ−/− mice, but not for RPTPγ−/− mice (Supplemental Figure 2). Thus, kidneys expressed RPTPγ protein, for which our antibody is specific. Figure 1 shows cryosections of kidney cortex from WT versus RPTPγ−/− mice. For both, the sodium-potassium adenosine triphosphatase showed the expected reactivity at the PT BL membrane (Figure 1, A and C), including the extensive infoldings that nearly surround a specialized extracellular space into which PT cells deposit reabsorbed fluid before moving into the blood vessels. The RPTPγ antibody (Figure 1, B and D) reacted only with PTs from WT mice, predominantly staining portions of the BL membrane that face blood vessels, not the infoldings (Supplemental Figure 3 shows additional control experiments). Thus, RPTPγ in the PT is ideally situated to monitor blood—not reabsorbate—composition.
Figure 1.
PTs express RPTPγ on the portion of the basalateral membrane that faces blood vessels. (A–D) Immunofluorescent staining of cortical sections of kidneys from WT or RPTPγ−/− mice, showing predominantly PTs. The sodium-potassium adenosine triphosphatase (Na+-K+ ATPase) antibody (green, BL membrane marker) stains all regions of the BL membrane in PTs from WT and RPTPγ−/− mice. Our RPTPγ antibody (red) establishes that RPTPγ protein was present only in the subset of the PT BL membrane facing the blood vessels in WT PTs. Images are representative of data obtained from three mice per group. Scale bar =20 μm.
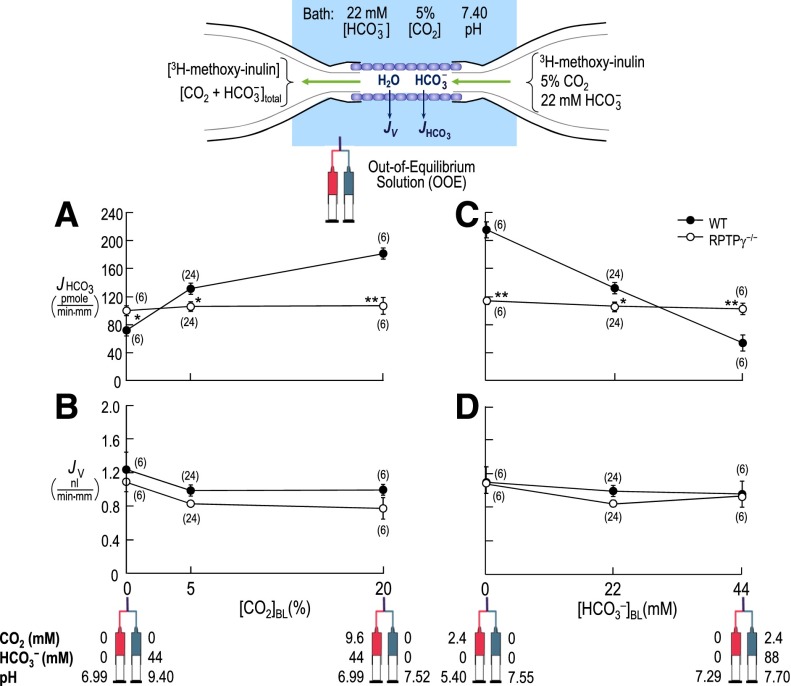
With the RPTPγ−/− mouse,13 it is possible to explore the role of RPTPγ in acid-base homeostasis. Figure 2 summarizes experiments on isolated, perfused PTs from WT versus RPTPγ−/− mice. The filled circles in Figure 2A show, for WT tubules, the dependence of JHCO3 on [CO2]BL, as we used OOE technology to fix [HCO3−]BL and pHBL. As noted previously for PTs from rabbits4 and mice,14 lowering [CO2]BL to <5% (the physiologic value) decreased JHCO3, whereas raising [CO2]BL increased JHCO3—responses appropriate for stabilizing pHa during “respiratory”/[CO2]a acid-base disturbances. The open circles, representing RPTPγ−/− tubules, reveal a depressed JHCO3 at 5% CO2 and, more strikingly, a complete insensitivity to changes in [CO2]BL. Figure 2B shows that for both WT and RPTPγ−/− tubules, [CO2]BL changes did not significantly affect fluid-volume reabsorption (JV). The WT data further confirm previous results from rabbits4 and mice14 by implying that PTs compensate for JNaHCO3 changes (Figure 2A) with reciprocal JNaCl changes; in the intact animal, this compensation would stabilize blood volume and BP.
Figure 2.
Isolated PTs from RPTPγ knockout mice do not respond to changes in either [CO2]BL or [HCO3−]BL. (A) Dependence of JHCO3, in WT versus RPTPγ−/− PTs, on BL [CO2], using OOE solutions to vary [CO2]BL at a fixed [HCO3−]BL of 22 mM and a fixed pHBL of 7.40 (37°C). (B) Dependence of JV on [CO2]BL in the same tubules as in panel A. (C) Dependence of JHCO3, in WT versus RPTPγ−/− PTs, on [HCO3−]BL, using OOE solutions to vary [HCO3−]BL at a fixed [CO2]BL of 5% and a fixed pHBL of 7.40. (D) Dependence of JV on [HCO3−]BL in the tubules from panel C.
Figure 2, C and D, is analogous to Figure 2, A and B, except here we varied [HCO3−]BL at a fixed [CO2]BL and pHBL. Figure 2C shows that, for WT tubules, JHCO3 varied reciprocally with [HCO3−]BL—the appropriate compensation for “metabolic”/[HCO3−]a disturbances, extending previous work on rabbit PTs.4 However, RPTPγ−/− tubules were completely insensitive to changes in [HCO3−]BL. Previous work with rabbit4 PTs showed that JV is insensitive to changes in [HCO3−]BL, again implying that JNaHCO3 and JNaCl vary reciprocally. Figure 2D confirms this result for PTs from WT mice.
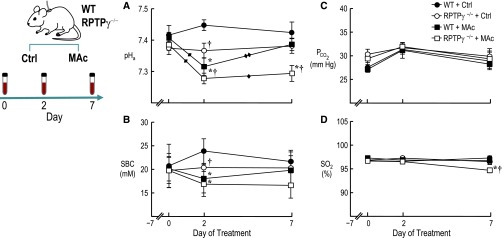
Because the knockout of RPTPγ eliminates the ability of PTs to respond to acute changes in [HCO3−]BL, we hypothesized that RPTPγ−/− mice also have a reduced ability to defend pHa during chronic metabolic acidosis (MAc). Figure 3 summarizes experiments in which we cannulated the carotid arteries of WT or RPTPγ−/− mice at day −7 and, after recovery, sequentially sampled arterial blood from conscious mice. On day 0, RPTPγ−/− mice had a mean pHa that was not significantly different from that of WT mice in this (Figure 3A) or a previous15 study. Thus, even though PTs from RPTPγ−/− mice show a modest decrease in JHCO3 at 5% CO2/22 mM HCO3− (Figure 2, A and C), RPTPγ−/− mice maintained a normal pHa, perhaps because of PT ammoniagenesis and modest compensation by nephron segments downstream of the PT.
Figure 3.
RPTPγ knockout mice cannot appropriately defend against chronic metabolic acidosis. Blood was sampled at days 0, 2, and 7. (A) pHa. (B) PCO2. (C) Standard bicarbonate concentration (SBC). (D) Oxygen saturation of hemoglobin (SO2, %). Values are the mean±SEM (n=8–16). *P<0.05 versus genotype-matched control (i.e., WT+MAc versus WT+Ctrl, or RPTPγ−/−+MAc versus RPTPγ−/−+Ctrl). †P<0.05 versus water-matched WT (RPTPγ−/−+Ctrl versus WT+Ctrl, or RPTPγ−/−+MAc versus WT+MAc). Single diamond denotes a statistically significance change from day 0; two diamonds denote significant change from day 2 (within the respective treatment group, P<0.05). Please refer to the Supplemental Material for additional details on time-dependent statistical differences.
Immediately following day 0 arterial sampling, we either continued to provide normal drinking water (control) or switched to water containing 1% ammonium chloride (wt/vol) to elicit MAc.16 On control water, both WT and RPTPγ−/− mice maintained a stable pHa over the 7 days (Figure 3A). However, a slight increase in the WT value on day 2 led to a difference that reached statistical significance, suggesting a modest defect in net acid excretion in RPTPγ−/− mice. Compared with day 0, on day 2 mice challenged with MAc exhibited substantial pHa declines, greater in RPTPγ−/− than WT. Compared with day 2, on day 7, pHa for WT+MAc mice recovered, whereas RPTPγ−/−+MAc mice continued to show substantial acidosis. As expected, the calculated standard [HCO3−] (Figure 3B) exhibited a pattern similar to that of pHa, whereas PCO2 (Figure 3C) and hemoglobin-oxygen saturation (Figure 3D) underwent less remarkable changes. (Supplemental Table 1 provides all arterial blood gas data, including full statistical analyses, and Supplemental Tables 2 and 3 provide blood chemistry and hematology data). Thus, RPTPγ−/− mice cannot appropriately defend pHa or standard [HCO3−] when challenged by MAc.
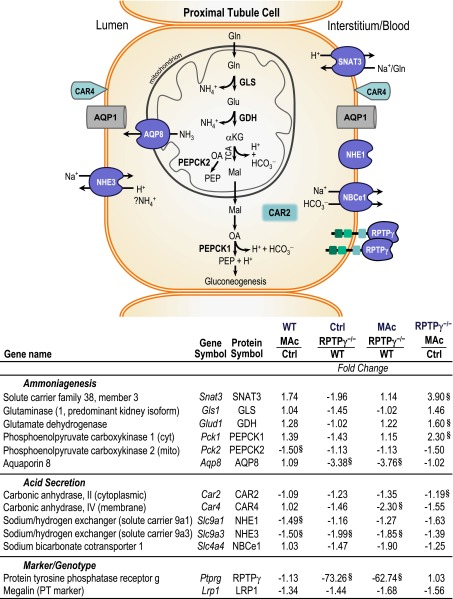
To further characterize the RPTPγ−/− phenotype at MAc day 7, we used quantitative PCR to assess a panel of gene targets related to ammoniagenesis and PT hydrogen (H+) secretion. Regarding ammoniagenesis, increased PT metabolism of plasma glutamine during MAc would increase ammonium excretion and thereby compensate for the increased acid load.17 As expected, we observed a trend for MAc to upregulate Snat3, Glud1, and Pck1 in WT tissue (Figure 4). The knockout tended to downregulate ammoniagenesis genes with control mice but produced a far more robust response to MAc. Interestingly, MAc did not upregulate Aqp8, and the knockout markedly reduced Aqp8. Regarding H+ secretion, MAc in WT tissue downregulated Slc9a1 and Slc9a3 and had no significant effect on the other components. MAc in RPTPγ−/− tissue tended to downregulate all genes involved in PT H+ secretion.
Figure 4.
RPTPγ gene deletion downregulates ammoniagenesis genes at baseline and exaggerates the response to MAc. The diagram summarizes proteins potentially involved in the adaptive response to 7 days of MAc. Relative gene expression was assessed in kidney tissue by quantitative PCR. Data are presented as fold change of gene expression between the groups indicated. §P<0.05 based on relative expression values (n=4–5). αKG, α-ketoglutarate; Gln, glutamine; Glu, glutamate; Mal, malate; Na+, sodium; NH3, ammonia; NH4+, ammonium; OA, oxaloacetate; PEP, phosphoenolpyruvate; TCA, tricarboxylic acid cycle. Columns 2 and 3 of the table present the gene and protein abbreviations, respectively.
Established acid-base sensors18 include soluble adenylyl cyclase19 and soluble guanylyl cyclase,20,21 which sense intracellular [HCO3−]; pHo-sensitive ion channels22–25; pHo-sensitive G-protein–coupled receptors26–29; and the tyrosine kinase Pyk2, which responds to decreased pHi.30 However, none can account for the ability of the PT to sense [CO2]o and [HCO3−]o.4 Our work suggests a new class of acid-base sensor, RPTPγ. The CALD domains of RPTPγ6 and RPTPζ31—homologous to the noncanonical CAs (VIII, X, and XI)—lack the full complement of His residues believed essential for enzymatic activity. Indeed, it is not clear how a CALD could sense CO2 versus HCO3− if it were interconverting them. The most straightforward hypothesis is that CO2 and HCO3− compete for binding to the CALD of RPTPγ and that CO2–CALD or HCO3−–CALD promotes dimerization and thus inactivation of this tyrosine phosphatase.32 Recent PT work shows that acid-base disturbances lead to specific changes in tyrosine phosphorylation of ErbB1/2.33
Previous work provided limited insight into specific signaling roles for RPTPγ, which has been implicated as a tumor-suppressor gene.6 The initial characterization of RPTPγ−/− mice revealed minor behavioral changes,13 with more recent work suggesting that RPTPγ knockdown has an antidepressive effect.34 Other studies show that a portion of the CALD surface—distant from regions homologous to the CO2/HCO3− binding sites of canonical CAs—interacts with contactins,35,36 implying a role in cell–cell interactions. The present work suggests a specific signaling role to RPTPγ and reveals robust PT and whole-animal phenotypes consistent with the molecular data. Finally, our work raises the question of whether RPTPζ and the noncanonical CAs (VIII, X, and XI) also serve as CO2/HCO3− sensors.
Concise Methods
Immunoblotting and Immunostaining
We generated a novel rabbit polyclonal antibody specific for the CALD of the RPTPγ protein to assess expression by Western blotting and subcellular localization by immunofluorescent staining in mouse renal tissue.
RPTPγ−/− Mice
We used an established RPTPγ−/− mouse line,13 backcrossed with our WT line.
Perfusion of Isolated Mouse PTs
As described previously,14 the luminal perfusate always contained 5% CO2/22 mM HCO3− plus 14C-methoxyinulin as a volume marker. We used OOE technology to control [CO2] and [HCO3−] in the BL solution surrounding the PTs.4,12,37 We collected the fluid that had passed through the PT lumen and analyzed it for 14C-methoxyinulin by scintillation counting and for total CO2 using an enzyme-linked microfluorometry approach and computed JV and JHCO3.14,38
Serial Assessment of Arterial Blood in Mice Challenged with MAc
Mice were implanted with indwelling catheters in the left carotid artery to permit serial sampling. Following 7 days of recovery from surgery, mice were dosed via drinking water with 1% ammonium chloride for 7 days to induce MAc.
Transcription Profile of Targeted Genes
Gene expression was assessed using total RNA isolated from mouse kidneys harvested following the 7-day MAc study. Quantitative PCR was performed at Case Western University’s Gene Expression & Genotyping facility using validated TaqMan assays from a commercial vendor.
Please refer to the Supplemental Material for detailed information on methods.
Disclosures
The research described in this article was completed while Margaret Chandler was an employee at Case Western Reserve University. The opinions expressed in this article are the authors' own and do not reflect the view of the National Institutes of Health, the US Department of Health and Human Services, or the US Government.
Supplementary Material
Acknowledgments
We thank Joseph Schlessinger (Yale University) for his generous gift of the RPTPγ−/− mice, Frank D'Angelo for preparation of kidney tissue, Sue-Ann Mentone (Yale University) for preparing cryosections, Xiaoqin Chen and Tracy A. McElfresh of the Mouse Physiological Phenotyping Core, and Gerald Babcock for his role as laboratory manager. We also thank Dr. Mark Parker and Dr. Ulrich Hopfer for insightful discussions about the data. Gene expression experiments were facilitated by the Gene Expression and Genotyping Facility of the Case Comprehensive Cancer Center (P30 CA43703).
This work was supported by National Institutes of Health grant DK81567 (to W.F.B.) and a Scientist Development grant (0735432N) from the American Heart Association (to Y.Z.). L.A.S. was supported by a postdoctoral fellowship from the National Kidney Foundation.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Receptor Protein Tyrosine Phosphatase γ, CO2 Sensing in Proximal Tubule and Acid Base Homeostasis,” on pages 2543–2545.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015040439/-/DCSupplemental.
References
- 1.Roos A, Boron WF: Intracellular pH. Physiol Rev 61: 296–434, 1981 [DOI] [PubMed] [Google Scholar]
- 2.Chesler M: Regulation and modulation of pH in the brain. Physiol Rev 83: 1183–1221, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Parker MD, Boron WF: The divergence, actions, roles, and relatives of sodium-coupled bicarbonate transporters. Physiol Rev 93: 803–959, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou Y, Zhao J, Bouyer P, Boron WF: Evidence from renal proximal tubules that HCO3− and solute reabsorption are acutely regulated not by pH but by basolateral HCO3− and CO2. Proc Natl Acad Sci U S A 102: 3875–3880, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou Y, Bouyer P, Boron WF: Role of a tyrosine kinase in the CO2-induced stimulation of HCO3− reabsorption by rabbit S2 proximal tubules. Am J Physiol Renal Physiol 291: F358–F367, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Barnea G, Silvennoinen O, Shaanan B, Honegger AM, Canoll PD, D’Eustachio P, Morse B, Levy JB, Laforgia S, Huebner K, Musacchi JM, Sap J, Schlessinger J: Identification of a carbonic anhydrase-like domain in the extracellular region of RPTP gamma defines a new subfamily of receptor tyrosine phosphatases. Mol Cell Biol 13: 1497–1506, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brazeau P, Gilman A: Effect of plasma CO2 tension on renal tubular reabsorption of bicarbonate. Am J Physiol 175: 33–38, 1953 [DOI] [PubMed] [Google Scholar]
- 8.Relman AS, Etsten B, Schwartz WB: The regulation of renal bicarbonate reabsorption by plasma carbon dioxide tension. J Clin Invest 32: 972–978, 1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dorman PJ, Sullivan WJ, Pitts RF: The renal response to acute respiratory acidosis. J Clin Invest 33: 82–90, 1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cogan MG: Effects of acute alterations in PCO2 on proximal HCO3−, Cl-, and H2O reabsorption. Am J Physiol 246: F21–F26, 1984 [DOI] [PubMed] [Google Scholar]
- 11.Chan YL, Giebisch G: Relationship between sodium and bicarbonate transport in the rat proximal convoluted tubule. Am J Physiol 240: F222–F230, 1981 [DOI] [PubMed] [Google Scholar]
- 12.Zhao J, Hogan EM, Bevensee MO, Boron WF: Out-of-equilibrium CO2/HCO3− solutions and their use in characterizing a new K/HCO3 cotransporter. Nature 374: 636–639, 1995 [DOI] [PubMed] [Google Scholar]
- 13.Lamprianou S, Vacaresse N, Suzuki Y, Meziane H, Buxbaum JD, Schlessinger J, Harroch S: Receptor protein tyrosine phosphatase gamma is a marker for pyramidal cells and sensory neurons in the nervous system and is not necessary for normal development. Mol Cell Biol 26: 5106–5119, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou Y, Bouyer P, Boron WF: Role of the AT1A receptor in the CO2-induced stimulation of HCO3− reabsorption by renal proximal tubules. Am J Physiol Renal Physiol 293: F110–F120, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Iversen NK, Malte H, Baatrup E, Wang T: The normal acid-base status of mice. Respir Physiol Neurobiol 180: 252–257, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Biver S, Belge H, Bourgeois S, Van Vooren P, Nowik M, Scohy S, Houillier P, Szpirer J, Szpirer C, Wagner CA, Devuyst O, Marini AM: A role for Rhesus factor Rhcg in renal ammonium excretion and male fertility. Nature 456: 339–343, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Nowik M, Lecca MR, Velic A, Rehrauer H, Brändli AW, Wagner CA: Genome-wide gene expression profiling reveals renal genes regulated during metabolic acidosis. Physiol Genomics 32: 322–334, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Brown D, Wagner CA: Molecular mechanisms of acid-base sensing by the kidney. J Am Soc Nephrol 23: 774–780, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Y, Cann MJ, Litvin TN, Iourgenko V, Sinclair ML, Levin LR, Buck J: Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science 289: 625–628, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Sun L, Wang H, Hu J, Han J, Matsunami H, Luo M: Guanylyl cyclase-D in the olfactory CO2 neurons is activated by bicarbonate. Proc Natl Acad Sci U S A 106: 2041–2046, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schulz S, Wedel BJ, Matthews A, Garbers DL: The cloning and expression of a new guanylyl cyclase orphan receptor. J Biol Chem 273: 1032–1037, 1998 [DOI] [PubMed] [Google Scholar]
- 22.Chatelain FC, Bichet D, Douguet D, Feliciangeli S, Bendahhou S, Reichold M, Warth R, Barhanin J, Lesage F: TWIK1, a unique background channel with variable ion selectivity. Proc Natl Acad Sci U S A 109: 5499–5504, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.L’Hoste S, Barriere H, Belfodil R, Rubera I, Duranton C, Tauc M, Poujeol C, Barhanin J, Poujeol P: Extracellular pH alkalinization by Cl-/HCO3− exchanger is crucial for TASK2 activation by hypotonic shock in proximal cell lines from mouse kidney. Am J Physiol Renal Physiol 292: F628–F638, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski M: A proton-gated cation channel involved in acid-sensing. Nature 386: 173–177, 1997 [DOI] [PubMed] [Google Scholar]
- 25.Vulcu SD, Liewald JF, Gillen C, Rupp J, Nawrath H: Proton conductance of human transient receptor potential-vanilloid type-1 expressed in oocytes of Xenopus laevis and in Chinese hamster ovary cells. Neuroscience 125: 861–866, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Ludwig M-G, Vanek M, Guerini D, Gasser JA, Jones CE, Junker U, Hofstetter H, Wolf RM, Seuwen K: Proton-sensing G-protein-coupled receptors. Nature 425: 93–98, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Sun X, Yang LV, Tiegs BC, Arend LJ, McGraw DW, Penn RB, Petrovic S: Deletion of the pH sensor GPR4 decreases renal acid excretion. J Am Soc Nephrol 21: 1745–1755, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mohebbi N, Benabbas C, Vidal S, Daryadel A, Bourgeois S, Velic A, Ludwig M-G, Seuwen K, Wagner CA: The proton-activated G protein coupled receptor OGR1 acutely regulates the activity of epithelial proton transport proteins. Cell Physiol Biochem 29: 313–324, 2012 [DOI] [PubMed] [Google Scholar]
- 29.Ishii S, Kihara Y, Shimizu T: Identification of T cell death-associated gene 8 (TDAG8) as a novel acid sensing G-protein-coupled receptor. J Biol Chem 280: 9083–9087, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Li S, Sato S, Yang X, Preisig PA, Alpern RJ: Pyk2 activation is integral to acid stimulation of sodium/hydrogen exchanger 3. J Clin Invest 114: 1782–1789, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levy JB, Canoll PD, Silvennoinen O, Barnea G, Morse B, Honegger AM, Huang JT, Cannizzaro LA, Park SH, Druck T, Huebner K, Sap J, Ehrlich M, Musacchio JM, Schlessinger J: The cloning of a receptor-type protein tyrosine phosphatase expressed in the central nervous system. J Biol Chem 268: 10573–10581, 1993 [PubMed] [Google Scholar]
- 32.Barr AJ, Ugochukwu E, Lee WH, King ONF, Filippakopoulos P, Alfano I, Savitsky P, Burgess-Brown NA, Müller S, Knapp S: Large-scale structural analysis of the classical human protein tyrosine phosphatome. Cell 136: 352–363, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skelton LA, Boron WF: Effect of acute acid-base disturbances on ErbB1/2 tyrosine phosphorylation in rabbit renal proximal tubules. Am J Physiol Renal Physiol 305: F1747–F1764, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang W, Savelieva KV, Tran DT, Pogorelov VM, Cullinan EB, Baker KB, Platt KA, Hu S, Rajan I, Xu N, Lanthorn TH: Characterization of PTPRG in knockdown and phosphatase-inactive mutant mice and substrate trapping analysis of PTPRG in mammalian cells. PLoS One 7: e45500, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bouyain S, Watkins DJ: Identification of tyrosine phosphatase ligands for contactin cell adhesion molecules. Commun Integr Biol 3: 284–286, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bouyain S, Watkins DJ: The protein tyrosine phosphatases PTPRZ and PTPRG bind to distinct members of the contactin family of neural recognition molecules. Proc Natl Acad Sci U S A 107: 2443–2448, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao J, Zhou Y, Boron WF: Effect of isolated removal of either basolateral HCO3− or basolateral CO2 on HCO3− reabsorption by rabbit S2 proximal tubule. Am J Physiol Renal Physiol 285: F359–F369, 2003 [DOI] [PubMed] [Google Scholar]
- 38.McKinney TD, Burg MB: Biocarbonate and fluid absorption by renal proximal straight tubules. Kidney Int 12: 1–8, 1977 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.