Abstract
A specific biomarker that can separate active renal vasculitis from other causes of renal dysfunction is lacking, with a kidney biopsy often being required. Soluble CD163 (sCD163), shed by monocytes and macrophages, has been reported as a potential biomarker in diseases associated with excessive macrophage activation. Thus, we hypothesized that urinary sCD163 shed by crescent macrophages correlates with active glomerular inflammation. We detected sCD163 in rat urine early in the disease course of experimental vasculitis. Moreover, microdissected glomeruli from patients with small vessel vasculitis (SVV) had markedly higher levels of CD163 mRNA than did those from patients with lupus nephritis, diabetic nephropathy, or nephrotic syndrome. Both glomeruli and interstitium of patients with SVV strongly expressed CD163 protein. In 479 individuals, including patients with SVV, disease controls, and healthy controls, serum levels of sCD163 did not differ between the groups. However, in an inception cohort, including 177 patients with SVV, patients with active renal vasculitis had markedly higher urinary sCD163 levels than did patients in remission, disease controls, or healthy controls. Analyses in both internal and external validation cohorts confirmed these results. Setting a derived optimum cutoff for urinary sCD163 of 0.3 ng/mmol creatinine for detection of active renal vasculitis resulted in a sensitivity of 83%, specificity of 96%, and a positive likelihood ratio of 20.8. These data indicate that urinary sCD163 level associates very tightly with active renal vasculitis, and assessing this level may be a noninvasive method for diagnosing renal flare in the setting of a known diagnosis of SVV.
Keywords: immunology, ANCA, glomerulonephritis, nephrology, renal injury, vasculitis
Crescentic GN is the histologic hallmark of small vessel vasculitis (SVV) with renal involvement. The main causes of renal vasculitis are antiANCA vasculitis and antiglomerular basement membrane (GBM) disease. Traditional biomarkers such as ANCA titers have limited value in identifying relapse as rising titers have been reported in up to 40% of patients without new or worsening disease activity.1 Commonly measured inflammatory markers such as C‑reactive protein are limited by lack of specificity, as they are affected by other diseases including concomitant infection. Other tests such as urine sediment analysis and proteinuria can be helpful in detecting early relapse of renal vasculitis. However, proteinuria may also indicate chronic renal scarring rather than active disease. Hematuria is similarly nonspecific because this can persist for months or years despite clinical remission: Magrey et al. reported that 25% of patients with sustained remission had persistent hematuria after a median follow-up of 38 months.2 Use of serum creatinine level cannot distinguish active renal vasculitis from other causes of renal dysfunction, and there may be substantial loss of function prior to an observed rise. Kidney biopsy, an invasive procedure associated with potential morbidity, is therefore usually required to definitively diagnose worsening renal vasculitis.
CD163 is a glycosylated membrane protein exclusively expressed on monocytes and macrophages3 that acts as a scavenger of hemoglobin/haptoglobin complexes.4 It is enzymatically cleaved to form soluble CD163 (sCD163) via ectodomain shedding in response to proinflammatory stimuli such as LPS.5 The exact function of sCD163 is unknown, although it may have anti-inflammatory properties as purified sCD163 inhibits phorbol ester-induced T lymphocyte activation in a dose-dependent manner.6 Serum sCD163 level has recently gained traction as a biomarker in diseases associated with excessive macrophage activation and proliferation such as Gaucher disease,7,8 hemophagocytic syndrome,9 and celiac disease.10
Macrophages are the most frequent inflammatory cell type in glomerular crescents.11 We hypothesized that activated macrophages infiltrating the glomerulus during evolution of crescentic renal vasculitis would shed sCD163 into the urinary space, where it could be measured and serve as a clinically relevant biomarker of active renal vasculitis.
Results
CD163 is Expressed in Kidneys in a Rat Model of ANCA Vasculitis and is Detected in the Urine
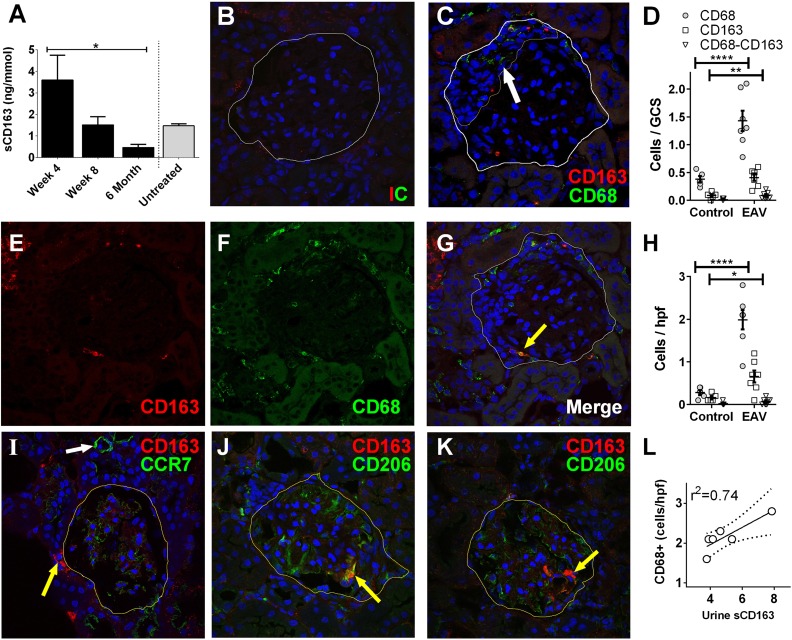
The experimental autoimmune vasculitis (EAV) model was used to examine kidney expression of CD163 in ANCA vasculitis.12 Rats immunized with human myeloperoxidase (MPO) analyzed at day 28 had proliferative GN and occasional early crescent formation (Supplemental Figure 1). We examined urinary sCD163 excretion over a time course (Figure 1A) and surprisingly found that, although histologic evidence of disease is most severe at day 56, urine sCD163 level was highest at day 28 (mean±SD: 3.6±1.1 ng/mmol). The level returned to that observed in control animals by day 56 (1.5±0.4 ng/mmol) and continued to fall further by 6 months (0.5±0.1 ng/mmol). At day 56, rats had fibrinoid necrosis and crescentic GN, as described previously,13 and CD163+ cells were present in both the glomerular and tubulointerstitial compartments (Figure 1, B–H). To further phenotype the CD163+ cells, we stained EAV kidney tissue for CCR7 (macrophage subtype M1 marker) and CD206 (M2 marker, Figure 1, I–K). CD163 did not colocalize with CCR7, but was variably colocalized with CD206, with CD163+CD206– cells also being present. The level of urine sCD163 was correlated with the degree of macrophage infiltration (Figure 1L).
Figure 1.
CD163 is present in the urine and kidneys of rats with EAV. (A) Urine was collected from healthy control rats (n=3) and rats 28 days (n=5), 56 days (n=6), and 186 days (n=3) after induction of EAV. sCD163 levels were determined by ELISA. Data are presented as mean sCD163 n /mmol creatinine ±SEM. One-way ANOVA and Dunn’s multiple comparison test were used to test for linear trend (*P<0.05). (B, C, E–G, I–K) Confocal images of glomeruli from rats with EAV dual stained with antibodies to the macrophage marker CD68 (C, F, G, green) and CD163 (red). Panels (B) and (C) depict representative images from tissue stained with isotype control and test antibody, respectively. The white line indicates Bowman’s capsule, while the fine yellow line in panel (C) indicates a glomerular crescent (white arrow). Panels (E) and (F) show confocal images in the CD163 and CD68 channels, respectively, while panel (G) shows the merged image. Although most cells are positive for one of the markers only, cells are also identified with dual staining (yellow arrow). Panel (I) shows dual staining with CD163 (yellow arrow) and CCR7 (green); there was no colocalization between the two markers, CCR7 being most strongly expressed in small blood vessels (white arrow). Panels (J) and (K) show staining with CD163 and CD206 (green), showing colocalization in panel (J, arrow) and lack of colocalization in panel (K, arrow). (D, H) Using 5 uM kidney sections from EAV and control rats, cells staining positive for CD68, CD163 or both markers were counted blind in 30 consecutive glomeruli (D) and ten ×40 fields containing tubulointerstitial compartment only (H), (*P<0.05; **P<0.01; ****P<0.001). (L) The average number of cells per high power field (hpf) in rats with EAV was correlated with the urine sCD163 level in the same animal. GCS, glomerular cross section.
CD163 is Highly Expressed in the Kidneys of Patients with Vasculitis
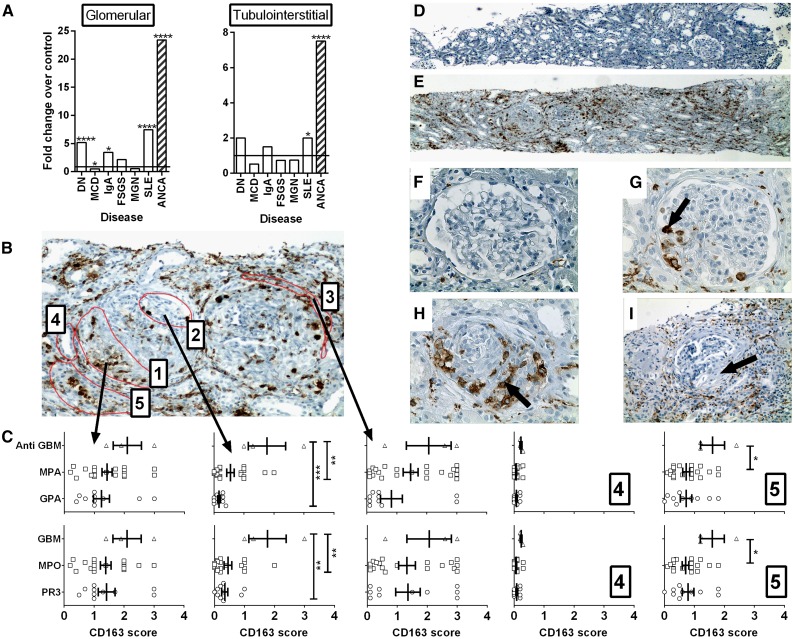
To assess the level of CD163 expression in the kidney of patients with renal vasculitis we first measured CD163 mRNA in microdissected glomerular and tubulointerstitial compartments in diabetic nephropathy, minimal change disease (MCD), IgA nephropathy, FSGS, membranous nephropathy (MGN), lupus nephritis, and ANCA vasculitis (Figure 2). Of all conditions examined, CD163 was most highly expressed in the glomeruli and tubulointerstitium of patients with ANCA vasculitis (23.4-fold and 7.5-fold change above control, respectively; P<0.001). We then confirmed expression of CD163 protein in kidney biopsy samples from patients with renal vasculitis by immunohistochemistry (Figure 2, B–I). High expression of CD163 was observed in both the glomeruli and interstitium of patients with active renal vasculitis. Interestingly, CD163 was observed in glomeruli that appeared unaffected based on histologic markers. Patients with anti-GBM disease exhibited the highest CD163 expression in all five compartments scored, particularly in histologically unaffected glomerular tufts. CD163 expression was similar in MPO and proteinase 3-ANCA positive patients. However, when patients were separated based on diagnosis rather than autoantibody specificity, patients with granulomatosis with polyangiitis were found to have relatively low levels of kidney CD163 expression (Figure 2C). Very occasional CD163+ cells were noted in healthy kidney from live transplant donors (Figure 2E) and healed fibrous crescents exhibited very little CD163 staining (Figure 2I).
Figure 2.
CD163 is highly expressed in the kidneys of patients with vasculitis. (A) RNA was extracted from microdissected glomerular and tubulointerstitial compartments from patients with diabetic nephropathy (DN), minimal change disease (MCD), IgA nephropathy (IgA), FSGS, MGN, lupus nephritis (SLE), and ANCA vasculitis. The degree of expression of the CD163 gene compared with microdissected healthy control kidney was determined by Affymetrix microarrays. Bars represent fold changes compared with the respective controls. (****q<0.01%; q<5%) (b) Paraffin-embedded human kidney sections from patients with vasculitis were stained for CD163 protein by immunohistochemistry and scored blind according to the location of cells with each of five regions: (1) within regions of fibrinoid necrosis or crescent formation, (2) within regions of apparently normal glomeruli, (3) in the periglomerular region, (4) within tubules, and (5) in the interstitial compartment. (C) CD163 scores in each of the respective five regions stratified by clinical diagnosis (upper graphs) and antibody specificity (lower graphs), (P<0.05; **P<0.01; ***P<0.001). (D–I) Images depict representative low power (×40 magnification) views of healthy control (D) and vasculitic (E) kidney, alongside high power (×400) views of healthy control kidney (F), a glomerulus with mild vasculitic injury (G, arrow), a severely affected glomerulus with established crescent formation (H, arrow), and a glomerulus with a fibrous crescent from previous vasculitic injury (I, arrow, ×200). MPA, microscopic polyangiitis; GPA, granulomatosis with polyangiitis.
Urinary sCD163 Levels are Elevated in Active Renal Vasculitis
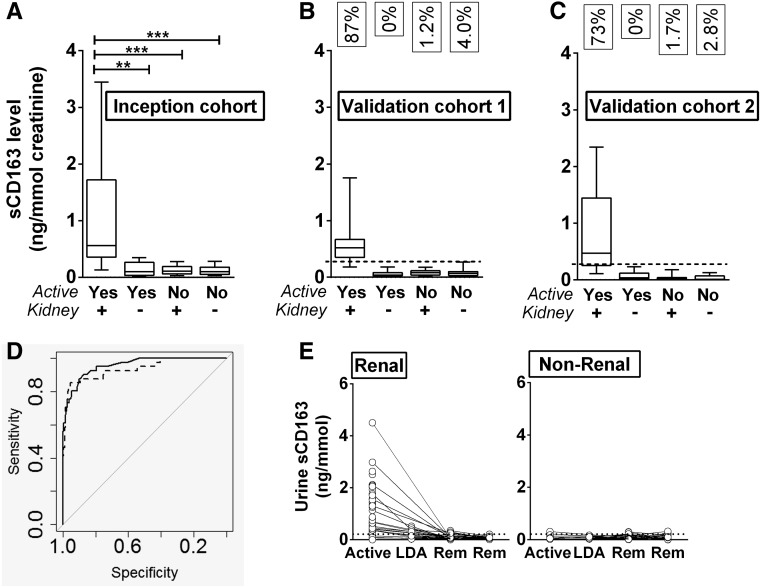
To test our hypothesis that the urinary level of sCD163 could act as a diagnostic measure for active renal vasculitis, we measured urinary sCD163 in an inception cohort of 177 subjects (14.6% with active renal vasculitis [n=26], 5.9% with active extrarenal vasculitis [n=11], and 79.4% in remission [n=140], Table 1). In order to account for differences in the concentration of urine between individuals, all sCD163 values were normalized to urinary creatinine. Normalized urinary sCD163 levels were significantly elevated in patients with active renal vasculitis (0.56 ng/mmol) compared with patients with active extrarenal vasculitis (0.12 ng/mmol), remission renal vasculitis (0.11 ng/mmol), and remission extrarenal vasculitis (0.1 ng/mmol) (Figure 3A). To assess whether this finding was a surrogate of hematuria (reflecting monocyturia), we assayed sCD163 in control urine spiked with serial dilutions of blood and correlated the result with urine dipstick analysis of hematuria. Addition of blood to urine only led to a rise in sCD163 level when the concentration of blood in urine was >2%. The urine appeared frankly blood-stained down to a concentration of 0.7% and was dipstick positive for blood down to 0.1%, levels at which sCD163 was undetectable (Supplemental Figure 2C). In order to assess the stability of sCD163 in urine, samples were stored under various conditions and aliquots were taken for repeated analyses. There was no significant change in sCD163 level after 1 week of storage at room temperature, 4°C, or –20°C, and the sCD163 level was stable for up to four freeze–thaw cycles. Freeze–thaw cycles beyond this caused a significant decline in sCD163 level (Supplemental Figure 2, A and B).
Table 1.
Demographic and clinical information for patients and controls
| Variable | Inception | Validation Cohort 1 | Validation Cohort 2 | External Validation (n=52) | Disease Controls | Healthy Controls | P Value | ||
|---|---|---|---|---|---|---|---|---|---|
| Patients | Healthy Controls | ||||||||
| n | 177 | 155 | 133 | 39 | 13 | 865 | 55 | ||
| Age (median, range) | 63.5 (21.2–90.2) | 60.3 (16.7–95.3) | 59.9 (18.1–91.0) | 58.6 (31.3–81.9) | 53.8 (34.6–64.1) | 55.4 (16.6–87.3) | 52.1 | 0.03 | |
| Male, n (%) | 87 (49) | 97 (62.6) | 80 (60.1) | 16 (41.1) | 6 (46.2) | 411 (47.6) | 20 (36.3) | <0.001 | |
| Diagnosis, n (%) | 0.07 | ||||||||
| GPA | 101 (57.1) | 94 (60.6) | 57 (42.9) | 28 (71.8) | GN | 30 | |||
| MPA | 54 (31.1) | 49 (31.6) | 59 (44.3) | 11 (28.2) | Non-GN | 54 | |||
| EGPA | 12 (6.8) | 6 (3.9) | 8 (6.0) | 0 (0) | ITU: sepsis ±AKI | 32 | |||
| Anti-GBM Disease | 3 (1.7) | 3 (1.9) | 5 (3.8) | 0 (0) | ITU: No sepsis + AKI | 286 | |||
| Double Positive | 6 (3.4) | 3 (1.9) | 4 (3.0) | 0 (0) | ITU: No sepsis/No AKI | 463 | |||
| Disease Characteristics, n (%) | 0.01a | ||||||||
| Active (renal) | 26 (14.6) | 15 (9.7) | 33 (24.8) | 24 (61.5) | |||||
| Active (extrarenal) | 11 (5.9) | 9 (5.8) | 6 (4.5) | 3 (7.7) | |||||
| Remission | 140 (79.4) | 131 (84.5) | 94 (70.7) | 12 (30.8) | |||||
| Kidney function, n (%) | 0.3b | ||||||||
| eGFR<30 | 35 (19.8) | 34 (21.9) | 33 (24.8) | 14 (35.9) | 258 (29.8) | ||||
| eGFR 30–60 | 50 (28.2) | 49 (31.6) | 44 (33.1) | 10 (25.6) | 313 (36.2) | ||||
| eGFR>60 | 92 (52) | 72 (46.5) | 56 (42.1) | 15 (38.5) | 294 (34.0) | ||||
| Dialysis | 9 (5.1) | 3 (1.9) | 12 (9.0) | N/A | 20 (2.3) | ||||
P values show significance between patient groups in inception, internal validation, and external validation cohorts. GPA, granulomatosis with polyangiitis; MPA, microscopic polyangiitis; EGPA, eosinophilic granulomatosis with polyangiitis; ITU, Intensive therapy unit.
Comparing inception, validation 1, and validation 2 cohorts.
Comparing SVV cohorts.
Figure 3.
sCD163 is elevated in the urine of those with active renal vasculitis, but not active extrarenal vasculitis. sCD163 levels were measured by ELISA in an inception cohort (n=177) (A) and two validation cohorts (n=155+133) (B, C). Graphs show comparison of levels found in active renal vasculitis, active extrarenal vasculitis, remission (Rem) renal vasculitis, and remission extrarenal vasculitis. Data are presented as median sCD163 ng/mmol creatinine with interquartile range. The boxes in panels (B) and (C) indicate the fraction of positive samples in each group using the optimum cutoff of 0.3 ng/mmol. Nonparametric one-way ANOVA (Kruskal-Wallis test) and Dunn’s multiple comparison test were used to test for significance of each group compared with the active renal vasculitis group (**P<0.01, ***P<0.001). (D) ROC curves calculated from normalized (solid line) and non-normalized (dashed line) data derived from the inception cohort depicting the ability of sCD163 to detect active renal vasculitis. The respective areas under the curves were 0.94 and 0.96, respectively. (E) sCD163 was measured by ELISA in serial samples during periods of active disease, low disease activity (LDA), and remission (Rem). Graphs show sCD163 levels over time in renal and extrarenal vasculitis patients. Lines depict samples from the same subject at different time points.
Serum sCD163 is Not Increased in Patients with Active Vasculitis
In order to assess whether serum levels of sCD163 could predict vasculitis disease activity we measured this in active SVV patients (n=59), patients in remission (n=184), disease controls (n=37), and healthy controls (n=57). Serum sCD163 concentration did not differ significantly between patients with active vasculitis (median 387 ng/ml), remission vasculitis (313 ng/ml), disease controls (298 ng/ml), and healthy controls (287 ng/ml) (Supplemental Figure 3). Furthermore, serum and urine sCD163 levels did not correlate (Supplemental Figure 3).
Internal Validation of the Association between Elevated Urine sCD163 and Active Renal Vasculitis
Using inception cohort data, we generated a receiver–operator characteristic (ROC) curve to examine the ability of urinary sCD163 to identify active renal vasculitis in patients with a known diagnosis (Figure 3D). The area under the ROC curve was 0.94, indicating excellent biomarker potential. We used this curve to define an optimum cutoff value of 0.3 ng/mmol creatinine and applied this to two validation cohorts of 155 and 133 subjects (Table 2). These cohorts provided virtually identical results to the inception cohort, with sCD163 significantly elevated in the urine of patients with active renal vasculitis compared with patients with active extrarenal vasculitis and remission patients with previous renal or extrarenal vasculitis (Figure 3, B and C). The chosen cutoff accurately diagnosed active renal disease in 87% and 73% of SVV patients in the two cohorts. Conversely, none of the patients with active extrarenal vasculitis were positive. False-positive rates in remission patients were 1.5% and 3.4%, respectively, for patients with previous renal and extrarenal vasculitis. Stratification of values by ANCA specificity (proteinase 3 versus MPO) revealed no difference in the ability of sCD163 to identify active renal vasculitis (Supplemental Figure 4). Biomarker statistics for diagnosis of active renal vasculitis within the overall vasculitis cohorts are summarized in Table 3. Notable values are specificity of 96% and positive likelihood ratio of 20.8. The non-normalized (to creatinine) urine sCD163 values also performed well, although specificity (92%) and positive likelihood ratio (10.0) were less than for the normalized values (Table 3). In individuals in whom sCD163 was measured in serial samples following presentation with active disease, the level fell rapidly with treatment, and remained low. In those with active extrarenal vasculitis, the level was low at all time points (Figure 3E). To assess the relationship between renal CD163 protein expression and the urine sCD163 level, we identified 25 cases with renal tissue obtained at approximately the same time as the test urine sample. Of these, sufficient glomerular tissue was available for analysis from 17 patients and extraglomerular tissue was available from 20. There was a correlation between the number of CD163+ cells in glomeruli and the linked level of urine sCD163 (Supplemental Figure 5). The correlation with extraglomerular staining was weaker.
Table 2.
Generation of optimum cutoff in inception cohort, with application to two separate validation cohorts
| Cohort Analysed | AUC | Cutoff | Sensitivity | Specificity | PPV | NPV | PLR | NLR |
|---|---|---|---|---|---|---|---|---|
| Inception | ||||||||
| sCD163 normalized | 0.96 | 0.3 | 0.96 | 0.94 | 0.69 | 0.99 | 14.7 | 0.05 |
| Validation | ||||||||
| sCD163 normalized | 0.97 | 0.3 | 0.87 | 0.98 | 0.81 | 0.99 | 43.5 | 0.13 |
| Validation 2 | ||||||||
| sCD163 normalized | 0.94 | 0.3 | 0.73 | 0.98 | 0.92 | 0.92 | 36.5 | 0.28 |
Statistics were determined by first analyzing data from the inception cohort to define optimum cutoff, and applying this to the validation cohorts. AUC, area under curve; PPV, positive predictive value; NPV, negative predictive value; PLR, positive likelihood ratio; NLR, negative likelihood ratio.
Table 3.
Biomarker attributes of normalized and non-normalized urine sCD163 for discriminating active renal vasculitis from remission
| sCD163 Value Analysed | AUC | Cutoff | Sensitivity | Specificity | PPV | NPV | PLR | NLR |
|---|---|---|---|---|---|---|---|---|
| sCD163 normalized | 0.93 | 0.3 | 0.83 | 0.96 | 0.8 | 0.97 | 20.75 | 0.17 |
| sCD163 non-normalized | 0.91 | 1.3 | 0.80 | 0.92 | 0.64 | 0.96 | 10.0 | 0.22 |
Statistics were determined by analyzing combined data from both the inception and validation cohorts. AUC, area under curve; PPV, positive predictive value; NPV, negative predictive value; PLR, positive likelihood ratio; NLR, negative likelihood ratio.
External Validation of the Association between Elevated Urine sCD163 and Active Renal Vasculitis
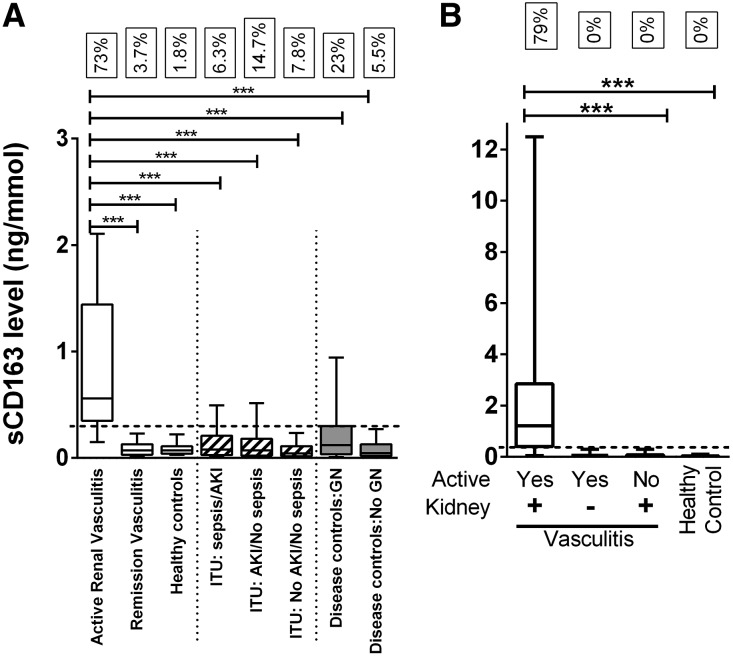
An independent Dutch cohort comprising 52 subjects (39 SVV patients and 13 healthy controls) was interrogated to further validate our findings. These analyses confirmed the ability of urinary sCD163 to identify active renal disease in SVV patients. sCD163 was significantly elevated in the urine of patients with active renal vasculitis compared with remission patients with previous renal involvement, and healthy controls (Figure 4B). Using the cutoff value of 0.3 ng/mmol, 79% of patients with active renal vasculitis were positively diagnosed. No false-positive cases were seen in patients with active extrarenal vasculitis, remission renal vasculitis, or healthy controls.
Figure 4.
sCD163 is elevated in the urine of patients with active renal vasculitis recruited from an independent cohort and compared with a range of control groups. (A) The data from the inception and validation cohorts (n=465) were combined and compared with healthy controls (n=55) and a diverse disease control group comprising samples from patients in the intensive care unit with sepsis with or without AKI (n=32), AKI without sepsis (n=286), and no AKI or sepsis (n=463), in addition to a nonICU disease control group with (n=30) and without (n=54) nonvasculitic GN. The boxes refer to the fraction of cases with a urine sCD163 level >0.3 ng/mmol (dotted line). (B) sCD163 levels were also measured by ELISA in an independent external validation cohort (n=52). Graph showing comparison of levels found in active renal vasculitis (Act), active extrarenal vasculitis, remission renal vasculitis (Rem), and healthy controls. Data are presented as median sCD163 ng/mmol creatinine with interquartile range. Nonparametric one-way ANOVA (Kruskal-Wallis test) and Dunn’s multiple comparison test were used to test for significance of each group compared with the active renal vasculitis group (***P<0.001).
Patients with kidney disease without active crescentic GN do not present with elevated urinary sCD163
In order to examine the specificity of elevated urinary sCD163 for active renal vasculitis, we measured the urine sCD163 level in a series of disease control groups (Figure 4A, Table 1). Elevated urine sCD163 levels (above cutoff of 0.3 ng/mmol creatinine) were identified in a small number of critically ill patients in the intensive care unit, with 6.3% of patients with sepsis and AKI, 14.7% of patients with AKI but no sepsis, and 7.8% of patients with no AKI or sepsis testing positive. Urinary sCD163 was not significantly elevated in a cohort of disease controls without GN (n=54) or in healthy controls (n=55). However, one quarter of disease controls with nonvasculitic GN, in whom variable degrees of macrophage infiltration would be expected, had an elevated level (n=30).
Urine sCD163 is a significantly better biomarker for active renal vasculitis than urine protein excretion rate
Using the internal validation cohorts, we tested the utility of urine sCD163 in the identification of active renal vasculitis against urine protein-to-creatinine ratio (PCR), using a PCR cutoff of 15 mg/mmol (Table 4). On its own, urine sCD163 was superior by all measures of biomarker performance including sensitivity and specificity, which acts a measure of false-positive rate. Combining urine sCD163 with urine PCR did not provide a statistically significant improvement in biomarker performance versus the use of sCD163 alone. In a small subset of individuals for whom samples were available from an incident histologically proven renal flare, the biomarker precision of urine sCD163, c-reactive protein, hematuria, and ANCA titer were compared (Supplemental Table 1). Urine sCD163 was superior to the other three biomarkers by all measures of biomarker performance.
Table 4.
Biomarker comparison of urine sCD163 with urine PCR and combination sCD163/PCR for discriminating active renal vasculitis from remission
| Comparator Variable | AUC | Cutoff | Sensitivity | Specificity | PPV | NPV | PLR | NLR |
|---|---|---|---|---|---|---|---|---|
| sCD163 normalized | 0.91 | 0.3 | 0.83 | 0.97 | 0.89 | 0.95 | 30.6 | 0.17 |
| PCR | 0.68 | 15 | 0.97 | 0.39 | 0.3 | 0.97 | 1.6 | 0.08 |
| sCD163+PCR | 0.93 | 0.8 | 0.97 | 0.89 | 0.95 | 29.33 | 0.21 | |
| Bootstrap P-value | 0.07 |
Statistics were determined by analyzing data from a subset of cases from the combined cohort. The bootstrap test compared the AUC sCD163 and PCR. AUC, area under curve; PPV, positive predictive value; NPV, negative predictive value; PLR, positive likelihood ratio; NLR, negative likelihood ratio.
Discussion
We have identified a highly specific association between active renal vasculitis and the level of urinary sCD163. There is a strong biologic rationale for this finding as this macrophage marker is strongly expressed in glomerular crescents. The shedding of this protein from the glomerular cell surface directly into the urine makes it attractive as a potential biomarker. Our key finding was elevated urinary sCD163 levels in patients with active renal vasculitis compared with vasculitis patients in remission, disease controls, and healthy controls. Those with active extrarenal vasculitis were indistinguishable from all control groups in terms of sCD163 levels, despite the presence of severe vasculitic manifestations, such as alveolar hemorrhage. Moderately elevated levels of urine sCD163 were detected in a small number of disease controls. These individuals had severe nephrotic syndrome due to MCD or MGN, suggesting that in this setting sCD163 may spill over from the blood. Caution will, therefore, need to be applied in patients with very heavy proteinuria.
Urine sCD163 was also elevated in some patients with nonvasculitic forms of GN, suggesting that other forms of macrophage-rich glomerular disease are also characterized by urinary sCD163 excretion. Of note, CD163+ macrophages have recently been shown to accumulate in the kidney of patients with lupus nephritis, to correlate with histologic activity and to be increased in the urine of these patients.14 The goal of the current study was to diagnose active renal vasculitis in those with a known diagnosis of SVV, not to distinguish SVV from other causes of GN, although our results suggest that this test may have utility in these conditions also. Of note, the biomarker statistics were generated without reference to utility of urine microscopy for casts and dysmorphic red cells, which was not routinely documented in the biomarker dataset, so the superior performance of urine sCD163 over conventional tests may be less marked if urine microscopy is used. There were no significant differences in serum sCD163 among the experimental groups, supporting our hypothesis that increased urinary sCD163 observed in active renal vasculitis is due to increased local shedding from the glomerulus.
We found sCD163 to be maximally elevated in the urine of rats with EAV 28 days after induction of disease, a time point when renal inflammation is developing. The degree of glomerular inflammation, as measured by conventional histologic methods, is relatively subtle at this time point, with maximum histologic renal damage occurring at 8 weeks post immunization. Return of urine sCD163 levels to baseline by day 56 indicates that glomerular CD163 shedding occurs relatively early in the pathologic vasculitic process. This suggests that it may serve as an early indicator of active disease and if incorporated into routine patient testing could allow therapeutic intervention prior to structural injury occurring.
Su et al. reported that urine sCD163 was elevated in patients with sepsis, particularly in those with AKI (area under ROC curve to differentiate AKI from non-AKI was 0.69).15 The average level of non-normalized urine sCD163 in their cohort (74.8 ng/ml) was two-log higher than that observed in our sepsis/AKI cohort (0.4 ng/ml). The study by Su et al. used a different ELISA product (IQ Products) to that used in our study (DuoSet, R&D Systems, Minneapolis, MN). Of note, the R&D DuoSet ELISA has been shown by Moller et al. to result in lower values than the IQ Products kit.16 Indeed, the serum levels we observed (mean 441 ng/ml) were lower than the reference range suggested by Moller et al. (890–3950 ng/ml). This lack of standardization, with commercial assays systematically reporting lower values than Moller’s in-house automated assay, will clearly need to be clarified prior to its introduction as a clinical test. Notwithstanding this, the massively elevated urine level found in these patients with sepsis is interesting and remains unexplained. Su et al. also found a correlation between serum and urine sCD163 (correlation coefficient 0.51, P<0.001), in contrast to our findings. Indeed, in our study population, which was 10 times larger, we found a correlation coefficient of 0.15, suggesting minimal correlation between urine and serum values. The most likely reason for this is the study setting of severe sepsis, which could conceivably nonspecifically increase renal permeability to sCD163, and which may also partially account for the markedly elevated urine level. However, in our cohort of critically ill patients with sepsis, only occasional cases were noted to have an elevated level, and never to the extent observed by Su et al.
A urinary biomarker that could reliably identify active renal vasculitis in patients already diagnosed with SVV would reduce the need for expensive and invasive kidney biopsies. In this context, several proteins have been investigated as biomarkers of active disease in vasculitis, including alpha‑1 acid glycoprotein, kidney injury molecule‑1, fractalkine, neutrophil gelatinase-associated lipocalin, and monocyte chemoattractant protein‑1 (MCP‑1).17,18 Of these, MCP‑1 has shown most promise as a biomarker for active vasculitis. This chemokine was elevated in the urine, but not the serum of patients with active or persistent renal vasculitis, and levels were found to decline with treatment.18 Lieberthal et al. demonstrated that, in discriminating active renal vasculitis, MCP‑1 had a specificity of 94% and a sensitivity of 89% (AUC=0.93, positive likelihood ratio of 8.5 and negative likelihood ratio of 0.07),17 values that are close to those obtained in our study.
sCD163 is a highly stable protein, making it an attractive clinical biomarker. In whole blood it is stable for 24 and 48 hours at room temperature and 4°C, respectively, while in plasma it is stable for weeks at 4°C and several years at –20°C.16 Similarly, we have found that in urine sCD163 is stable for at least 1 week at room temperature. This stability means that variations in the collection and processing of samples are unlikely to be a determining factor in the assay result. This represents a significant advantage over MCP‑1, which degrades quickly. MCP‑1 is actively produced by intrinsic renal cells and leukocytes in response to inflammatory stimuli, resulting in monocyte chemoattraction, cells which themselves are likely to express CD163. Therefore, MCP‑1 and sCD163 measurements reflect subtly different elements of a similar process; their use in combination may further increase diagnostic precision.
The excellent biomarker characteristics of urinary sCD163 in identifying active renal vasculitis, the availability of high-quality detection antibody, and its stability during sample storage suggest that this biomarker could now be developed for clinical use. Most biomarker development programs rely upon panels of proteins or metabolites; the ability of urine sCD163 alone (or normalized to creatinine) to identify active renal vasculitis suggests that it is ideal for development as a point-of-care dipstick test and may have additional use in tracking induction therapy response, allowing more tailored and less toxic treatment regimens, or potentially to triage patients with undifferentiated AKI in settings where there may be delay in obtaining serological or histologic diagnosis.
Concise Methods
Induction of EAV
EAV, a model of MPO ANCA vasculitis, was induced in rats, as previously described, with slight amendments.13 Briefly, 6-week-old Wistar–Kyoto (WKY) rats were immunized with 3.2 mg/kg human MPO (kind gift from Biovitrum, Sweden) in CFA along with 1 µg of Pertussis toxin (Sigma-Aldrich, St. Louis, MO). Rats were boosted with 4 µg LPS and 0.1 mg/kg MPO (without adjuvant) after 28 and 35 days, respectively. Control animals were left unimmunized. Urine samples were collected at several time points during EAV progression by placing rats in metabolic cages for 24 hours. Urine samples were centrifuged at 2000 ×g for 10 minutes at 4°C and stored at –80°C until analysis. sCD163 levels were measured in neat urine using a rat sCD163/soluble hemoglobin scavenger receptor kit (BlueGene), following the manufacturer’s instructions. Rats were sacrificed at day 28 and 56 post immunization and renal sections were stained with hematoxylin and eosin and periodic acid Schiff stains. Animal studies were approved by local ethics committee and complied with a project license granted by the Irish Medicines Board.
Immunohistochemistry of EAV Renal Tissue
Kidneys harvested from rats 56 days after EAV induction were sectioned and blocked with 20% normal goat serum. Sections were dual stained with antibodies specific for rat CD68 (rabbit polyclonal; Abcam, Inc., Cambridge, MA) and rat CD163 (mouse anti-rat ED2, AbD Serotec, used for all rat CD163 staining) followed by Alexa Fluor 568 goat anti-mouse IgG (Life Technologies, Carlsbad, CA) and Alexa Fluor 488 goat anti-rabbit IgG (Abcam, Inc.). Sections were also dual stained with antibodies specific for rat CCR7 (rabbit mAb; Abcam, Inc.) or rat CD206 (rabbit polyclonal; Abcam, Inc.) and rat CD163, followed by Alexa Fluor goat anti-rabbit IgG 488 and Alexa Fluor goat anti-mouse IgG 568, as above. Nuclei were stained with Hoechst 33342 (Life Technologies). The numbers of CD68+ and CD163+ cells per glomerulus were scored blindly for at least 30 glomeruli and ten ×40 fields (without glomeruli) using a fluorescent microscope (Eclipse 90i, Nikon, Tokyo, Japan). Images were obtained using a confocal microscope (Carl Zeiss LSM 510).
Clinical Recruitment and Assessment
Patients with SVV (comprising granulomatosis with polyangiitis, microscopic polyangiitis, eosinophilic granulomatosis with polyangiitis, and anti-GBM disease), healthy controls, and disease controls were recruited through the Rare Kidney Disease (RKD) Biobank. Disease controls (Supplemental Table 2) were divided into those with GN in whom glomerular macrophage infiltration may be expected, those without GN, and intensive care unit patients with nonimmune-mediated AKI, multisystem failure, and sepsis. To address the latter, we tested a large number of urine samples from the Prospective Validation of Acute Kidney Injury Biomarkers and Definitions in Critically Ill Patients study. Samples were archived until analysis in the RKD Biobank and the linked registry was used to define clinical details. The study was approved by the institutional review board (reference 2012/38/04) and all recruits provided written, informed consent. All vasculitis patients were classified according to the Chapel Hill consensus classification criteria.19 The healthy control group were self-reported as healthy and had a normal urine dipstick examination. Vasculitis disease activity was recorded using the Birmingham Vasculitis Activity Score (BVAS).20 Active vasculitis was considered to be present with a BVAS score ≥0. Active renal vasculitis was defined as BVAS>0 with one or more renal items, including the presence of hematuria by urine dipstick; urine samples were not routinely assessed for the presence of casts or dysmorphic red cells. Patients with both newly diagnosed and flaring disease were included in the analysis. The presence or absence of active vasculitis was adjudicated without knowledge of the sCD163 level by reference to the clinical state and response to therapy one month after obtaining the sample. For the purpose of assessment of biomarker performance, we randomly assigned each recruit to either an inception or one of two validation cohorts prior to measurement of sCD163 levels.
CD163 Staining of Human Kidney Sections
All recruits undergoing a clinically indicated kidney biopsy at one of the study sites (Beaumont Hospital) were further analyzed by immunohistochemistry of renal tissue to determine the degree and pattern of CD163 expression. Formalin fixed paraffin wax embedded renal tissue sections were run with the ultraView Universal DAB detection kit run on the Benchmark XT (Roche, Basel, Switzerland) automated immunostaining machine. Cell Conditioning 1 (CC1; Roche) was used for 30 minutes at 96°C to retrieve the antigen. Mouse antihuman mAb CD163 (10D6; Abcam, Inc.) and ref ab74604 (Abcam, Inc.) were used at a dilution of 1/20 for 40 minutes at 37°C. CD163 expression was quantified using the technique of Zhao et al. Expression was assessed within areas of fibrinoid necrosis/crescent formation, within normal appearing glomeruli, in the periglomerular region, within tubules, and in the interstitial space.11 Scores were assigned as follows: 0=no CD163+ cells, 1=1–5 CD163+ cells, 2=6–10 CD163+ cells and 3=>10 CD163+ cells. Values were calculated per glomerular cross section for each of the three glomerular regions, and by estimating the fraction of tubular or interstitial compartments with CD163+ cells and multiplying this by the score. To derive a quantitative measure to compare with the urine sCD163 value we performed image analysis on samples with a paired urine sCD163 value using the Visiopharm Integrator System (Visiopharm, Hoersholm, Denmark) (Supplemental Figure 5). The number of nuclei associated with CD163 staining was quantified in glomerular, obsolescent glomerular, and extraglomerular (renal cortex) compartments, the same threshold being applied to all sections.
Microdissection and CD163 mRNA Quantification in Human Kidney
Human renal biopsy specimens and Affymetrix microarray expression data were procured within the framework of the European Renal cDNA Bank–Kröner-Fresenius Biopsy Bank.21,22 Biopsies were obtained from patients after informed consent and with approval of the local ethics committees. Following renal biopsy, the tissue was transferred to RNase inhibitor and microdissected into glomerular and tubular fragments. Total RNA was isolated from microdissected glomeruli, reverse transcribed, and linearly amplified according to a protocol previously reported.23 The microarray expression data used in this study came from individual patients with diabetic nephropathy, MCD, IgA nephropathy (IgA), FSGS, MGN, and ANCA vasculitis. Pretransplantation kidney biopsies from living donors were used as control renal tissue. Fragmentation, hybridization, staining, and imaging were performed according to the Affymetrix Expression Analysis Technical Manual (Affymetrix, Santa Clara, CA). CEL file normalization was performed with the Robust Multichip Average method using RMAExpress (version 1.0.5) and the human Entrez-Gene custom CDF annotation from Brain Array version 18 (http://brainarray.mbni.med.umich.edu/Brainarray/default.asp). To identify differentially expressed genes the Significance Analysis of Microarrays method was applied using TiGR (MeV, version 4.8.1).24 A q-value <5% was considered to be statistically significant.
Measurement of sCD163 in Human Serum and Urine Samples
Venous blood was collected into serum tubes and allowed to clot at room temperature before being centrifuged at 1500×g for 10 minutes at 4°C. Serum was removed and stored at –80°C until assayed. Urine samples were centrifuged at 2000×g for 10 minutes at 4°C, with storage of the supernatant at –80°C until assay. Samples from the Groningen validation cohort were processed slightly different. Venous blood was centrifuged at 2000×g for 10 minutes, after which serum was collected and stored at –20°C until assay. Urine was centrifuged for 15 minutes at 1200×g, supernatant was collected, diluted 1:1 in PBS, and stored at –20°C until assay. Serum and urine sCD163 levels were determined by ELISA (R&D Systems, human sCD163 DuoSet, DY1607) following the manufacturer’s instructions. Serum and urine samples were diluted 1:400 and 1:4, respectively. We normalized the urine sCD163 level to the creatinine level as determined by a modified Jaffe technique.
Investigation of the Impact of Hematuria on Urine sCD163 Level
To assess whether the urine sCD163 was merely a surrogate marker of hematuria, we obtained urine from a healthy control (confirmed previously as negative for sCD163) and spiked in serial dilutions of anticoagulated blood. At each dilution the urine was tested for blood by dipstick and by visual inspection for macroscopic hematuria. After incubation at room temperature for 1 hour, the urine was centrifuged and sCD163 levels measured as described above.
Assessment of the Impact of Prolonged Storage and Freeze–Thaws on sCD163 Level
To assess stability of sCD163 under normal urine handling conditions we first prepared a series of aliquots from a urine sample with known elevated sCD163 level and stored them at room temperature, 4°C, –20°C, and -80°C for up to 1 week. Aliquots were removed at 24 and 168 hours for sCD163 assay (n=6 per condition), with the level being normalized to the level in the original sample. We then took stored urine from the same patient and subjected aliquots to between one and six freeze–thaw cycles to assess the effect on sCD163 level.
Statistical Analyses
Analyses were performed using GraphPad Prism version 6.01 for Windows (GraphPad Software, San Diego, CA), SPSS (IBM, version 21, Amrok) and R (R Foundation for Statistical Computing, version 3.1.1, Vienna, Austria). Data were expressed as median±interquartile range and P-values <0.05 were considered significant. Differences between two groups were analyzed using the Mann–Whitney U-test, while differences between three or more groups were analyzed using Kruskal–Wallis test with a post hoc Dunn test. To assess the ability of sCD163 to predict active renal vasculitis over urine protein excretion rate, ROC curves for sCD163, urine PCR, and their combination were estimated using data from the combined cohorts. Note that the full cohort was not available for this analysis, so the optimum sCD163 cutoff was slightly different. Logistic regression was used to estimate the combination of sCD163 and urine protein excretion rate. Inference on differences between ROC curves was performed using bootstrap resampling. The point on the curves that maximized the sum of sensitivity and specificity was chosen as a cutoff for predicting active renal vasculitis. For PCR, the standard laboratory cutoff of 15 mg/mmol was used. Optimum cutoffs were then used to predict active renal vasculitis in the validation cohort and estimate sensitivity, specificity, positive/negative predictive value, positive/negative likelihood ratios.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors wish to acknowledge contributors to the Rare Kidney Disease registry and Biobank, including Valerie Logan, Liam Casserley, Peter Conlon, Declan DeFreitas, Conall O’Seaghda, Colm Magee, Tom Crotty, John Holian, Alan Watson, Dearbhla Kelly, Radzi Rodzlan, Niall Conlon, Louise Ryan, Dervla Connaughton, Emily Naylor, Claire Foley, Sharon Casey, Yvelynne Kelly, and Ann-Marie O’Sullivan. Also, Eoin Cotter for assistance with the AKI Biobank.
This study was funded by Science Foundation Ireland award SFI 11/Y/B2093, Health Research Board grant POR/2011/128 and the Dublin Centre for Clinical Research Network (CRF/2007/1).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “The Search for a Biomarker of Relapse in ANCA-Associated Vasculitis,” on pages 2551–2553.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015050511/-/DCSupplemental.
References
- 1.Tomasson G, Grayson PC, Mahr AD, Lavalley M, Merkel PA: Value of ANCA measurements during remission to predict a relapse of ANCA-associated vasculitis--a meta-analysis. Rheumatology (Oxford) 51: 100–109, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Magrey MN, Villa-Forte A, Koening CL, Myles JL, Hoffman GS: Persistent hematuria after induction of remission in Wegener granulomatosis: a therapeutic dilemma. Medicine (Baltimore) 88: 315–321, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Fabriek BO, Dijkstra CD, van den Berg TK: The macrophage scavenger receptor CD163. Immunobiology 210: 153–160, 2005. 010 [DOI] [PubMed] [Google Scholar]
- 4.Kristiansen M, Graversen JH, Jacobsen C, Sonne O, Hoffman HJ, Law SK, Moestrup SK: Identification of the haemoglobin scavenger receptor. Nature 409: 198–201, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Møller HJ, Nielsen MJ, Maniecki MB, Madsen M, Moestrup SK: Soluble macrophage-derived CD163: a homogenous ectodomain protein with a dissociable haptoglobin-hemoglobin binding. Immunobiology 215: 406–412, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Högger P, Sorg C: Soluble CD163 inhibits phorbol ester-induced lymphocyte proliferation. Biochem Biophys Res Commun 288: 841–843, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Møller HJ, de Fost M, Aerts H, Hollak C, Moestrup SK: Plasma level of the macrophage-derived soluble CD163 is increased and positively correlates with severity in Gaucher’s disease. Eur J Haematol 72: 135–139, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Adly AA, Ismail EA, Ibraheem TM: Macrophage-derived soluble CD163 level in young patients with Gaucher disease: relation to phenotypes, disease severity and complications. Int Immunopharmacol 24: 416–422, 2015 [DOI] [PubMed] [Google Scholar]
- 9.Schaer DJ, Schleiffenbaum B, Kurrer M, Imhof A, Bächli E, Fehr J, Moller HJ, Moestrup SK, Schaffner A: Soluble hemoglobin-haptoglobin scavenger receptor CD163 as a lineage-specific marker in the reactive hemophagocytic syndrome. Eur J Haematol 74: 6–10, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Daly A, Walsh C, Feighery C, O’Shea U, Jackson J, Whelan A: Serum levels of soluble CD163 correlate with the inflammatory process in coeliac disease. Aliment Pharmacol Ther 24: 553–559, 2006. x [DOI] [PubMed] [Google Scholar]
- 11.Zhao L, David MZ, Hyjek E, Chang A, Meehan SM: M2 macrophage infiltrates in the early stages of ANCA-associated pauci-immune necrotizing GN. Clin J Am Soc Nephrol 10: 54–62, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Little MA, Smyth CL, Yadav R, Ambrose L, Cook HT, Nourshargh S, Pusey CD: Antineutrophil cytoplasm antibodies directed against myeloperoxidase augment leukocyte-microvascular interactions in vivo. Blood 106: 2050–2058, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Little MA, Smyth L, Salama AD, Mukherjee S, Smith J, Haskard D, Nourshargh S, Cook HT, Pusey CD: Experimental autoimmune vasculitis: an animal model of anti-neutrophil cytoplasmic autoantibody-associated systemic vasculitis. Am J Pathol 174: 1212–1220, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Endo N, Tsuboi N, Furuhashi K, Maruyama S, Matsuo S: Association of glomerular macrophage phenotypes and urinary soluble cd163 with disease activity in human lupus nephritis. [Abstract] J Am Soc Nephrol 25: SA-PO472, 2014 [Google Scholar]
- 15.Su L, Feng L, Liu C, Jiang Z, Li M, Xiao K, Yan P, Jia Y, Feng D, Xie L: Diagnostic value of urine sCD163 levels for sepsis and relevant acute kidney injury: a prospective study. BMC Nephrol 13: 123, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Møller HJ: Soluble CD163. Scand J Clin Lab Invest 72: 1–13, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Lieberthal JG, Cuthbertson D, Carette S, Hoffman GS, Khalidi NA, Koening CL, Langford CA, Maksimowicz-McKinnon K, Seo P, Specks U, Ytterberg SR, Merkel PA, Monach PA Vasculitis Clinical Research Consortium : urinary biomarkers in relapsing antineutrophil cytoplasmic antibody-associated vasculitis. J Rheumatol 40: 674–683, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tam FW, Sanders JS, George A, Hammad T, Miller C, Dougan T, Cook HT, Kallenberg CG, Gaskin G, Levy JB, Pusey CD: Urinary monocyte chemoattractant protein-1 (MCP-1) is a marker of active renal vasculitis. Nephrol Dial Transplant 19: 2761–2768, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, Flores-Suarez LF, Gross WL, Guillevin L, Hagen EC, Hoffman GS, Jayne DR, Kallenberg CG, Lamprecht P, Langford CA, Luqmani RA, Mahr AD, Matteson EL, Merkel PA, Ozen S, Pusey CD, Rasmussen N, Rees AJ, Scott DG, Specks U, Stone JH, Takahashi K, Watts RA: 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum 65: 1–11, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Stone JH, Hoffman GS, Merkel PA, Min YI, Uhlfelder ML, Hellmann DB, Specks U, Allen NB, Davis JC, Spiera RF, Calabrese LH, Wigley FM, Maiden N, Valente RM, Niles JL, Fye KH, McCune JW, St Clair EW, Luqmani RA International Network for the Study of the Systemic Vasculitides (INSSYS) : A disease-specific activity index for Wegener’s granulomatosis: modification of the Birmingham Vasculitis Activity Score. Arthritis Rheum 44: 912–920, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Cohen CD, Frach K, Schlöndorff D, Kretzler M: Quantitative gene expression analysis in renal biopsies: a novel protocol for a high-throughput multicenter application. Kidney Int 61: 133–140, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Martini S, Nair V, Keller BJ, Eichinger F, Hawkins JJ, Randolph A, Böger CA, Gadegbeku CA, Fox CS, Cohen CD, Kretzler M European Renal cDNA Bank C-PROBE Cohort CKDGen Consortium : Integrative biology identifies shared transcriptional networks in CKD. J Am Soc Nephrol 25: 2559–2572, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen CD, Klingenhoff A, Boucherot A, Nitsche A, Henger A, Brunner B, Schmid H, Merkle M, Saleem MA, Koller KP, Werner T, Gröne HJ, Nelson PJ, Kretzler M: Comparative promoter analysis allows de novo identification of specialized cell junction-associated proteins. Proc Natl Acad Sci U S A 103: 5682–5687, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tusher VG, Tibshirani R, Chu G: Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A 98: 5116–5121, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.