Abstract
BACKGROUND:
Trial discontinuation and nonpublication represent potential waste in research resources and lead to compromises in medical evidence. Pediatric trials may be particularly vulnerable to these outcomes given the challenges encountered in conducting trials in children. We aimed to determine the prevalence of discontinuation and nonpublication of randomized clinical trials (RCTs) conducted in pediatric populations.
METHODS:
Retrospective, cross-sectional study of pediatric RCTs registered in ClinicalTrials.gov from 2008 to 2010. Data were collected from the registry and associated publications identified (final search on September 1, 2015).
RESULTS:
Of 559 trials, 104 (19%) were discontinued early, accounting for an estimated 8369 pediatric participants. Difficulty with patient accrual (37%) was the most commonly cited reason for discontinuation. Trials were less likely to be discontinued if they were funded by industry compared with academic institutions (odds ratio [OR] 0.46, 95% confidence interval [CI] 0.27–0.77). Of the 455 completed trials, 136 (30%) were not published, representing 69 165 pediatric participants. Forty-two unpublished trials posted results on ClinicalTrials.gov. Trials funded by industry were more than twice as likely to result in nonpublication at 24 and 36 months (OR 2.21, 95% CI 1.35–3.64; OR 3.12, 95% CI 1.6–6.08, respectively) and had a longer mean time to publication compared with trials sponsored by academia (33 vs 24 months, P < .001).
CONCLUSIONS:
In this sample of pediatric RCTs, discontinuation and nonpublication were common, with thousands of children exposed to interventions that did not lead to informative or published findings. Trial funding source was an important determinant of these outcomes, with both academic and industry sponsors contributing to inefficiencies.
What’s Known on This Subject:
Discontinuation and nonpublication of randomized clinical trials raise both ethical and scientific concerns. Previous research has shown that these practices are common among clinical trials performed in adults but are less well defined in pediatric populations.
What This Study Adds:
Our study is an extensive examination of discontinuation and nonpublication of pediatric RCTs. The high rates of both these outcomes indicate that there is substantial waste of both human and financial resources in current pediatric clinical research.
Randomized controlled trials (RCTs) provide the highest level of evidence to inform clinical practice and are dependent on the enrollment of human subjects who volunteer for participation, even though they may not directly benefit from the study findings.1 Trial sponsors have an obligation to participants not only to minimize potential harms but also to conduct research with the highest ethical standards and rigor and report study findings publicly and in a timely fashion.1–3 Failure to do so represents a breach of contract with participants and a waste of limited human and material resources. Furthermore, the nonpublication of trial findings compromises the available medical evidence by distorting the apparent safety and efficacy of interventions, and undermining clinical guidelines and evidence-based clinical practice.1,4–6
Over the past 2 decades, substantial investments have been made to increase transparency and accountability in human subject research. In 1997, the US Congress passed the Food and Drug Administration (FDA) Modernization Act, which mandates public access to information about clinical trials for patients with “serious or life-threatening” medical illnesses.7 This was followed by the establishment in 2000 of ClinicalTrials.gov, a Web-based, publicly available registry of clinical studies. A number of policies and regulations have made registration of interventional trials obligatory, and registration has become standard practice. ClinicalTrials.gov is the most comprehensive database of clinical trials conducted in the United States and internationally,8,9 and there is evidence suggesting that it has positively influenced the reporting of clinical trials involving human subjects.10,11
Despite these heightened ethical and legislative mandates, the discontinuation and nonpublication of clinical trials remains common.12–17 Pediatric trials may be particularly vulnerable to these outcomes because they face unique challenges in terms of concerns around testing interventions in children and the logistics of recruiting and consenting research subjects in collaboration with parents and caretakers. In addition, there has historically been more limited funding allotted to pediatric research, both by industry and nonprofit sponsors, posing additional challenges to the successful conduct of trials.18 Analyses of trial discontinuation and nonpublication have primarily focused on adult populations,12–16 and the prevalence of these outcomes for pediatric RCTs remains less well defined.17 Accordingly, the aim of this study was to determine the frequency of trial discontinuation and nonpublication for RCTs conducted in pediatric populations.
Methods
Data Source
We conducted a cross-sectional study of randomized pediatric clinical trials registered in ClinicalTrials.gov. Trial entries provide details on the study population, intervention type, start and completion dates, funding source, design characteristics, and current recruitment status. Investigators must periodically update the record,19 with public availability of archived versions tracking all changes and additions to the entry.
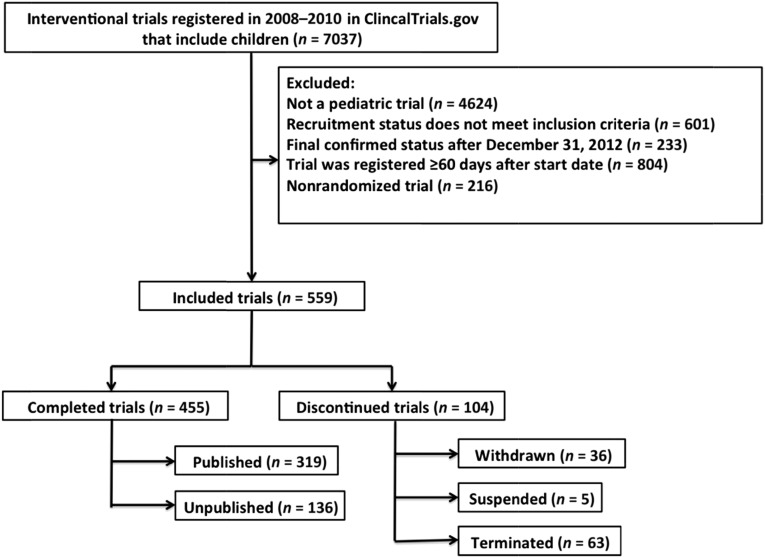
We limited our analyses to randomized trials studying children (birth–age 17 years) and registered between January 1, 2008, and December 31, 2010, and selected trials that were completed or had been discontinued by December 31, 2012 (Fig 1). This corresponded to recruitment statuses of “completed,” “terminated,” “withdrawn,” or “suspended” (Supplemental Table 7).20 Trials that were registered >60 days after the start date were excluded to avoid biases in the sample related to investigator decisions after initial trial findings.13
FIGURE 1.
Selection of pediatric randomized controlled trials.
The ClinicalTrials.gov query was performed on a single day (February 2, 2015) to account for ongoing updates to database records. The Institutional Review Board at Boston Children’s Hospital determined that this study was exempt from review.
Definitions and Data Characterization
Definitions for data elements in ClinicalTrials.gov were used as per the Glossary of Common Site Terms20 and ClinicalTrials.gov Protocol Data Element Definitions21 (Supplemental Table 7). Certain data elements were further categorized for the purposes of our analysis.
All conditions under study were classified using a modified version of the 2010 Global Burden of Disease hierarchical disease and injury cause list.22 The age of study participants was categorized into “preterm, newborn, and infant,” “toddler and preschool,” “school age,” “adolescent,” and “mixed ages” on the basis of the age eligibility criteria and the details provided in the trial description.
Organizations listed as sponsors and collaborators of a study considered the funders of the study (see Supplemental Table 7). In cases where >1 sponsor was listed, the lead sponsor was considered to be the primary funder.20,21,23 Primary funding sources are categorized in ClinicalTrials.gov as industry, government (National Institutes of Health and other US federal agencies), or “other,” which includes academic institutions, nonprofit research networks, and non–US government sponsors. We reviewed all trials that were designated as “other” and identified those that were funded by an academic institution to create a new funding variable. Trials were therefore considered to be funded by “industry,” “academic institutions,” or “other,” which included all government-funded trials (including non–US federal agencies), as well as the remaining trials originally labeled as “other.”
Trials were characterized as small (<100 participants), midsize (100–499 participants), or large (>500 participants).13 Archived entries of each trial were queried to extract the planned enrollment figures before commencement of subject recruitment and the actual number of enrolled participants at the time of trial completion or discontinuation.24
Time to publication was defined as the interval between “primary completion date” (Supplemental Table 7), and the date the publication appeared in print or as an electronic publication, whichever occurred first. If the primary completion date was missing (n = 2), the study “completion date” was used.25
Reasons for trial discontinuation were tabulated based on data provided in ClinicalTrials.gov entries and e-mail correspondence with study investigators when this information was missing or unclear. Conduct problems were defined as technical or logistical issues compromising trial completion. Difficulties with obtaining approval by institutional review boards or other regulatory bodies were considered regulatory issues. Trial discontinuation due to safety or efficacy findings, or changes in standard of care, were considered informative terminations.26
Publication Search
All trial entries were reviewed to identify publications automatically added to the trial record via the national clinical trial (NCT) identifier number. If a publication was not listed in the publication field of the entry, Medline was searched via PubMed independently by both authors (NP and FB) using NCT number, trial title, author names, institutions, and study keywords. Articles were linked to the corresponding trials based on comparison of the trial data provided in the registry entry and in the abstract or full manuscript, when necessary. If a publication was not identified in Medline, the same search protocol was used in Embase and GoogleScholar. For industry-sponsored trials, we also reviewed company Web sites for information on trial publications.
If we were unable to match a trial to a publication, we attempted to contact study investigators and sponsors to inquire about publication status. E-mail addresses were collected from the registry entries and from previous publications by the investigators. A standardized e-mail was sent, with 1 follow-up e-mail if no response was received.12 For trials that listed only a sponsoring company name, responsible individuals were contacted by e-mail, online form, or telephone as per company Web site instructions.
We considered a trial published if it was associated with a peer-reviewed manuscript describing trial findings.12,13 Trials were considered unpublished if we were unable to identify a publication and trial investigators informed us that the trial was unpublished, did not respond to our inquiries, or did not have valid contact information that we were able to locate.12 A final search for all trials without publications was completed on September 1, 2015, allowing for a minimum of 32 months for manuscript submission, review, and publication.
Statistical Analyses
χ2 tests were used to assess associations between trial characteristics and trial completion and publication status. We used logistic regression models to assess the impact of primary funding source on trial discontinuation and on trial nonpublication at 24 and 36 months after trial completion, controlling for trial design variables previously shown to impact trial completion and eventual publication.12–14 These variables were prespecified and included intervention type, age of study participants, masking, and sample size. Age of study participants was not found to be significant in any of the models and was dropped from the final models to minimize overfitting. This did not substantially change the results. Student t test was used to compare time to publication according to funding source. Statistical significance was set at P < .05. All statistical analyses were conducted by using SAS (version 9.4, SAS Institute, Inc, Cary, NC).
Results
Trial Characteristics
We identified 559 randomized pediatric clinical trials that met inclusion criteria for our analysis (Fig 1). Nearly a quarter of all trials studied childhood vaccines and another 8% examined other interventions for common childhood infectious diseases (Table 1). Drugs/biologics (67.8%) were the most frequently investigated interventions (Table 2). The predominant sources of funding were academic institutions (43.8%) and industry (48.7%). Fifty-three percent of trials were designed as double-blind studies, with 33.5% conducted as open-label trials. The median planned sample size was 159 (interquartile range 60–424) with 37.9% of trials anticipating enrollment of <100 participants and 21.5% planning to enroll >500 participants.
TABLE 1.
Disease Categories Addressed in 559 Pediatric RCTs
| Condition Category | Pediatric Trials, n (%) |
|---|---|
| Allergy and atopy | 18 (3.2) |
| Anesthesia, critical care, and surgery | 34 (6.1) |
| Cardiovascular and circulatory diseases | 18 (3.2) |
| Chronic respiratory disease | 25 (4.5) |
| Common childhood vaccines | 135 (24.2) |
| Diabetes and endocrine diseases | 40 (7.2) |
| Diarrheal illnesses, lower respiratory infections, meningitis, and other common infectious diseases | 45 (8.1) |
| Digestive and liver disease | 13 (2.3) |
| Genetic and metabolic disease | 9 (1.6) |
| HIV/AIDS and tuberculosis | 9 (1.6) |
| Mental and behavioral disorders | 63 (11.3) |
| Musculoskeletal disorders | 6 (1.1) |
| Neglected tropical diseases | 26 (4.7) |
| Neonatal and infant medicine | 56 (10.0) |
| Neoplasm | 8 (1.4) |
| Neurologic disorders | 8 (1.4) |
| Nutrition and nutritional deficiencies | 27 (4.8) |
| Unintentional injuries | 6 (1.1) |
| Other | 13 (2.3) |
TABLE 2.
Characteristics of Completed and Discontinued RCTs
| All Trials (n = 559), n (%) | Completed Trials (n = 455), n (%) | Discontinued Trials (n = 104), n (%) | P | |
|---|---|---|---|---|
| Intervention | .81 | |||
| Behavioral | 55 (9.8) | 47 (10) | 8 (7.7) | |
| Drug/Biologic | 379 (67.8) | 304 (66.8) | 75 (72.1) | |
| Device/Procedure | 37 (6.6) | 32 (7.0) | 5 (4.8) | |
| Dietary supplement | 45 (8.1) | 37 (8.1) | 8 (7.7) | |
| Other | 43 (7.7) | 35 (7.7) | 8 (7.7) | |
| Age of study participants | .60 | |||
| Preterm, newborn, and infant | 145 (25.9) | 119 (26.2) | 26 (25.0) | |
| Toddler and preschool | 119 (21.3) | 101 (22.2) | 18 (17.3) | |
| School age | 68 (12.2) | 57 (12.5) | 11 (10.6) | |
| Adolescent | 60 (10.7) | 46 (10.1) | 14 (13.5) | |
| Mixed ages | 167 (29.9) | 132 (29.0) | 35 (33.7) | |
| Primary funding source | .044 | |||
| Academic institution | 245 (43.8) | 188 (41.3) | 57 (54.8) | |
| Industry | 272 (48.7) | 231 (50.8) | 41 (39.4) | |
| Other | 42 (7.5) | 36 (7.9) | 6 (5.8) | |
| Year registered | .84 | |||
| 2008 | 199 (35.6) | 161 (35.4) | 38 (36.5) | |
| 2009 | 202 (36.1) | 163 (35.8) | 39 (37.5) | |
| 2010 | 158 (28.3) | 131 (28.8) | 27 (26.0) | |
| Trial phasea | .16 | |||
| Phase 1 | 29 (5.2) | 26 (5.7) | 3 (2.9) | |
| Phase 2 | 115 (20.6) | 89 (19.6) | 26 (25.0) | |
| Phase 3 | 186 (33.3) | 159 (35.0) | 27 (26.0) | |
| Phase 4 | 81 (14.5) | 61 (13.4) | 20 (19.2) | |
| Unknown | 148 (26.5) | 120 (26.4) | 28 (26.9) | |
| Masking | .22 | |||
| Open label | 187 (33.5) | 155 (34.1) | 32 (30.8) | |
| Single blind | 77 (13.8) | 67 (14.7) | 10 (9.6) | |
| Double bind | 295 (52.8) | 233 (51.2) | 62 (59.6) | |
| Planned sample size | <.001 | |||
| <100 participants | 212 (37.9) | 161 (35.4) | 51 (49.0) | |
| 100–499 participants | 227 (40.6) | 182 (40.0) | 45 (43.3) | |
| >500 participants | 120 (21.5) | 112 (24.6) | 8 (7.7) |
Phase 0 trials (n = 5) included as phase 1. Trials described as phase 1/2 (n = 17) were categorized as phase 2, and trials described as phase 2/3 (n = 18) were categorized as phase 3.
Discontinuation of Pediatric Clinical Trials
A total of 104 trials (19%) were discontinued. Thirty-six were withdrawn before participant recruitment, whereas 5 were suspended and 63 terminated after participants had already been enrolled. In total, an estimated 8369 children were enrolled in trials that were never completed. Patient accrual (n = 38, 36.5%) was cited to be the most common reason, followed by conduct problems (n = 13, 12.5%) and informative termination, (n = 13, 12.5%). Notably, funding issues were the least likely to be cited as reasons for trial discontinuation (n = 5, 4.8%; Table 3).
TABLE 3.
Reasons for Discontinuation of 104 RCTs
| Reason | n, (%) |
|---|---|
| Patient accrual | 38 (36.5) |
| Conduct problemsa | 13 (12.5) |
| Informative terminationb | 13 (12.5) |
| Company/business decision | 9 (8.7) |
| Principle investigator left | 8 (7.7) |
| Regulatory issuec | 8 (7.7) |
| Funding issue | 5 (4.8) |
| None reported or unclear | 10 (9.6) |
Includes technical difficulties and logistical issues.
Includes changes in standard of care and safety or efficacy findings.
Includes issues with institutional review board or other regulatory body, including the FDA.
In univariate analyses, primary funding source (P = .044), and planned sample size (P < .001) were found to be significant determinants of trial discontinuation (Table 2). Fewer trials funded by industry were discontinued (39.4%) compared with those with academic affiliations (54.8%). In multivariate analysis, funding source and sample size remained significant determinants of trial discontinuation (Table 4). Trials primarily funded by industry were less likely to result in discontinuation compared with those funded by academic sources (odds ratio [OR] 0.46, 95% confidence interval [CI] 0.27–0.77, P = .004). Larger trials were also less likely to be discontinued (OR 0.999, 95% CI 0.998–1.0).
TABLE 4.
Multivariate Analysis of Factors Associated With Discontinuation of RCTs
| OR (95% CI) | P | |
|---|---|---|
| Intervention | ||
| Behavioral | Reference | |
| Drug/biologic | 2.15 (0.88–5.23) | .09 |
| Device/procedure | 0.95 (0.28–3.24) | .94 |
| Dietary supplement | 1.15 (0.37–3.60) | .81 |
| Other | 1.54 (0.50–4.70) | .45 |
| Primary funding source | ||
| Academic institution | Reference | |
| Industry | 0.46 (0.27–0.77) | .004 |
| Other | 0.47 (0.18–1.22) | .12 |
| Masking | ||
| Open label | Reference | |
| Single blind | 0.62 (0.28–1.39) | .25 |
| Double blind | 0.71 (0.67–1.82) | .72 |
| Planned sample size/enrollment | 0.999 (0.998–1.00) | .008 |
Nonpublication of Pediatric Clinical Trials
Among all trials that were completed, 136 (29.8%) remained unpublished after a mean of 58 months between trial completion and publication search. These trials accounted for an estimated 69 165 pediatric trial participants, representing 27% of the total study population among completed trials. Among these unpublished trials, 42 (30.8%) had results posted in the registry.
In univariate analyses, intervention type and primary funding source were found to be associated with nonpublication at 24 months; at 36 months, primary funding source was associated with nonpublication (Table 5). In multivariate analyses, industry funding was associated with a greater than twofold increase in the odds of nonpublication at 24 months compared with academic institutions (OR 2.21, 95% CI 1.35–3.64; P = .002) and a greater than threefold increase at 36 months (OR 3.12, 95% CI 1.60–6.08, P < .001) (Table 6).
TABLE 5.
Characteristics of Published and Unpublished RCTs 24 and 36 Months After Trial Completion
| Published at 24 mo (n = 262), n (%) | Unpublished at 24 mo (n = 193), n (%) | P | Published at 36 moa (n = 354), n (%) | Unpublished at 36 moa (n = 95), n (%) | P | |
|---|---|---|---|---|---|---|
| Intervention | .01 | .11 | ||||
| Behavioral | 34 (13.0) | 13 (6.7) | 41 (11.6) | 5 (5.3) | ||
| Drug/biologic | 159 (60.7) | 145 (75.1) | 226 (63.8) | 73 (76.8) | ||
| Device/procedure | 24 (9.2) | 8 (4.2) | 29 (8.2) | 3 (3.2) | ||
| Dietary supplement | 23 (8.8) | 14 (7.3) | 30 (8.5) | 7 (7.4) | ||
| Other | 22 (8.4) | 13 (6.7) | 28 (7.9) | 7 (7.4) | ||
| Age of study participants | .60 | .64 | ||||
| Preterm, newborn, and infant | 67 (25.6) | 52 (26.9) | 87 (24.6) | 31 (32.6) | ||
| Toddler and preschool | 64 (24.4) | 37 (19.2) | 80 (22.6) | 19 (20.0) | ||
| School age | 34 (13.0) | 23 (11.9) | 46 (13.0) | 11 (11.6) | ||
| Adolescent | 23 (8.8) | 23 (11.9) | 37 (10.5) | 9 (9.5) | ||
| Mixed ages | 74 (28.2) | 58 (30.1) | 104 (29.4) | 25 (26.3) | ||
| Primary funding source | <.001 | <.001 | ||||
| Academic institution | 130 (49.6) | 58 (30.1) | 164 (46.3) | 22 (23.2) | ||
| Industry | 110 (42.0) | 121 (62.7) | 162 (45.8) | 66 (69.5) | ||
| Other | 22 (8.4) | 14 (7.3) | 28 (7.9) | 7 (7.4) | ||
| Trial phaseb | .16 | .06 | ||||
| Phase 1 | 14 (5.3) | 12 (6.2) | 18 (5.1) | 7 (7.4) | ||
| Phase 2 | 53 (20.2) | 36 (18.7) | 67 (18.9) | 20 (21.1) | ||
| Phase 3 | 81 (30.9) | 78 (40.4) | 120 (33.9) | 38 (40.0) | ||
| Phase 4 | 35 (13.4) | 26 (13.5) | 44 (12.4) | 16 (16.8) | ||
| Unknown | 79 (30.2) | 41 (21.2) | 105 (29.7) | 14 (3.1) | ||
| Masking | .29 | .19 | ||||
| Open label | 94 (35.9) | 61 (31.6) | 124 (35.0) | 29 (30.5) | ||
| Single blind | 42 (16.0) | 25 (13.0) | 56 (15.8) | 10 (10.5) | ||
| Double blind | 126 (48.1) | 107 (55.4) | 174 (49.2) | 56 (59.0) | ||
| Actual sample size | .07 | .64 | ||||
| <100 participants | 109 (41.6) | 60 (31.1) | 134 (37.9) | 32 (33.7) | ||
| 100–499 participants | 96 (36.6) | 85 (44.0) | 138 (39.0) | 42 (44.2) | ||
| >500 participants | 57 (21.8) | 48 (24.9) | 82 (23.2) | 21 (22.1) |
Six trials were excluded from this analysis because 36 mo had not elapsed between completion date and time that publication search was conducted.
Phase 0 trials (n = 5) were included as Phase 1. Trials described as phase 1/2 (n = 15) were categorized as phase 2, and trials described as phase 2/3 (n = 15) were categorized as phase 3.
TABLE 6.
Multivariate Analysis of Factors Associated with Nonpublication of RCTs at 24 and 36 months After Trial Completion
| Unpublished at 24 mo, OR (95% CI) | P | Unpublished at 36 mo, OR (95% CI)a | P | |
|---|---|---|---|---|
| Intervention | ||||
| Behavioral | Reference | Reference | ||
| Drug/biologic | 1.36 (0.61–2.99) | .45 | 1.09 (0.36–3.35) | .88 |
| Device/procedure | 0.81 (0.29–2.28) | .69 | 0.72 (0.15–3.33) | .67 |
| Dietary supplement | 1.50 (0.57–3.94) | .42 | 1.66 (0.45–6.10) | .44 |
| Other | 1.12 (0.41–3.02) | .83 | 1.13 (0.30–4.35) | .85 |
| Primary funding source | ||||
| Academia | Reference | Reference | ||
| Industry | 2.21 (1.35–3.64) | .002 | 3.12 (1.60–6.08) | <.001 |
| Other | 1.37 (0.64–2.93) | .42 | 1.95 (0.74–5.15) | .18 |
| Masking | ||||
| Open label | Reference | Reference | ||
| Single blind | 1.15 (0.62–2.16) | .65 | 0.97 (0.43–2.20) | .94 |
| Double blind | 1.19 (0.77–1.84) | .44 | 1.26 (0.75–2.14) | .38 |
| Enrolled participants/ actual sample size | 1.00 (1.00–1.00) | .82 | 1.00 (1.00–1.00) | .31 |
Six trials were excluded from this analysis because 36 mo had not elapsed between completion date and time that publication search was conducted.
The mean time to publication for all trials was 29 months (95% CI 28–31 months), with a longer mean time to publication for trials funded by industry compared with academic institutions (33 months vs 24 months, respectively, P < .001).
Discussion
Our study demonstrates that among interventional trials conducted in children, trial discontinuation and nonpublication are common. We found that 19% of trials were discontinued, two-thirds of which had already enrolled participants at the time of trial termination. Poor recruitment and problems with the conduct of the trial were among the most commonly reported reasons for trial discontinuation. Furthermore, after a mean of 58 months since completion, 30% of trials remained unpublished. In all, >69 000 children, representing nearly a third of the total population enrolled in completed trials, were exposed to interventions without subsequent publication of trial findings. Our study shows that trial sponsors were an important determinant of these outcomes, with trials funded by industry less likely to be discontinued but more likely to remain unpublished 24 and 36 months after trial completion.
Children have historically been underrepresented in clinical trials compared with adults, and a number of FDA policies aim to incentivize and increase the study of interventions in pediatric populations. These include the Best Pharmaceuticals for Children Act and the Pediatric Research Equity Act, which have been credited with increasing the number of pediatric drug trials and the number of drug labels that contain pediatric safety and efficacy information.18,27,28 Our findings indicate that once trials are initiated, additional focus is needed to maximize the knowledge gain from pediatric trial participation.
A number of trials were discontinued for reasons considered informative, including preliminary safety and efficacy findings or changes in the standard of care that occurred after the trial had been initiated. Such termination likely prevents further wasted resources and may be unavoidable at a certain baseline rate. However, there may be opportunities to reduce noninformative trial discontinuation, such as poor patient accrual and technical or logistical issues with trial conduct. Difficulties with trial enrollment have previously been documented among adult trials and cited as the most common factor for trial discontinuation.12,14,26 The rate of discontinuation of pediatric trials (19%) was comparable to rates found in adult populations (21%–25%),12,14 indicating that the potential challenges encountered with pediatric patient recruitment do not appear to increase the odds of trial discontinuation.
Trials sponsored by industry were less likely to be discontinued. This may be related to additional financial and human resources available in industry-funded trials, such as research coordinators to manage patient recruitment or technical infrastructure to facilitate trial conduct. For academic trials in particular, investigators and research oversight committees should be accountable for ensuring that clinical trials are feasible and have the material and human resources available to achieve the proposed goals.
The nonpublication of trial findings represents a violation of the ethical imperative to share results of trials that involve human subjects and also introduces publication bias into the medical literature.1,4–6 Nonpublication has been examined across a range of trial types, with rates predominantly between 25% and 35%.12–17 This is consistent with our findings of a nonpublication rate of 30%. Trial nonpublication is particularly concerning given the limited availability of volunteers for clinical trials and the high rates of trial termination due to difficulties in participant accrual. Similar to previous work, we found that industry sponsorship was associated with nonpublication and delay in publication of trial results.6,12–14,16,17 However, nonpublication was also high for trials funded by academic institutions, which arguably have an even greater mandate to uphold the standards of clinical trial reports underpinning evidence-based clinical decision-making. This finding is in accordance with previous work that has shown poor rates of dissemination of clinical trial findings across leading academic medical centers.29
The Declaration of Helsinki, which is the central document governing regulation of human subjects research, states that investigators are responsible for the public dissemination of trial results involving human participants, regardless of the findings.3 There is some evidence that trial registration has contributed to an increase in the publication of trials with negative results, thus curbing publication bias related to preferential reporting of positive findings.10,11 However, given persistent high rates of trial nonpublication across funding types, additional mechanisms are needed to increase trial publication or make trial results publicly available to facilitate analysis and reporting by other investigators. One such initiative is RIAT (Restoring Invisible and Abandoned Trials), which has garnered support from a number of high-profile journals.30 This proposal invites researchers with unpublished trials to signal their intent to publish the trial within a year or else provide public access to their trial results and offer the opportunity to independent investigators to become “restorative authors.”31,32
It is of note that the FDA Amendments Act of 2007 requires study results to be reported but does not specifically require publication. Trials can therefore fulfill this mandate through results reporting in ClinicalTrials.gov. Of note, 42 of the 136 unpublished trials reported results on ClinicalTrials.gov, although these data were not always interpretable because they were often without statistical analysis or without clarification of the hypothesis tested, making it difficult to draw substantive conclusions. Although any dissemination of results has value, we chose to focus on whether trial results were published in peer-reviewed journals because this represents the most widely accessible and commonly used information source for physicians seeking to apply trial results to their clinical practice. The peer-review process also ensures that trial results are rigorously scrutinized and ensures appropriate interpretation of the results.
Several limitations should be noted when interpreting our findings. This study analyzed only trials registered in ClincialTrials.gov; it is possible that there were additional pediatric interventional trials that were not captured in our analysis. The rate of nonregistration of pediatric trials is unknown, but it is unlikely that these trials would have a higher rate of completion or publication given federal and editorial policies mandating registration. It should also be noted that information in the registry is provided by investigators and sponsors, and we were not able to verify the accuracy of the trial data. This issue is mitigated in part by automated data validity checks and manual review by ClinicalTrials.gov staff to ensure data accuracy before public posting.33 There were missing data in the registry such as trial phase and reasons for trial discontinuation, which we were unable to complete despite efforts to contact investigators. Finally, it is possible that we did not identify all publications associated with trials in our cohort. However, we employed a rigorous approach, consisting of a standardized search protocol applied to 3 separate publication databases and performed by 2 investigators independently. These searches were further augmented with investigator queries, making missed publications unlikely.
Conclusions
We have found that pediatric clinical trials are frequently discontinued or the results are not published. Thousands of children have participated in these trials, representing considerable inefficiencies and waste of financial and human resources. Although policies and initiatives have been implemented to increase the number of pediatric trials and improve the standards of trial reporting overall, further action is needed to ensure that the participation of all children in clinical trials contributes to our scientific knowledge.
Acknowledgments
All authors have completed the International Committee of Medical Journal Editors uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; and no other relationships or activities that could appear to have influenced the submitted work.
Glossary
- CI
confidence interval
- FDA
Food and Drug Administration
- NCT
national clinical trial
- OR
odds ratio
- RCT
randomized controlled trial
Footnotes
Drs Pica and Bourgeois conceptualized and designed the study, carried out data collection and initial analyses, drafted the initial manuscript, reviewed and revised the manuscript, and approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Dr Bourgeois was supported by a grant from the National Institute of Child Health and Human Development (1R21HD072382). Dr Pica was supported by the Fred Lovejoy House-Staff Research and Education Fund at Boston Children’s Hospital. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.DeAngelis CD, Drazen JM, Frizelle FA, et al. ; International Committee of Medical Journal Editors . Clinical trial registration: a statement from the International Committee of Medical Journal Editors. JAMA. 2004;292(11):1363–1364 [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization WHO statement on public disclosure of clinical trial results. International Clinical Trials Registry Platform (ICRTP). 2015. Available at: www.who.int/ictrp/results/reporting/en/. Accessed June 6, 2015
- 3.World Medical Association WMA Declaration of Helsinki—ethical principles for medical research involving human subjects. 2013. Available at: www.wma.net/en/30publications/10policies/b3/. Accessed June 6, 2015
- 4.Zarin DA, Tse T. Medicine. Moving toward transparency of clinical trials. Science. 2008;319(5868):1340–1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mathieu S, Boutron I, Moher D, Altman DG, Ravaud P. Comparison of registered and published primary outcomes in randomized controlled trials. JAMA. 2009;302(9):977–984 [DOI] [PubMed] [Google Scholar]
- 6.Bourgeois FT, Murthy S, Mandl KD. Outcome reporting among drug trials registered in ClinicalTrials.gov. Ann Intern Med. 2010;153(3):158–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.US National Institutes of Health History, policies and laws (ClinicaTrials.gov). 2015. Available at: https://clinicaltrials.gov/ct2/about-site/history. Accessed June 3, 2015
- 8.US National Library of Medicine National Institutes of Health Launches ClinicalTrials.gov Results Database. 2013. Available at: www.nlm.nih.gov/news/expanded_clinicaltrials.html. Accessed June 12, 2015
- 9.ClinicalTrials.gov Available at: https://clinicaltrials.gov/. Accessed June 16, 2015
- 10.Emdin C, Odutayo A, Hsiao A, et al. Association of cardiovascular trial registration with positive study findings: Epidemiological Study of Randomized Trials (ESORT). JAMA Intern Med. 2015;175(2):304–307 [DOI] [PubMed] [Google Scholar]
- 11.Kaplan RM, Irvin VL. Likelihood of null effects of large NHLBI clinical trials has increased over time. PLoS One. 2015;10(8):e0132382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chapman SJ, Shelton B, Mahmood H, Fitzgerald JE, Harrison EM, Bhangu A. Discontinuation and non-publication of surgical randomised controlled trials: observational study. BMJ. 2014;349:g6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones CW, Handler L, Crowell KE, Keil LG, Weaver MA, Platts-Mills TF. Non-publication of large randomized clinical trials: cross sectional analysis. BMJ. 2013;347:f6104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kasenda B, von Elm E, You J, et al. Prevalence, characteristics, and publication of discontinued randomized trials. JAMA. 2014;311(10):1045–1051 [DOI] [PubMed] [Google Scholar]
- 15.Manzoli L, Flacco ME, D’Addario M, et al. Non-publication and delayed publication of randomized trials on vaccines: survey. BMJ. 2014;348:g3058. [DOI] [PubMed] [Google Scholar]
- 16.Ross JS, Mulvey GK, Hines EM, Nissen SE, Krumholz HM. Trial publication after registration in ClinicalTrials.Gov: a cross-sectional analysis. PLoS Med. 2009;6(9):e1000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shamliyan T, Kane RL. Clinical research involving children: registration, completeness, and publication. Pediatrics. 2012;129(5). Available at: www.pediatrics.org/cgi/content/full/129/5/e1291 [DOI] [PubMed] [Google Scholar]
- 18.Congressional Childhood Cancer Caucus BPCA and PREA reauthorization. 2011. Available at: http://childhoodcancer-mccaul.house.gov/issue/bpca-and-prea-reauthorization. Accessed July 2, 2015
- 19.US National Institutes of Health FDAAA 801 requirements. 2015. Available at: https://clinicaltrials.gov/ct2/manage-recs/fdaaa. Accessed June 3, 2015
- 20.US National Institutes of Health Glossary of common site terms. 2015. Available at: https://clinicaltrials.gov/ct2/about-studies/glossary. Accessed June 2, 2015, 2015.
- 21.US National Institutes of Health ClinicalTrials.gov protocol data element definitions. 2015. Available at: http://prsinfo.clinicaltrials.gov/definitions.html. Accessed June 2, 2015
- 22.Murray CJ, Ezzati M, Flaxman AD, et al. GBD 2010: design, definitions, and metrics. Lancet. 2012;380(9859):2063–2066 [DOI] [PubMed] [Google Scholar]
- 23.Califf RM, Zarin DA, Kramer JM, Sherman RE, Aberle LH, Tasneem A. Characteristics of clinical trials registered in ClinicalTrials.gov, 2007–2010. JAMA. 2012;307(17):1838–1847 [DOI] [PubMed] [Google Scholar]
- 24.Williams RJ, Tse T, DiPiazza K, Zarin DA. Terminated trials in the ClinicalTrials.gov results database: evaluation of availability of primary outcome data and reasons for termination. PLoS One. 2015;10(5):e0127242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderson ML, Chiswell K, Peterson ED, Tasneem A, Topping J, Califf RM. Compliance with results reporting at ClinicalTrials.gov. N Engl J Med. 2015;372(11):1031–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carlisle B, Kimmelman J, Ramsay T, MacKinnon N. Unsuccessful trial accrual and human subjects protections: an empirical analysis of recently closed trials. Clin Trials. 2015;12(1):77–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benjamin DK Jr, Smith PB, Sun MJ, et al. Safety and transparency of pediatric drug trials. Arch Pediatr Adolesc Med. 2009;163(12):1080–1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Field MJ, Boat TF, eds. Safe and Effective Medicines for Children: Pediatric Studies Conducted Under the Best Pharmaceuticals for Children Act and the Pediatric Research Equity Act. Washington, DC: National Academies Press; 2012 [PubMed] [Google Scholar]
- 29.Chen R, Desai NR, Ross JS, et al. Publication and reporting of clinical trial results: cross sectional analysis across academic medical centers. BMJ. 2016;352:i637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loder E, Godlee F, Barbour V, Winker M; PLOS Medicine editors . Restoring the integrity of the clinical trial evidence base. BMJ. 2013;346:f3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doshi P, Dickersin K, Healy D, Vedula SS, Jefferson T. Restoring invisible and abandoned trials: a call for people to publish the findings. BMJ. 2013;346:f2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Treasure T, Monson K, Fiorentino F, Russell C. The CEA Second-Look Trial: a randomised controlled trial of carcinoembryonic antigen prompted reoperation for recurrent colorectal cancer. BMJ Open. 2014;4(5):e004385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.US National Library of Medicine . ClinicalTrials.gov and related projects: improving access to information about clinical studies [press release LHNCBC2013]