Abstract
Urinary hepcidin may have protective effects against AKI. However, renal handling and the potential protective mechanisms of hepcidin are not fully understood. By measuring hepcidin levels in plasma and urine using mass spectrometry and the kidney using immunohistochemistry after intraperitoneal administration of human hepcidin-25 (hhep25) in C57Bl/6N mice, we showed that circulating hepcidin is filtered by the glomerulus and degraded to smaller isoforms detected in urine but not plasma. Moreover, hepcidin colocalized with the endocytic receptor megalin in proximal tubules, and compared with wild-type mice, megalin-deficient mice showed higher urinary excretion of injected hhep25 and no hepcidin staining in proximal tubules that lack megalin. This indicates that hepcidin is reaborbed in the proximal tubules by megalin dependent endocytosis. Administration of hhep25 concomitant with or 4 hours after a single intravenous dose of hemoglobin abolished hemoglobin-induced upregulation of urinary kidney injury markers (NGAL and KIM-1) and renal Interleukin-6 and Ngal mRNA observed 24 hours after administration but did not affect renal ferroportin expression at this point. Notably, coadministration of hhep25 and hemoglobin but not administration of either alone greatly increased renal mRNA expression of hepcidin-encoding Hamp1 and hepcidin staining in distal tubules. These findings suggest a role for locally synthesized hepcidin in renal protection. Our observations did not support a role for ferroportin in hhep25-mediated protection against hemoglobin–induced early injury, but other mechanisms of cellular iron handling may be involved. In conclusion, our data suggest that both systemically delivered and locally produced hepcidin protect against hemoglobin-induced AKI.
Keywords: hepcidin, iron, megalin, hemoglobin, acute kidney injury
The iron–regulating peptide hormone, hepcidin, has recently been proposed as a potential biomarker to predict AKI in patients undergoing coronary artery bypass grafting (CABG). Diverse clinical studies showed an association between high urinary hepcidin concentration and reduced risk of AKI in these patients.1–3 AKI after CABG can be induced or mediated through iron released from increased amounts of heme in the kidney via hemolysis and/or ischemia-reperfusion injury (IRI).4 Because of its important role in iron homeostasis,5 these observations prompted the hypothesis that hepcidin may have protective effects against iron-mediated AKI. Its mechanisms of action remain elusive, but may include (1) binding of luminal iron,6–8 thus promoting iron excretion, or (2) increased sequestration of renal iron in ferritin in the epithelial cells, thereby reducing serum and luminal iron levels.1,3,9 Both mechanisms would reduce the formation of iron–induced oxidative stress and subsequent cell damage.
A better understanding of the renal handling of hepcidin is needed to fully understand these findings and guide the introduction of hepcidin in the diagnosis and/or treatment of AKI. For instance, the source of urinary hepcidin during CABG is not completely understood. Hepcidin is reported to be present in the plasma in a protein-bound form (specifically bound to α2-macroglobulin and nonspecifically bound to albumin) and a freely circulating form; the ratio between the bound and unbound forms of hepcidin in plasma is still under debate.10,11 The (unbound) hepcidin is freely filtered across the glomerular membrane, which was shown in two murine studies in which radiolabeled human hepcidin (hhep) was administered intraperitoneally and intravenously and measured in urine.12,13 Indeed, plasma hepcidin levels were increased compared with baseline after CABG, which may have contributed to the elevated urinary hepcidin levels observed in these patients. However, plasma hepcidin levels were not associated with development of AKI.2 Therefore, it has been postulated that local renal production may contribute to the high urinary hepcidin levels and ultimately, the renoprotective effect observed.1 Hepcidin has been shown to be synthesized in the mouse kidney, specifically in the distal nephron segment.14,15 However, it is unclear whether this locally synthesized hepcidin is excreted in the urine. Other than uncertainties concerning the origin of urinary hepcidin during CABG, gaps exist in our common knowledge on renal hepcidin handling. On the basis of the low fractional excretion of hepcidin assessed in healthy humans, it is proposed that hepcidin may be reabsorbed in the proximal tubule,16,17 possibly via megalin-mediated endocytosis.18 However, solid evidence for such a mechanism is lacking. Moreover, it is known that full-length hhep25 can be degraded into smaller isoforms, which can be detected in human urine under physiologic conditions.19–21 These smaller hepcidin forms do not elicit a hypoferremic response,13,22 but it is currently unknown whether, in vivo, they retain other biologic functions that have been identified for hepcidin-25 (e.g., host defense or metal binding).6–8,21
To further substantiate tubular reabsorption and degradation of hhep25, we administered hhep25 to wild-type and megalin-deficient mice. Here, we show, for the first time, reabsorption of hepcidin in the proximal tubules via megalin and breakdown of hepcidin into the smaller isoforms in the kidney. Subsequently, we show a protective effect of administered hhep25 against early hemoglobin–mediated kidney injury, which does not seem to comprise ferroportin degradation. Interestingly, we also show that locally synthesized hepcidin may be involved in renal protection.
Results
Circulating Hepcidin Is Reabsorbed in the Renal Proximal Tubule Via Megalin but Also Degraded in the Tubular Lumen
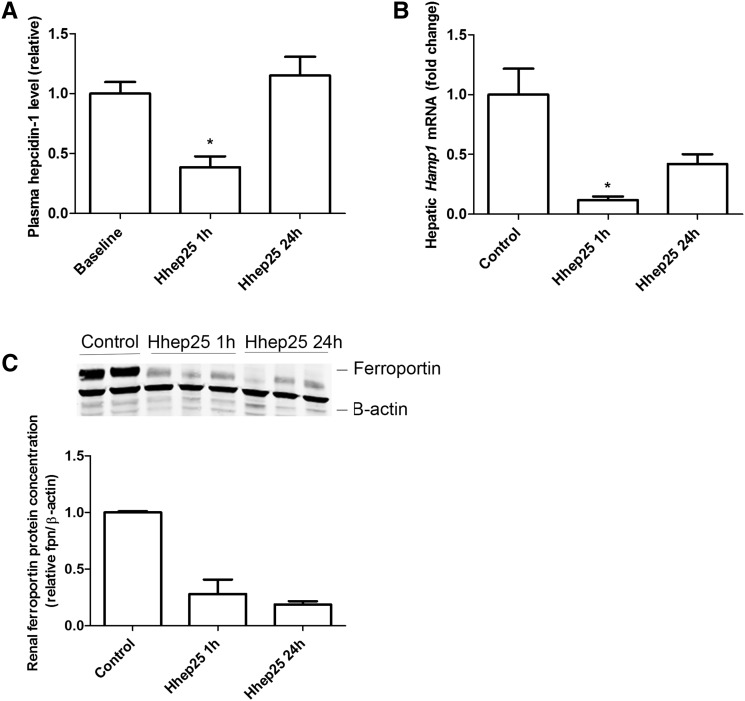
To determine renal handling of circulating hepcidin, wild–type C57Bl/6 mice were administered a single intraperitoneal injection of hhep25. We confirmed rapid plasma clearance of hhep25, because hhep25 was detectable in plasma 1 hour but not 24 hours after administration (data not shown).13 Plasma levels of endogenous hepcidin-1 and hepatic Hamp1 mRNA expression levels were significantly reduced compared with baseline or control 1 hour after hhep25 administration but returned to baseline or control values after 24 hours (Figure 1, A and B). The negative feedback of hhep25 on endogenous hepcidin-1 levels suggests that hhep25 is biologically active, which is in agreement with the findings by Rivera et al.13 Administered hhep25 was also biologically active in the kidney, which was shown by a reduction in renal ferroportin protein levels (Figure 1C), the known target of hepcidin.5
Figure 1.
Hhep25 administration in mice reduces endogenous plasma hepcidin-1, hepatic Hamp1 mRNA, and renal ferroportin protein expression. Relative plasma levels of (A) endogenous hepcidin-1, (B) hepatic Hamp1 mRNA expression, and (C) renal ferroportin protein expression in controls and 1 and 24 hours after hhep25 administration. Original Western blots are shown in C, upper panel, and their quantification is shown in C, lower panel. *P<0.05 compared with baseline or control by one-way ANOVA with Bonferroni multiple comparisons test.
To investigate the contribution of megalin to proximal tubular reabsorption of filtered hepcidin, we applied two approaches by studying renal handling of hhep25 in (1) megalin-deficient mice (megalinf/f,Cre)17 and (2) wild-type mice in which megalin was pharmacologically blocked using succinylated gelatine, a plasma expander (Gelofusin: B. Braun Medical).23 Reduced Megalin mRNA expression and increased urinary hepcidin-1 excretion in megalinf/f,Cre mice compared with their nondeficient transgenic littermates (megalinf/f,wt mice) was confirmed as previously reported (Supplemental Figure 1, A and B).18 In addition, increased hepcidin-1 excretion was also observed in wild-type mice after treatment with succinylated gelatin compared with baseline (Supplemental Figure 1C).
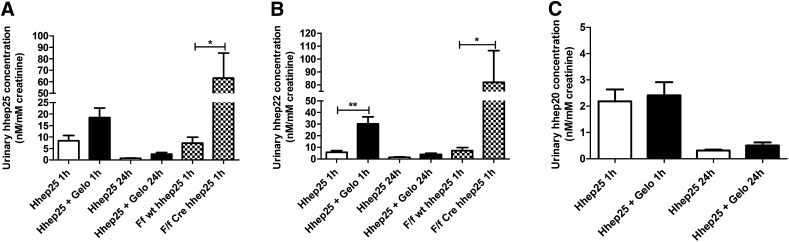
Urinary concentration of hhep25 was highest 1 hour after administration (Figure 2A), suggesting that the excretion rate of hhep25 in urine is relatively fast. Reduction in megalin–mediated protein reabsorption via genetic knockdown (megalinf/f,Cre mice) or succinylated gelatin treatment resulted in increased urinary concentrations of hhep25, which was significant in megalinf/f,Cre mice (P<0.05) compared with their controls (Figure 2A). Interestingly, in addition to hhep25, we also detected hhep22 and to a lesser extent, hhep20, cleavage products of hhep25, which followed the same urinary excretion pattern as hhep25 (Figure 2, B and C). These N–truncated hepcidin isoforms could not be detected in plasma at any time point, suggesting that the isoforms are formed in the renal tubular lumen. This corroborates observations in humans, where under physiologic conditions, hepcidin isoforms are present in the urine but are not present or are present at very low concentrations in plasma.20,24,25
Figure 2.
Increased urinary excretion of hhep25 and isoforms hhep22 and hhep20 in succinylated gelatin-treated and megalin deficient mice. Urinary concentration per millimolar creatinine of (A) hhep25, (B) hhep22, and (C) hhep20 after 1 and 24 hours in wild-type mice (white bars), mice pretreated with succinylated gelatin (Gelo) (black bars), and megalinf/f,wt mice and megalinf/f,Cre mice (checkered bars; n=4–5 per group). *P<0.05 using t test; **P<0.01 using t test.
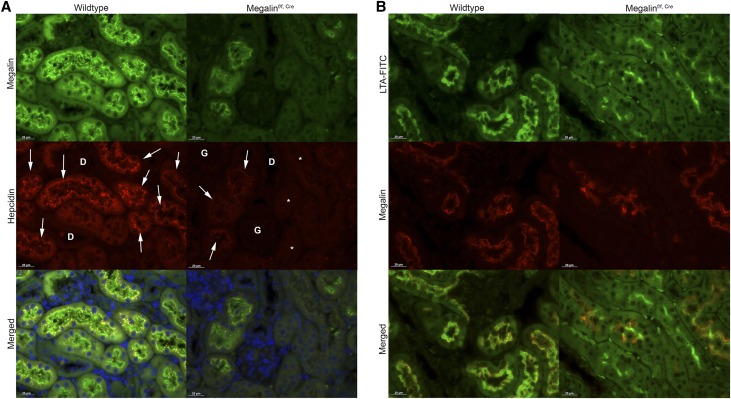
Double staining for hepcidin and megalin by immunohistochemistry revealed hepcidin and megalin colocalization in endocytic vesicles at the apical site of the proximal tubules (Figure 3A, arrows). Proximal tubular localization of megalin was confirmed by colocalization with the fluorescently labeled lectin Lotus Tetragonolobus Agglutinin (LTA)-FITC (Figure 3B), which is a marker for the brush border. Hepcidin staining was not observed in untreated control mice (Supplemental Figure 2), and in megalinf/f,Cre mice, hepcidin was only detectable in tubules that still contain megalin (compare arrows with asterisks in Figure 3A). Hence, our observations strongly suggest that hepcidin is reabsorbed in the proximal tubules via megalin-mediated endocytosis.
Figure 3.
Filtered hepcidin is reabsorbed in proximal tubules via megalin-mediated endocytosis. Double staining of (A) megalin and hepcidin (indicated by arrows) and (B) megalin and Lotus Tetragonolobus Agglutinin (LTA)-FITC in wild-type and megalinf/f,Cre mice treated with hhep25 for 1hour. D, distal tubule; G, glomeruli. Scalebar, 20 μm. *Megalin-deficient tubules without hepcidin.
Administered Hepcidin Reduces Early Hemoglobin–Mediated Kidney Injury
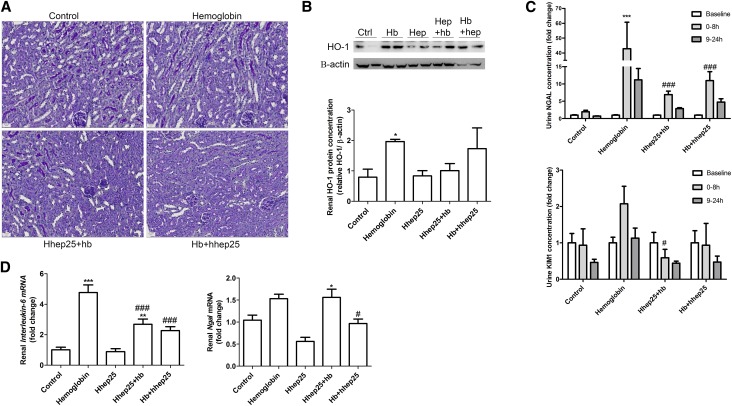
To investigate a protective effect of urinary hepcidin against hemoglobin–mediated kidney injury, we administered hhep25 to wild-type mice at the same time or 4 hours after a single intravenous injection of human hemoglobin. Urine was collected in two time slots after hemoglobin injection: 0–8 and 9–24 hours. The single injection of hemoglobin did not lead to overt kidney injury 24 hours after administration as determined by histology (Figure 4A). Furthermore, terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick end labeling did not reveal evidence for apoptosis (data not shown). However, we could observe significantly increased renal HO-1 protein expression (Figure 4B) (P<0.05), which is indicative of intracellular hemoglobin catabolism.26 In addition, hemoglobin injection resulted in significantly increased concentrations of the early urinary marker for kidney injury neutrophil gelatinase–associated lipocalin27 (NGAL; P<0.001 for 0–8 hours) (Figure 4C) and to a lesser extent, kidney injury molecule 128 (KIM-1) (Figure 4C) as well as significantly increased renal mRNA expression levels of interleukin-6 (IL-6) (Figure 4D) (P<0.001), another early indicator of kidney injury,29 and a minor increase in renal Ngal mRNA expression (Figure 4D). Administration of hhep25 simultaneously with or 4 hours after hemoglobin significantly reduced 0- to 8-hour urine NGAL (P<0.001) and KIM-1 concentrations (P<0.05), renal IL-6 mRNA expression levels (P<0.001), and renal Ngal mRNA expression levels (P<0.05) compared with hemoglobin injection alone. In addition, renal HO-1 protein expression was reduced, albeit not significantly, in mice treated with hemoglobin and hhep25 compared with hemoglobin treatment alone (Figure 4B). In conclusion, addition of hhep25 was able to abolish early kidney injury induced by hemoglobin.
Figure 4.
Addition of hhep25 reduces hemoglobin–mediated early kidney injury. Periodic acid–Schiff staining of (A) renal sections, (B) renal HO-1 protein expression, (C) urinary NGAL and KIM-1, and (D) renal mRNA expression of IL-6 and Ngal in control, hemoglobin-treated, hhep25-treated, or hhep25 administered at the same time (hhep25+hb) or 4 hours after hemoglobin (hb+hhep25) –treated mice. Representative blots are shown in B. For B and D, one-way ANOVA with Bonferroni multiple comparisons test was used, and for C, two-way ANOVA with Bonferroni multiple comparisons test was used. Hb, hemoglobin. *P<0.05 compared with control; **P<0.01 compared with control; ***P<0.001 compared with control; #P<0.05 compared with hemoglobin; ###P<0.001 compared with hemoglobin.
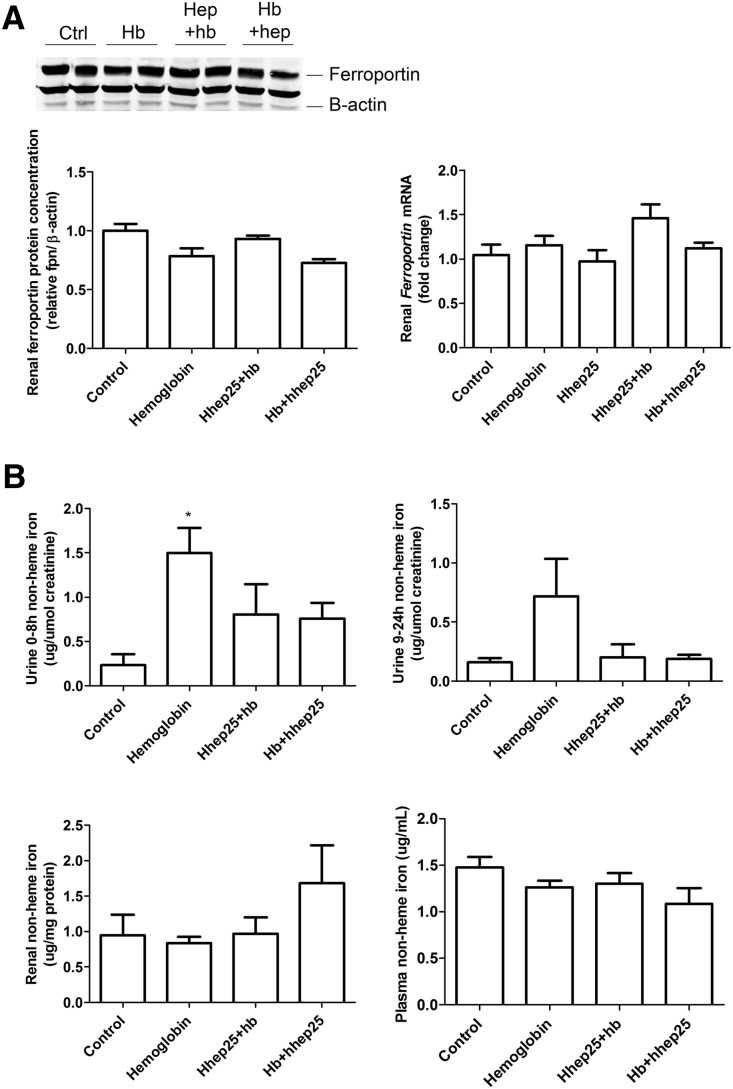
Although a single injection of hhep25 was able to reduce renal ferroportin protein concentrations at 24 hours after injection (Figure 1C), there was no reduction in renal ferroportin protein concentration or Ferroportin mRNA expression after 24 hours when hhep25 was administered during hemoglobin–mediated kidney injury (Figure 5A). This suggests that hhep25 may exert its protective effects via other mechanisms. Possible mechanisms for hepcidin-mediated protection against AKI include cellular sequestration of reactive iron and/or enhanced excretion of luminal iron through binding to hepcidin.1,3,6–9 Urinary non-heme iron levels (Figure 5B) were significantly increased by hemoglobin treatment at 0–8 hours (P<0.05) but reduced again by additional hhep25 administration (not significantly). A nonsignificant trend toward an increase in renal non-heme iron levels was observed in single intravenous injection of human hemoglobin administered 4 hours after hhep25–treated mice, whereas plasma non-heme iron levels remained unaffected by any treatment (Figure 5B). Together, these data, although not significant, point in the direction that addition of hhep25 leads to renal iron sequestration rather than enhanced iron excretion.
Figure 5.
Administration of hhep25 in hemoglobin-treated mice does not affect renal ferroportin expression and reduces urinary non-heme iron concentration. (A) Renal protein and mRNA expression of ferroportin and (B) non-heme iron levels in 0- to 8- and 9- to 24-hour urine, plasma, and kidney tissue. Representative blots are shown in A. Hb, hemoglobin. *P<0.05 compared with control by one-way ANOVA with Bonferroni multiple comparisons test.
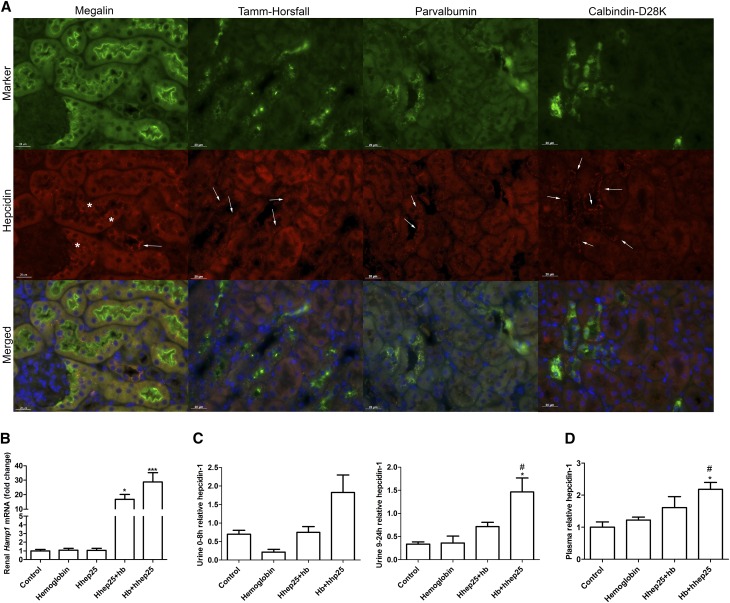
Strikingly, combined administration of hhep25 and hemoglobin resulted in hepcidin staining in nephron segments lacking megalin (Figure 6A, arrows), which was not observed in control, hemoglobin-treated, and hhep25-treated mice (Figure 3, Supplemental Figure 2). Costaining with markers for distal tubule segments showed that most of the hepcidin colocalized with calbindin-D28K (distal convoluted tubule, connecting tubule, and the first part of the collecting duct) and parvalbumin (distal convoluted tubule) and to a lesser extent, Tamm–Horsfall protein (thick ascending loop of Henle). Moreover, renal Hamp1 mRNA expression levels were significantly increased in hemoglobin groups treated with hhep25 (P<0.05 and P<0.001) compared with control (Figure 6B), suggesting hepcidin-1 synthesis. Urinary hepcidin-1 concentrations were also increased in mice given hhep25 4 hours after hemoglobin injection compared with control, which was significant for the 9- to 24-hour urine (P<0.05) (Figure 6C). However, it cannot be concluded that the increased urinary hepcidin excretion originated from the increased synthesis in the kidney, because plasma hepcidin-1 levels were also increased in these mice (Figure 6D). The increased level of systemically circulating hepcidin-1, however, may explain the hepcidin staining observed in the proximal tubules in the hemoglobin and hhep25-treated mice. Because administered hhep25 was shown before to be undetectable in renal sections by immunohistochemistry at 24 hours after injection (data not shown), the proximal hepcidin staining (Figure 6A) probably reflects reabsorption of increased hepcidin-1 levels in the ultrafiltrate. Taken together, it seems that both proximal reabsorption of systemic hepcidin and distal hepcidin synthesis may be involved in protection against hemoglobin-mediated AKI.
Figure 6.
Increased renal hepcidin synthesis, urinary excretion and plasma concentration after hhep25 administration in hemoglobin-treated mice. (A) Representative images of distal tubule hepcidin staining (indicated by arrows) of a mouse treated simultaneously with hhep25 and hemoglobin (hhep25+hb) in colocalization with megalin, Tamm–Horsfall protein, parvalbumin, and calbindin-D28K. Scalebar, 20 μm. *Proximal tubule hepcidin staining. (B) Renal Hamp1 mRNA expression, (C) urinary hepcidin-1 excretion, and (D) plasma hepcidin-1 levels in control, hemoglobin-treated, hhep25+hb-treated, and hhep25 injection 4 hours after hemoglobin–treated (hb+hhep25) mice.*P<0.05 compared with control by one-way ANOVA with Bonferroni multiple comparisons test; ***P<0.001 compared with control by one-way ANOVA with Bonferroni multiple comparisons test. #P<0.05 compared with hemoglobin by one-way ANOVA with Bonferroni multiple comparisons test.
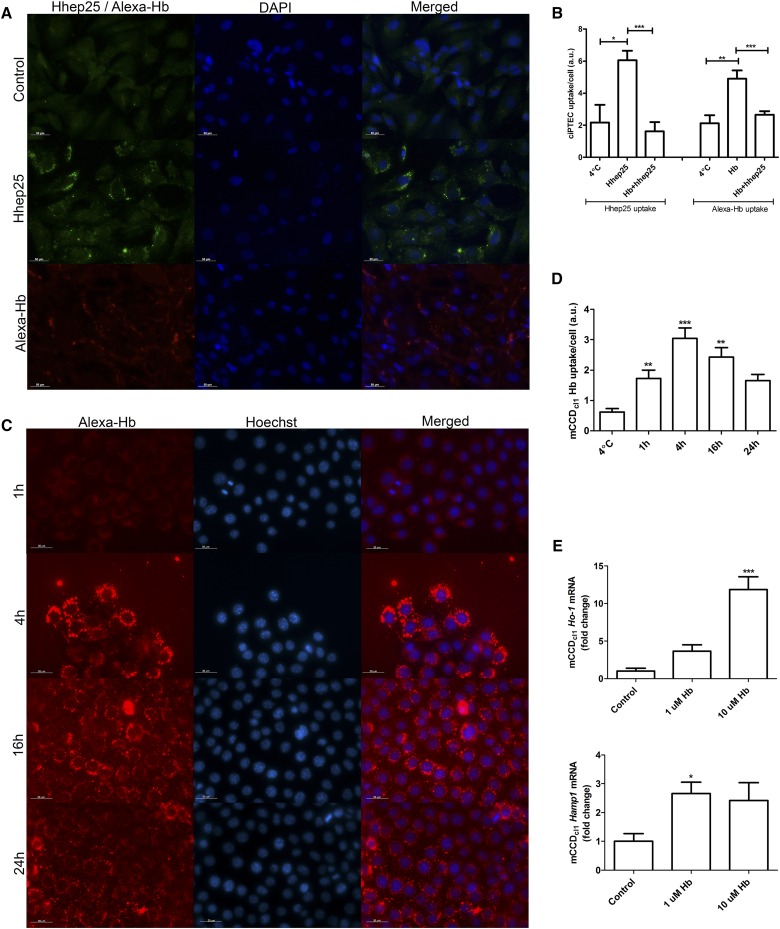
We hypothesize that the observed upregulation of distal nephron hepcidin-1 in the hemoglobin groups treated with hhep25 is caused by a competition of both hemoglobin and hhep25 for uptake by the proximal tubule via megalin, which causes more hemoglobin to pass through the nephron to the distal tubules, where it could induce Hamp1 mRNA expression. To test this hypothesis, we exploited two in vitro models. Using human conditionally immortalized proximal tubular epithelial cells (ciPTECs)30 that express megalin,31 we showed intracellular uptake of both hhep25 (20 nM) and Alexa546–labeled hemoglobin (Alexa-Hb; 5 nM) during 1 hour of incubation (Figure 7A). Incubation of ciPTECs with both compounds simultaneously for 1 hour resulted in significantly less uptake of both (P<0.001) compared with incubation with either one alone (Figure 7B), which indicates competition for uptake between hhep25 and hemoglobin. Alexa-Hb (1 nM) was also taken up by mouse cortical collecting duct cells (mCCDcl1s) (Figure 7C) by active transport as indicated by the significant increase in uptake compared with Alexa-Hb uptake at 4°C (Figure 7D). Moreover, incubation of mCCDcl1s with hemoglobin for 24 hours significantly increased mCCDcl1 Ho-1 mRNA expression (P<0.001) (Figure 7E), indicating intracellular metabolism of hemoglobin and increased Hamp1 mRNA expression (P<0.05).
Figure 7.
Competition between hhep25 and hemoglobin uptake in ciPTECs and hemoglobin-mediated induction of Ho-1 and Hamp1 in mCCDcl1s. (A) Uptake of hepcidin and Alexa-Hb in ciPTECs. Control indicates hepcidin staining in untreated ciPTECs. Scalebar, 50 μm. (B) Quantification of hepcidin and/or Alexa-Hb uptake in ciPTECs (n=3). (C) Intracellular uptake of Alexa-Hb in mCCDcl1s after 1, 4, 16, and 24 hours of incubation quantified in (D). mCCDcl1 mRNA expression of Ho-1 and Hamp1 (E) in controls (n=5) and after 24 hours of hemoglobin incubation (n=3). Hb, hemoglobin; hb+hhep25, simultaneous incubation with hemoglobin and hhep25. Scalebar, 30 μm. *P<0.05 compared with control or as indicated by one-way ANOVA with Bonferroni multiple comparisons test; **P<0.01 compared with control or as indicated by one-way ANOVA with Bonferroni multiple comparisons test; ***P<0.001 compared with control or as indicated by one-way ANOVA with Bonferroni multiple comparisons test.
Discussion
Urinary hepcidin is suggested to have protective effects against AKI. This study aimed to get more insight in renal hepcidin handling and assess its protective potential against hemoglobin-induced AKI. Collectively, our results show, for the first time, that circulating hepcidin is filtered by the glomerulus and subsequently, reabsorbed in the proximal tubule cells via megalin but also, degraded in the tubular lumen. Furthermore, a protective effect of hhep25 against hemoglobin–induced early kidney injury was shown, which may be mediated by mechanisms other than degradation of ferroportin. Moreover, our data suggest that both circulating filtered hepcidin that is reabsorbed in the proximal tubules and hepcidin synthesized in the distal parts of the nephron play a role in the defense against iron–mediated kidney injury.
Administered hhep25 was rapidly excreted in urine and partly reabsorbed via megalin in the proximal tubules. Other than hhep25, we also detected its smaller isoforms hhep22 and hhep20 in urine. Because excretion of hhep22 and to a lesser extent, hhep20 was increased in mice treated with succinylated gelatin and megalinf/f,Cre mice, it can be argued that hepcidin isoforms are also reabsorbed by megalin. Alternatively, the increase of the isoforms may be attributed to breakdown of the increased urinary hepcidin-25. Our previous observations support the notion that degradation of hepcidin into smaller isoforms is an active process, because ex vivo incubation of spiked hhep25 in urine did not yield any isoforms,32 whereas others have shown that liver and pancreas extracts were able to induce the formation of hhep20 and hhep22 from hhep25 through enzymatic reactions.33 Because the brush border membrane of the tubular epithelial cells contains many enzymes, including dipeptidylpeptidase IV,34 which has been shown to degrade hhep22 to hhep20,33 breakdown of hhep25 into isoforms may be facilitated within the tubular lumen. Moreover, because these isoforms retain the iron-binding properties in vivo, they could participate in protection of the kidney.6–8
Twenty-four hours after administration, a single dose of hemoglobin resulted in early kidney injury as indicated by the rise in urinary markers NGAL and KIM-1 and renal mRNA expression levels of IL-6 and Ngal. Overt histologic damage was absent in our model, which is in line with the majority of AKI observed in humans.35 Hhep25 administered together with or 4 hours after hemoglobin injection ameliorated this early kidney injury. Protective effects of hepcidin administration in a mouse model of IRI have recently been described by Scindia et al.36 In their study, renal injury was more pronounced compared with that in our study, and higher concentrations of hepcidin were administered (50 versus 10 μg in our study).36 Scindia et al.36 observed a reduction in IRI-induced apoptosis, oxidative stress, and inflammatory cell infiltration when hepcidin was administered 24 hours before IRI, possibly mediated by hepatic iron sequestration, a reduction in renal ferroportin protein levels, and concomitant increased renal H-ferritin protein concentration. In contrast, although we could show decreased renal ferroportin expression 24 hours after injection of hhep25 alone, coexposure or hhep25 treatment shortly after hemoglobin had no effect on renal ferroportin expression. These differences may be explained by the disparity in severity of kidney injury, the timing of hepcidin administration, and the experimental model used. Future studies should explore whether hepcidin can act via distinct mechanisms to protect the kidney from injury depending on the timing of treatment and type of iron–mediated kidney injury.
A possible mechanism underlying our observations is that hhep25 may bind reactive iron during hemoglobin-induced AKI, which can be released from hemoglobin in the kidney after internalization in proximal tubule cells by catabolism,37,38 the urine because of acidic conditions,39,40 or the plasma by peroxidases.41,42 During hemoglobin-induced AKI, circulating and filtered hepcidin may bind iron, which is, subsequently, internalized by the proximal tubules and as such, promotes renal cell sequestration. This is supported by the decrease of hemoglobin-induced elevation of urinary non-heme iron concentration. Moreover, ferroportin protein expression is upregulated by intracellular iron concentrations through a post-translational mechanism that comprises binding of the iron-regulatory protein to the iron-responsive element present on ferroportin mRNA.43,44 Therefore, increased intracellular iron concentrations as a result of hemoglobin catabolism may counteract the hhep25-mediated degradation of ferroportin. We did not observe any effect on renal ferroportin expression or non-heme iron levels by hemoglobin injection alone at 24 hours and may, therefore, have missed a transient upregulation of renal ferroportin early after hemoglobin injection. Overall, our data show that hhep25 blunts the hemoglobin-induced effects, including induction of kidney injury markers, renal HO-1 protein expression, and urinary non-heme iron concentrations. More studies are needed to elucidate the molecular mechanisms involved in hepcidin–mediated renal protection against hemoglobin and at multiple time points after hemoglobin administration.
Proximal tubules virtually do not synthesize hepcidin15 but reabsorb filtered circulating hepcidin, whereas the distal tubules can synthesize hepcidin14,15 but did not show uptake of the administered hhep25 in our studies. Interestingly, we observed that hhep25 injection in hemoglobin-treated mice led to a dramatic increase in renal Hamp1 mRNA expression and hepcidin immunostaining in the distal tubules. It has been postulated by Ho et al.1 that patients who cannot effectively upregulate their hepcidin response during IRI are more likely to develop clinically relevant AKI. In agreement with this clinical observation, it is possible that high urinary hepcidin levels originating from the circulation prevent injury in the first hours after the damaging insult, but subsequently, local hepcidin synthesis is required to maintain protection along the entire nephron. Indeed, we showed uptake of hemoglobin as well as hhep25 by proximal tubular epithelial cells and competition in uptake between both compounds. Moreover, we showed that the distal tubular epithelial cells of the cortical collecting duct take up and catabolize hemoglobin, which induces hepcidin synthesis. Our in vitro studies provide preliminary evidence for a potential coordinated interaction between proximal and distal tubules mediated by hepcidin during hemoglobin–mediated kidney injury, but future studies are warranted to further unravel such dynamics during AKI.
Overall, our observations serve as a basis for improved insights of the dynamics of hepcidin during CABG and AKI. The next step is to investigate the protective molecular mechanism of hepcidin in iron–mediated kidney injury and determine the role of locally synthesized hepcidin in kidney injury by unraveling the driving forces behind its transcription. Together, these insights will provide novel leads for protection against iron–mediated kidney injury.
Concise Methods
Animal Experiments
All experiments were approved by the local Animal Welfare Committee of the Radboudumc (DEC 2012–293) in accordance with the guidelines of the Principles of Laboratory Animal Care (National Institutes of Health). Male C57Bl/6N mice (8–11 weeks of age; Charles River Laboratories, Wilmington, MA) were housed under controlled conditions and randomly assigned to a treatment group. Unless stated otherwise, all experiments were carried out with five mice per group. Heterozygous megalin–deficient mice (ApoECre/gp330flox/w; referred to as megalinf/wt,Cre) were obtained from the Max Delbrück Center for Molecular Medicine (Berlin, Germany) and bred with C57Bl/6N mice to obtain homozygous megalin–deficient mice (megalinf/f,Cre).45
Synthetic hhep25 (Peptide International) was dissolved in saline, and 10 μg were administered to each animal via intraperitoneal injection. To investigate megalin–mediated renal hhep25 reabsorption, succinylated gelatin (Gelofusin; B.Braun Medical) was used to block the megalin receptor by injection of 4 mg in the tail vein 5 minutes before hhep25 injection in two additional groups.23 Succinylated gelatin alone (4×10 mg during 24 hours) was injected via intraperitioneal injection. Human hemoglobin (Sigma-Aldrich, St. Louis, MO) was dissolved in saline (20 mg/ml) and injected via the tail vein (250 μl per mouse).
Twenty-four hour urine samples were collected at baseline or immediately after treatment by means of metabolic cages (Techniplast GmbH) with pulverized standard chow and water ad libitum. Urine samples collected 1 hour after treatment were directly taken from the bladder. Protease inhibitors (Complete Mini; Roche Diagnostics, Indianapolis, IN) were added to urine samples, which were centrifuged for 10 minutes at 3000×g to remove debris. Mice were anesthetized with isoflurane and O2, and blood was taken directly from the heart. Kidney and liver tissues were collected in liquid nitrogen and stored at −80°C for protein and mRNA isolation and in 4% formalin O/N before imbedding in paraffin for histochemistry.
Genotyping Megalin-Deficient Mice
Mouse ear biopsies were taken after weaning, and HotShot DNA extraction was performed as described elsewhere.46 Primers for ApoECre included forward 5′- CCCAAGAAGAGGAAGGTG-3′ and reverse 5′-GCTGGCCCAAATGTTGCTG-3′. The ApoECre PCR protocol was as follows: 4 minutes at 95°C; 32 cycles of 30 seconds at 95°C, 30 seconds at 56°C, and 30 seconds at 72°C; and 10 minutes at 72°C. The ApoECre PCR resulted in a product of approximately 300 bp, which was visualized on a 1.5% agarose gel. For gp330flox/flox, we used three primers: F4a (5′-TAACTGGCAGCACCGTTGAGTG-3′), B4 (5′- GCAGAGGATTGTGTGAGAACCAAAC-3′), and TKP2 (5′- TGAAAACCACACTGCTCGATCCGGAAC-3′). The gp330flox/flox PCR protocol was as follows: 3 minutes at 95°C; 36 cycles of 30 seconds at 95°C, 40 seconds at 60°C, and 25 seconds at 72°C; and 10 minutes at 72°C. On a 1.5% agarose gel, two products can be visualized: a 480-bp band for the wild-type allele and/or a 550-bp band for the floxed allele.
Creatinine Determination
Urinary creatinine concentration was determined using the assay kit on the basis of the Jaffé method from Labor & Technik (LT-SYS 0251).
Hepcidin Analyses
The concentrations of hhep25 and also, endogenous mouse hepcidin-1 were determined in urine, plasma, and kidney homogenate using mass spectrometry.17,47 Briefly, 50 μl acetonitril (ACN) was added to a 25-μl sample, to which 5 μl internal standard (0.1 μM synthetic hhep-24; custom made; Peptide International) was added. The solution was mixed and centrifuged at 27,500×g for 5 minutes; 50 μl supernatant, 25 μl weak cation exchange beads (Macro-Prep Support Beads; Bio-Rad, Hercules, CA), and 150 μl binding buffer were combined and mixed thoroughly, and hepcidin was allowed to bind to the beads by incubation for 15 minutes on the rollerbank at RT. Beads were washed three times with 150 μl wash buffer before hepcidin was eluted from the beads using 50 μl elution buffer (50% ACN and 2% TFA) for 15 minutes on the roller bank. Of the prepared sample, 1.5 μl were spotted onto an MSP 96 Polished Steel Target Plate (Bruker Daltonics) followed by 1.5 μl energy-absorbing matrix (5 mg/mlα-cyano-4-hydroxy cinnamic acid; Bruker Daltonics). Plasma samples were pretreated with ACN but without beads. The supernatant obtained after centrifugation was spotted directly on the target plate. The sample and matrix were dried in N2 atmosphere and measured using matrix–assisted laser desorption/ionization time-of-flight mass spectrometry (Bruker Daltonics). Hepcidin was measured using the following settings: positive linear ion mode; 500 laser shot; initial laser power, 55%; offset, 30%; range, 30%; pulsed ion extraction, 250 ns; and mass range, 2000–10,000 m/z.
The concentration of hhep25 was calculated by comparing its mass peak height with that of the internal standard, which had a final concentration of 10 nM. Because the internal standard is of human origin and may behave slightly different in the mass spectrometry assay than mouse hepcidin, the concentrations of mouse hepcidin-1 are given as relative concentrations corrected for creatinine concentrations (for urine) and set to one for the baseline or control group.
RNA Isolation and Quantitative PCR
Frozen kidney and liver tissues were homogenized using a Mikro Dismembrator U (Sartorius Stedim) before RNA isolation by means of TRIzol (Life Technologies, Carlsbad, CA) according to manufacturer’s instructions.
Quantitative PCR was performed on a CFX96 (Bio-Rad) using 4 μl cDNA (10 ng/μl), 10 μl SYBR Green Mastermix (2×; Applied Biosystems, Foster City, CA), and 6 μl Primermix (containing 5 μl 100 μM forward primer, 5 μl 100 μM reversed primer, and 490 μl sterile water). Primers used are β-actin (housekeeping gene) forward 5′-GCTATGCTCTCCCTCACGCCA-3′; β-actin reversed 5′-CTCTTTGATGTCACGCACGAT-3′; Hamp1 forward 5′-TTGCGATACCAATGCAGAAG-3′; Hamp1 reversed 5′-GGATGTGGCTCTAGGCTATGTT-3′; Megalin forward 5′-CCTCCTTCACCTGCGACAAT-3′; Megalin reversed 5′-TCCCCGAAGCTTGACAGTTC-3′; Ngal forward 5′-GCCTCAAGGACGACAACATCA-3′; Ngal reversed 5′-TTCTCTGTCCCCACCGACCAATGC-3′; IL-6 forward 5′-GAGGATACCACTCCCAACAGACC-3′; IL-6 reversed 5′-AAGTGCATCATCGTTGTTCATACA-3′; Ferroportin forward 5′-CTACCATTAGAAGGATTGGATCC-3; and Ferroportin reversed 5′-CAAATGTCATAATCTGGC-3′. The PCR protocol was as follows: 7 minutes at 95°C and 40 cycles of 15 seconds at 95°C and 1 minute at 60°C, with a measurement at the end of each cycle. Fold change values compared with control or baseline were calculated with the 2−ddcT formula.
Protein Isolation and Western Blot
Frozen kidney tissue were homogenized using a Mikro Dismembrator U (Sartorius Stedim) in Tris-Sucrose buffer supplemented with protease inhibitors (Complete Mini), after which the samples were centrifuged at 10,000×g for 15 minutes at 4°C. The supernatant was collected and stored at −80°C until further use. Protein concentrations were determined using the Bio-Rad Protein Assay (Bio-Rad) according to the manufacturer’s instructions.
For Western blotting, 50 μg kidney protein was loaded on a 10% or 12.5% gel (Mini Protean TGX Precast Gel; Bio-Rad) and blotted on nitrocellulose membrane. Membranes were incubated in 1:1 PBS-Tween (0.1%): Odyssey blocking buffer O/N at 4°C with primary antibodies: rabbit anti–human ferroportin antibody (PAB15509; Abnova) diluted 1:2000, mouse anti–human β–actin antibody (A5441; Sigma-Aldrich) diluted 1:50,000, and rabbit anti–human HO-1 antibody (SPA895; Stressgen) diluted 1:1000. After washing with PBS-Tween (0.1%), the membranes were incubated with the secondary antibody in 1:1 PBS-Tween (0.1%): Odyssey blocking buffer for 1 hour at RT. For ferroportin, we used an Alexa Fluor680–labeled goat anti–rabbit antibody (A21109; Invitrogen, Carlsbad, CA) diluted 1:20,000; for β-actin, we used an IRDye 800–labeled goat anti–mouse antibody (610–132–121; Rockland Immunochemicals Inc., Gilbertsville, PA) diluted 1:20,000 or a peroxidase affinipure goat anti–mouse IgG (H&L; 115–035–003; The Jackson Laboratory, Bar Harbor, ME) diluted 1:2500. For HO-1, we used an HRP goat anti–rabbit IgG (7074; Cell Signaling Technology, Danvers, MA) diluted 1:10,000. Membranes were analyzed using an Odyssey Scanner (LI-COR Biosciences, Lincoln, NE) or by ECL (LAS 3000; Fujifilm, Tokyo, Japan), and quantification was performed using the Odyssey software or ImageJ software.
Non-heme Iron Assay
Total non-heme iron levels were measured in plasma, urine, and kidney tissue using the bathophenanthroline assay (adapted from the work by Torrance and Bothwell48). In short, iron is liberated by incubating the sample with an acid solution containing 0.1 g/ml trichloric acid and 25% (vol/vol) hydrochloric acid O/N at 65°C. Next, samples are incubated with bathopenantroline, and the absorbance is read at 535 nm. The concentration of non-heme iron is calculated from a standard curve prepared from ferrous iron sulfate.
Hemoglobin Assay
Hemoglobin concentrations were measured in plasma, urine, and kidney tissue using the hemoglobin assay kit (MAK115) from Sigma-Aldrich according to the manufacturer’s instructions.
Immunohistochemistry
Tissue sections were embedded in paraffin, and 4-μm sections were mounted on APES–covered glass slides. Periodic acid––Schiff staining was used for kidney sections for gross histology. Images were taken using the VisionTekDigital Microscope (Sakura). For double staining of hepcidin and the various markers, kidney sections were deparaffinized, and an antigen retrieval step was performed using citrate buffer (pH6) followed by washing with 0.1% PBS-Tween. Sections were then incubated with 1% BSA in PBS-Tween for 1 hour followed by the first primary antibody, a rabbit polyclonal antihepcidin antibody (ab30760; Abcam, Inc., Cambridge, MA), in a 1:200, 1:100, or 1:75 dilution O/N at 4°C or 1 hour at RT. The first secondary antibody, a goat anti–rabbit Alexa Fluor 568 (Invitrogen), was added in 1:200 dilution for 30 minutes. Lotus Tetragonolobus Agglutinin FITC (Vector Laboratories, Burlingame, CA) was added at this step at a 1:100 dilution. Sections were incubated O/N at 4°C or for 1 hour at RT with the second primary antibody, namely goat polyclonal antimegalin antibody (sc16478; Santa Cruz Biotechnology, Santa Cruz, CA) diluted 1:500, sheep anti–human Tamm–Horsfall (BT85–9500–54; Bio Trend) diluted 1:2000, goat anti–human parvalbumin (7449; Santa Cruz Biotechnology) diluted 1:2500, or mouse anti–bovine calbindin-D28K (C9848; Sigma-Aldrich) diluted 1:8000. The following secondary antibodies were used diluted 1:200: donkey anti–goat Alexa Fluor 488 antibody (A-11055; Invitrogen), biotin-SP–conjugated goat anti–mouse (115–066–003; The Jackson Laboratory), biotin-SP–conjugated donkey anti–goat (705–065–147; The Jackson Laboratory), and biotin-SP–conjugated donkey anti–sheep (713–066–147; The Jackson Laboratory). The signal of biotin–conjugated secondary antibodies was amplified with a fluorescent label using the Renaissance TSA Kit (PerkinElmer, Waltham, MA) according to the manufacturer’s instructions. Finally, for nuclear staining, DAPI (1:1000) was added to the sections for 5 minutes. Sections were fixed with fluorescent mounting medium (DAKO), dried, and stored at 4°C in the dark. Fluorescent staining was visualized using an Apotome.2 FL Microscope (Carl Zeiss).
ELISA
The concentrations of NGAL and KIM-1 were determined in mouse urine samples using the DuoSet ELISA Development Kits from R&D Systems (Minneapolis, MN; DY1857 for NGAL and DY1817 for KIM-1) according to the manufacturer’s protocol.
Cell Culture Experiments
The ciPTEC line was generated and cultured as described previously.30,49 For each experiment, cells were cultured at 33°C to 40% confluency followed by maturation for 7 days at 37°C in a 5% (vol/vol) CO2 atmosphere. Experiments were performed on cells with passage number between 33 and 50.
The mCCDcl1 line was established by Rossier and coworkers50 and cultured as described. Cells were used for experiments between passage 26 and 44.
To study uptake of hemoglobin in ciPTECs and mCCDcl1s, human hemoglobin (Sigma-Aldrich) was labeled with an Alexa Fluor 546 Protein Labeling Kit (Invitrogen) according to the manufacturer’s instructions. Cells were grown on glass coverslides and incubated with hhep25 (Peptide International), Alexa-Hb, or vehicle in serum free medium for the indicated time points at 37°C or 4°C. Cells were fixed with 4% paraformaldehyde and permeabilized with 0.5% TritonX-100 (ciPTEC) or 1% SDS (mCCDcl1). For detection of hhep25, a rabbit polyclonal antihepcidin antibody (ab30760; Abcam, Inc.) diluted 1:100 was applied for 2 hours at RT followed by 1:500 goat anti–rabbit Alexa Fluor 488 (A11008; Invitrogen) for 1 hour at RT. Cells were counterstained with 300 nM DAPI (Invitrogen; for ciPTEC) or 0.8 μg/ml Hoechst 33342 (mCCDcl1) for 5 minutes at RT. Uptake of hhep25 and/or Alexa-Hb was quantified using ImageJ (ciPTEC) or Metamorph (mCCDcl1) software as described previously.51
To determine whether hemoglobin exposure induced HO-1 and hepcidin mRNA synthesis, mCCDcl1s were incubated with 1 and 10 μM hemoglobin for 24 hours in serum free medium at 37°C. Quantitative PCR was performed as described above using the following primers: β-actin forward 5′-GCTATGCTCTCCCTCACGCCA-3′; β-actin reversed 5′-CTCTTTGATGTCACGCACGAT-3′; Hamp1 forward 5′- TTGCGATACCAATGCAGAAG-3′; Hamp1 reversed 5′- GGATGTGGCTCTAGGCTATGTT-3′; Ho-1 forward 5′- CCTCACTGGCAGGAAATCAT-3′; and Ho-1 reversed 5′- CCAGAGTGTTCATTCGAGCA-3′.
Statistical Analyses
Data were statistically analyzed using GraphPad Prism 5.03 software (GraphPad Software, La Jolla, CA) and presented as means±SEMs. Results were analyzed for statistically significant differences using the t test or one-way ANOVA with post hoc analysis wherever appropriate. A P value of <0.05 was considered statistically significant.
Disclosures
R.P.L.v.S., C.M.M.L., and D.W.S. are managing director, technician, and medical director, respectively, of the Hepcidinanalysis.cominitiative, which aims to serve the scientific and medical communities with high–quality hepcidin measurements (www.hepcidinanalysis.com).
Supplementary Material
Acknowledgments
We thank Sanne van Raaij for performing the Western blot analysis, Caro Bos for the immunostainings, and Stephanie Probst for assistance with the mCCDcl1 experiments.
We thank the Dutch Kidney Foundation for financial support as part of Innovation Grant IP12.81 (to J.F.M.W., R.M., and D.W.S.). This work was also supported by Deutsche Forschungsgemeinschaft grants TH345 (to F.T.) and Zentrum für Biomedizinische Ausbildung und Forschung (to F.T.).
Part of this work was presented during the European Renal Association - European Dialysis and Transplant Association Meeting May 31–June 3, 2014 in Amsterdam, The Netherlands; the European Iron Club Meeting September 11–14, 2014 in Verona, Italy; the Society of Toxicology Annual Meeting March 22–26, 2015 in San Diego, California; the biannual meeting of the International BioIron Society September 6–10, 2015 in Hangzhou, China; and the Kidney Week of the American Society of Nephrology November 3–8, 2015 in San Diego, California.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015040461/-/DCSupplemental.
References
- 1.Ho J, Reslerova M, Gali B, Gao A, Bestland J, Rush DN, Nickerson PW, Rigatto C: Urinary hepcidin-25 and risk of acute kidney injury following cardiopulmonary bypass. Clin J Am Soc Nephrol 6: 2340–2346, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prowle JR, Ostland V, Calzavacca P, Licari E, Ligabo EV, Echeverri JE, Bagshaw SM, Haase-Fielitz A, Haase M, Westerman M, Bellomo R: Greater increase in urinary hepcidin predicts protection from acute kidney injury after cardiopulmonary bypass. Nephrol Dial Transplant 27: 595–602, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Haase-Fielitz A, Mertens PR, Plass M, Kuppe H, Hetzer R, Westerman M, Ostland V, Prowle JR, Bellomo R, Haase M: Urine hepcidin has additive value in ruling out cardiopulmonary bypass-associated acute kidney injury: An observational cohort study. Crit Care 15: R186, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haase M, Bellomo R, Haase-Fielitz A: Novel biomarkers, oxidative stress, and the role of labile iron toxicity in cardiopulmonary bypass-associated acute kidney injury. J Am Coll Cardiol 55: 2024–2033, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J: Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 306: 2090–2093, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Farnaud S, Patel A, Evans RW: Modelling of a metal-containing hepcidin. Biometals 19: 527–533, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Gerardi G, Biasiotto G, Santambrogio P, Zanella I, Ingrassia R, Corrado M, Cavadini P, Derosas M, Levi S, Arosio P: Recombinant human hepcidin expressed in Escherichia coli isolates as an iron containing protein. Blood Cells Mol Dis 35: 177–181, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Farnaud S, Rapisarda C, Bui T, Drake A, Cammack R, Evans RW: Identification of an iron-hepcidin complex. Biochem J 413: 553–557, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Ho J, Lucy M, Krokhin O, Hayglass K, Pascoe E, Darroch G, Rush D, Nickerson P, Rigatto C, Reslerova M: Mass spectrometry-based proteomic analysis of urine in acute kidney injury following cardiopulmonary bypass: A nested case-control study. Am J Kidney Dis 53: 584–595, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Itkonen O, Stenman UH, Parkkinen J, Soliymani R, Baumann M, Hämäläinen E: Binding of hepcidin to plasma proteins. Clin Chem 58: 1158–1160, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Peslova G, Petrak J, Kuzelova K, Hrdy I, Halada P, Kuchel PW, Soe-Lin S, Ponka P, Sutak R, Becker E, Huang ML, Suryo Rahmanto Y, Richardson DR, Vyoral D: Hepcidin, the hormone of iron metabolism, is bound specifically to alpha-2-macroglobulin in blood. Blood 113: 6225–6236, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Huang ML, Austin CJ, Sari MA, Rahmanto YS, Ponka P, Vyora l D, Richardson DR: Hepcidin bound to α2-macroglobulin reduces ferroportin-1 expression and enhances its activity at reducing serum iron levels. J Biol Chem 288: 25450–25465, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rivera S, Nemeth E, Gabayan V, Lopez MA, Farshidi D, Ganz T: Synthetic hepcidin causes rapid dose-dependent hypoferremia and is concentrated in ferroportin-containing organs. Blood 106: 2196–2199, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kulaksiz H, Theilig F, Bachmann S, Gehrke SG, Rost D, Janetzko A, Cetin Y, Stremmel W: The iron-regulatory peptide hormone hepcidin: Expression and cellular localization in the mammalian kidney. J Endocrinol 184: 361–370, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Houamel D, Ducro t N, Lefebvre T, Daher R, Moulouel B, Sari MA, Letteron P, Lyoumi S, Millot S, Tourret J, Bouvet O, Vaulont S, Vandewalle A, Denamur E, Puy H, Beaumont C, Gouya L, Karim Z: Hepcidin as a major component of renal antibacterial defenses against uropathogenic Escherichia coli [published online ahead of print August 20, 2015]. J Am Soc Nephrol doi:ASN.2014101035 [DOI] [PMC free article] [PubMed]
- 16.Ganz T, Olbina G, Girelli D, Nemeth E, Westerman M: Immunoassay for human serum hepcidin. Blood 112: 4292–4297, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Swinkels DW, Girelli D, Laarakkers C, Kroot J, Campostrini N, Kemna EH, Tjalsma H: Advances in quantitative hepcidin measurements by time-of-flight mass spectrometry. PLoS One 3: e2706, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peters HP, Laarakkers CM, Pickkers P, Masereeuw R, Boerman OC, Eek A, Cornelissen EA, Swinkels DW, Wetzels JF: Tubular reabsorption and local production of urine hepcidin-25. BMC Nephrol 14: 70, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kemna EH, Tjalsma H, Podust VN, Swinkels DW: Mass spectrometry-based hepcidin measurements in serum and urine: Analytical aspects and clinical implications. Clin Chem 53: 620–628, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Kroot JJ, Tjalsma H, Fleming RE, Swinkels DW: Hepcidin in human iron disorders: Diagnostic implications. Clin Chem 57: 1650–1669, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Park CH, Valore EV, Waring AJ, Ganz T: Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J Biol Chem 276: 7806–7810, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Nemeth E, Preza GC, Jung CL, Kaplan J, Waring AJ, Ganz T: The N-terminus of hepcidin is essential for its interaction with ferroportin: Structure-function study. Blood 107: 328–333, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Eerd JE, Vegt E, Wetzels JF, Russel FG, Masereeuw R, Corstens FH, Oyen WJ, Boerman OC: Gelatin-based plasma expander effectively reduces renal uptake of 111In-octreotide in mice and rats. J Nucl Med 47: 528–533, 2006 [PubMed] [Google Scholar]
- 24.Campostrini N, Traglia M, Martinelli N, Corbella M, Cocca M, Manna D, Castagna A, Masciullo C, Silvestri L, Olivieri O, Toniolo D, Camaschella C, Girelli D: Serum levels of the hepcidin-20 isoform in a large general population: The Val Borbera study. J Proteomics 76: 28–35, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laarakkers CM, Wiegerinck ET, Klaver S, Kolodziejczyk M, Gille H, Hohlbaum AM, Tjalsma H, Swinkels DW: Improved mass spectrometry assay for plasma hepcidin: Detection and characterization of a novel hepcidin isoform. PLoS One 8: e75518, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nath KA, Haggard JJ, Croatt AJ, Grande JP, Poss KD, Alam J: The indispensability of heme oxygenase-1 in protecting against acute heme protein-induced toxicity in vivo. Am J Pathol 156: 1527–1535, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, Barasch J, Devarajan P: Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol 14: 2534–2543, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Bonventre JV, Vaidya VS, Schmouder R, Feig P, Dieterle F: Next-generation biomarkers for detecting kidney toxicity. Nat Biotechnol 28: 436–440, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen J, Hartono JR, John R, Bennett M, Zhou XJ, Wang Y, Wu Q, Winterberg PD, Nagami GT, Lu CY: Early interleukin 6 production by leukocytes during ischemic acute kidney injury is regulated by TLR4. Kidney Int 80: 504–515, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilmer MJ, Saleem MA, Masereeuw R, Ni L, van der Velden TJ, Russel FG, Mathieson PW, Monnens LA, van den Heuvel LP, Levtchenko EN: Novel conditionally immortalized human proximal tubule cell line expressing functional influx and efflux transporters . Cell Tissue Res 339: 449–457, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gorvin CM, Wilmer MJ, Piret SE, Harding B, van den Heuvel LP, Wrong O, Jat PS, Lippiat JD, Levtchenko EN, Thakker RV: Receptor-mediated endocytosis and endosomal acidification is impaired in proximal tubule epithelial cells of Dent disease patients. Proc Natl Acad Sci U S A 110: 7014–7019, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kartikasari AE, Roelofs R, Schaeps RM, Kemna EH, Peters WH, Swinkels DW, Tjalsma H: Secretion of bioactive hepcidin-25 by liver cells correlates with its gene transcription and points towards synergism between iron and inflammation signaling pathways. Biochim Biophys Acta 1784: 2029–2037, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Schranz M, Bakry R, Creus M, Bonn G, Vogel W, Zoller H: Activation and inactivation of the iron hormone hepcidin: Biochemical characterization of prohepcidin cleavage and sequential degradation to N-terminally truncated hepcidin isoforms. Blood Cells Mol Dis 43: 169–179, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Kenny AJ, Booth AG, George SG, Ingram J, Kershaw D, Wood EJ, Young AR: Dipeptidyl peptidase IV, a kidney brush-border serine peptidase. Biochem J 157: 169–182, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosen S, Stillman IE: Acute tubular necrosis is a syndrome of physiologic and pathologic dissociation. J Am Soc Nephrol 19: 871–875, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Scindia Y, Dey P, Thirunagari A, Liping H, Rosin DL, Floris M, Okusa MD, Swaminathan S: Hepcidin mitigates renal ischemia-reperfusion injury by modulating systemic iron homeostasis. J Am Soc Nephrol 26: 2800–2814, 2015 [DOI] [PMC free article] [PubMed]
- 37.Gburek J, Verroust PJ, Willnow TE, Fyfe JC, Nowacki W, Jacobsen C, Moestrup SK, Christensen EI: Megalin and cubilin are endocytic receptors involved in renal clearance of hemoglobin. J Am Soc Nephrol 13: 423–430, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Gozzelino R, Soares MP: Coupling heme and iron metabolism via ferritin H chain. Antioxid Redox Signal 20: 1754–1769, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paller MS: Hemoglobin- and myoglobin-induced acute renal failure in rats: Role of iron in nephrotoxicity. Am J Physiol 255: F539–F544, 1988 [DOI] [PubMed] [Google Scholar]
- 40.Zager RA, Gamelin LM: Pathogenetic mechanisms in experimental hemoglobinuric acute renal failure. Am J Physiol 256: F446–F455, 1989 [DOI] [PubMed] [Google Scholar]
- 41.Gutteridge JM: Iron promoters of the Fenton reaction and lipid peroxidation can be released from haemoglobin by peroxides. FEBS Lett 201: 291–295, 1986 [DOI] [PubMed] [Google Scholar]
- 42.Leaf DE, Rajapurkar M, Lele SS, Mukhopadhyay B, Rawn JD, Frendl G, Waikar SS: Increased plasma catalytic iron in patients may mediate acute kidney injury and death following cardiac surgery. Kidney Int 87: 1046–1054, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McKie AT, Marciani P, Rolfs A, Brennan K, Wehr K, Barrow D, Miret S, Bomford A, Peters TJ, Farzaneh F, Hediger MA, Hentze MW, Simpson RJ: A novel duodenal iron-regulated transporter, IREG1, implicated in the basolateral transfer of iron to the circulation. Mol Cell 5: 299–309, 2000 [DOI] [PubMed] [Google Scholar]
- 44.Zarjou A, Bolisetty S, Joseph R, Traylor A, Apostolov EO, Arosio P, Balla J, Verlander J, Darshan D, Kuhn LC, Agarwal A: Proximal tubule H-ferritin mediates iron trafficking in acute kidney injury. J Clin Invest 123: 4423–4434, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leheste JR, Melsen F, Wellner M, Jansen P, Schlichting U, Renner-Müller I, Andreassen TT, Wolf E, Bachmann S, Nykjaer A, Willnow TE: Hypocalcemia and osteopathy in mice with kidney-specific megalin gene defect. FASEB J 17: 247–249, 2003 [DOI] [PubMed] [Google Scholar]
- 46.Truett GE, Heeger P, Mynatt RL, Truett AA, Walker JA, Warman ML: Preparation of PCR-quality mouse genomic DNA with hot sodium hydroxide and tris (HotSHOT). Biotechniques 29: 52–54, 2000 [DOI] [PubMed] [Google Scholar]
- 47.Tjalsma H, Laarakkers CM, van Swelm RP, Theurl M, Theurl I, Kemna EH, van der Burgt YE, Venselaar H, Dutilh BE, Russel FG, Weiss G, Masereeuw R, Fleming RE, Swinkels DW: Mass spectrometry analysis of hepcidin peptides in experimental mouse models. PLoS One 6: e16762, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Torrance JD, Bothwell TH: A simple technique for measuring storage iron concentrations in formalinised liver samples. S Afr J Med Sci 33: 9–11, 1968 [PubMed] [Google Scholar]
- 49.Jansen J, Schophuizen CM, Wilmer MJ, Lahham SH, Mutsaers HA, Wetzels JF, Bank RA, van den Heuvel LP, Hoenderop JG, Masereeuw R: A morphological and functional comparison of proximal tubule cell lines established from human urine and kidney tissue. Exp Cell Res 323: 87–99, 2014 [DOI] [PubMed] [Google Scholar]
- 50.Gaeggeler HP, Gonzalez-Rodriguez E, Jaeger NF, Loffing-Cueni D, Norregaard R, Loffing J, Horisberger JD, Rossier BC: Mineralocorticoid versus glucocorticoid receptor occupancy mediating aldosterone-stimulated sodium transport in a novel renal cell line. J Am Soc Nephrol 16: 878–891, 2005 [DOI] [PubMed] [Google Scholar]
- 51.Abouhamed M, Wolff NA, Lee WK, Smith CP, Thévenod F: Knockdown of endosomal/lysosomal divalent metal transporter 1 by RNA interference prevents cadmium-metallothionein-1 cytotoxicity in renal proximal tubule cells. Am J Physiol Renal Physiol 293: F705–F712, 2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.