Abstract
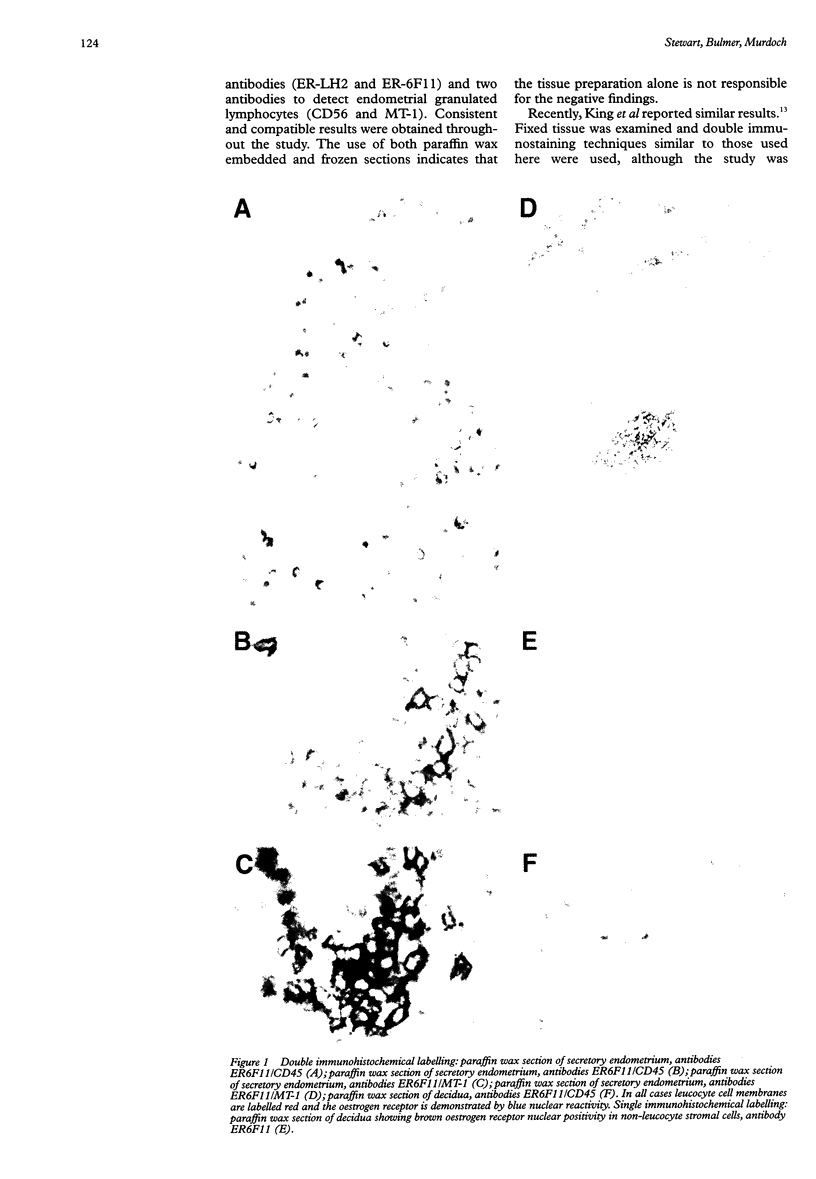
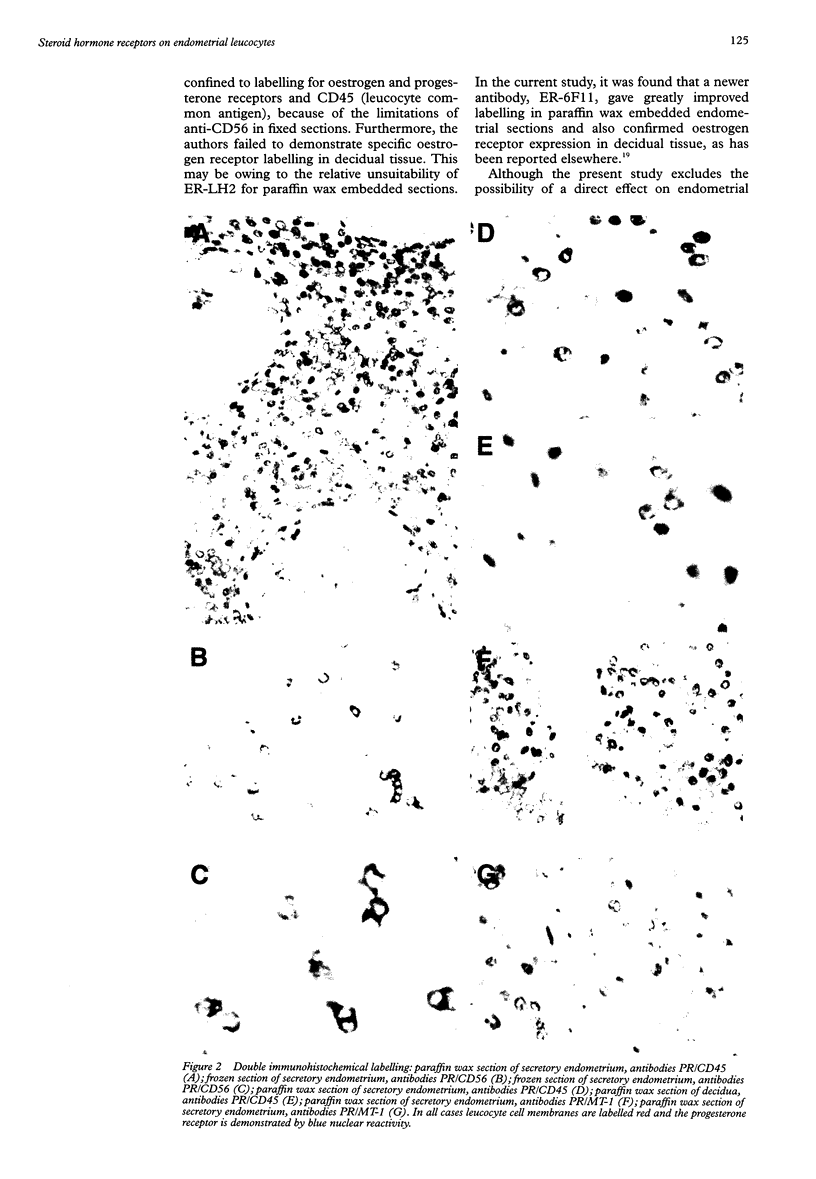
BACKGROUND: Stromal leucocyte populations in human endometrium comprise T cells, macrophages, and phenotypically unusual endometrial granulated lymphocytes. Their proportions vary during the menstrual cycle and, in particular, endometrial granulated lymphocytes increase in number in the late secretory phase. The stimulus responsible for these cyclical changes is unknown but it is likely that the steroid hormones oestrogen and progesterone play a role. AIMS: To define further the expression of steroid hormone receptors by leucocytes in non-pregnant and pregnant human endometrium. METHODS: Frozen and paraffin wax embedded sections of endometrium from non-pregnant women and early pregnancy decidua were labelled using single and double immunohistochemical techniques with monoclonal antibodies directed against oestrogen and progesterone receptors and various leucocyte subpopulations. RESULTS: Despite the prominence of CD56 positive endometrial granulated lymphocytes in late secretory phase endometrium and early pregnancy decidua, double immunohistochemical labelling showed no evidence of expression of either progesterone or oestrogen receptors by these cells or other endometrial leucocyte populations. CONCLUSIONS: Rather than acting directly, steroid hormones are likely to influence endometrial leucocyte populations indirectly via products of endometrial stromal or epithelial cells that express steroid hormone receptors.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amso N. N., Crow J., Shaw R. W. Comparative immunohistochemical study of oestrogen and progesterone receptors in the fallopian tube and uterus at different stages of the menstrual cycle and the menopause. Hum Reprod. 1994 Jun;9(6):1027–1037. doi: 10.1093/oxfordjournals.humrep.a138628. [DOI] [PubMed] [Google Scholar]
- Bulmer J. N., Hollings D., Ritson A. Immunocytochemical evidence that endometrial stromal granulocytes are granulated lymphocytes. J Pathol. 1987 Nov;153(3):281–288. doi: 10.1002/path.1711530313. [DOI] [PubMed] [Google Scholar]
- Bulmer J. N., Morrison L., Longfellow M., Ritson A., Pace D. Granulated lymphocytes in human endometrium: histochemical and immunohistochemical studies. Hum Reprod. 1991 Jul;6(6):791–798. doi: 10.1093/oxfordjournals.humrep.a137430. [DOI] [PubMed] [Google Scholar]
- Cattoretti G., Pileri S., Parravicini C., Becker M. H., Poggi S., Bifulco C., Key G., D'Amato L., Sabattini E., Feudale E. Antigen unmasking on formalin-fixed, paraffin-embedded tissue sections. J Pathol. 1993 Oct;171(2):83–98. doi: 10.1002/path.1711710205. [DOI] [PubMed] [Google Scholar]
- Coppens M. T., Dhont M. A., De Boever J. G., Serreyn R. F., Vandekerckhove D. A., Roels H. J. The distribution of oestrogen and progesterone receptors in the human endometrial basal and functional layer during the normal menstrual cycle. An immunocytochemical study. Histochemistry. 1993 Feb;99(2):121–126. doi: 10.1007/BF00571872. [DOI] [PubMed] [Google Scholar]
- Good R. G., Moyer D. L. Estrogen-progesterone relationships in the development of secretory endometrium. Fertil Steril. 1968 Jan-Feb;19(1):37–49. doi: 10.1016/s0015-0282(16)36543-8. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Kelly R. W., Illingworth P., Baldie G., Leask R., Brouwer S., Calder A. A. Progesterone control of interleukin-8 production in endometrium and chorio-decidual cells underlines the role of the neutrophil in menstruation and parturition. Hum Reprod. 1994 Feb;9(2):253–258. doi: 10.1093/oxfordjournals.humrep.a138491. [DOI] [PubMed] [Google Scholar]
- King A., Gardner L., Loke Y. W. Evaluation of oestrogen and progesterone receptor expression in uterine mucosal lymphocytes. Hum Reprod. 1996 May;11(5):1079–1082. doi: 10.1093/oxfordjournals.humrep.a019300. [DOI] [PubMed] [Google Scholar]
- King A., Wellings V., Gardner L., Loke Y. W. Immunocytochemical characterization of the unusual large granular lymphocytes in human endometrium throughout the menstrual cycle. Hum Immunol. 1989 Mar;24(3):195–205. doi: 10.1016/0198-8859(89)90060-8. [DOI] [PubMed] [Google Scholar]
- Klentzeris L. D., Bulmer J. N., Warren A., Morrison L., Li T. C., Cooke I. D. Endometrial lymphoid tissue in the timed endometrial biopsy: morphometric and immunohistochemical aspects. Am J Obstet Gynecol. 1992 Sep;167(3):667–674. doi: 10.1016/s0002-9378(11)91568-3. [DOI] [PubMed] [Google Scholar]
- Klentzeris L. D., Bulmer J. N., Warren M. A., Morrison L., Li T. C., Cooke I. D. Lymphoid tissue in the endometrium of women with unexplained infertility: morphometric and immunohistochemical aspects. Hum Reprod. 1994 Apr;9(4):646–652. doi: 10.1093/oxfordjournals.humrep.a138564. [DOI] [PubMed] [Google Scholar]
- Laird S. M., Li T. C., Bolton A. E. The production of placental protein 14 and interleukin 6 by human endometrial cells in culture. Hum Reprod. 1993 Jun;8(6):793–798. doi: 10.1093/oxfordjournals.humrep.a138144. [DOI] [PubMed] [Google Scholar]
- Laird S. M., Tuckerman E., Li T. C., Bolton A. E. Stimulation of human endometrial epithelial cell interleukin 6 production by interleukin 1 and placental protein 14. Hum Reprod. 1994 Jul;9(7):1339–1343. doi: 10.1093/oxfordjournals.humrep.a138706. [DOI] [PubMed] [Google Scholar]
- Li T. C., Rogers A. W., Dockery P., Lenton E. A., Cooke I. D. A new method of histologic dating of human endometrium in the luteal phase. Fertil Steril. 1988 Jul;50(1):52–60. doi: 10.1016/s0015-0282(16)60008-0. [DOI] [PubMed] [Google Scholar]
- Monterroso V. H., Hansen P. J. Regulation of bovine and ovine lymphocyte proliferation by progesterone: modulation by steroid receptor antagonists and physiological status. Acta Endocrinol (Copenh) 1993 Dec;129(6):532–535. doi: 10.1530/acta.0.1290532. [DOI] [PubMed] [Google Scholar]
- Neifeld J. P., Lippman M. E., Tormey D. C. Steroid hormone receptors in normal human lymphocytes. Induction of glucocorticoid receptor activity by phytohemagglutinin stimulation. J Biol Chem. 1977 May 10;252(9):2972–2977. [PubMed] [Google Scholar]
- Norton A. J. Microwave oven heating for antigen unmasking in routinely processed tissue sections. J Pathol. 1993 Oct;171(2):79–80. doi: 10.1002/path.1711710203. [DOI] [PubMed] [Google Scholar]
- Pace D., Morrison L., Bulmer J. N. Proliferative activity in endometrial stromal granulocytes throughout menstrual cycle and early pregnancy. J Clin Pathol. 1989 Jan;42(1):35–39. doi: 10.1136/jcp.42.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Press M. F., Udove J. A., Greene G. L. Progesterone receptor distribution in the human endometrium. Analysis using monoclonal antibodies to the human progesterone receptor. Am J Pathol. 1988 Apr;131(1):112–124. [PMC free article] [PubMed] [Google Scholar]
- Starkey P. M., Clover L. M., Rees M. C. Variation during the menstrual cycle of immune cell populations in human endometrium. Eur J Obstet Gynecol Reprod Biol. 1991 May 10;39(3):203–207. doi: 10.1016/0028-2243(91)90058-s. [DOI] [PubMed] [Google Scholar]
- Szekeres-Bartho J., Szekeres G., Debre P., Autran B., Chaouat G. Reactivity of lymphocytes to a progesterone receptor-specific monoclonal antibody. Cell Immunol. 1990 Feb;125(2):273–283. doi: 10.1016/0008-8749(90)90083-4. [DOI] [PubMed] [Google Scholar]
- Tabibzadeh S. S., Satyaswaroop P. G. Sex steroid receptors in lymphoid cells of human endometrium. Am J Clin Pathol. 1989 Jun;91(6):656–663. doi: 10.1093/ajcp/91.6.656. [DOI] [PubMed] [Google Scholar]
- Tabibzadeh S. Cytokines and the hypothalamic-pituitary-ovarian-endometrial axis. Hum Reprod. 1994 May;9(5):947–967. doi: 10.1093/oxfordjournals.humrep.a138621. [DOI] [PubMed] [Google Scholar]
- Van Voorhis B. J., Anderson D. J., Hill J. A. The effects of RU 486 on immune function and steroid-induced immunosuppression in vitro. J Clin Endocrinol Metab. 1989 Dec;69(6):1195–1199. doi: 10.1210/jcem-69-6-1195. [DOI] [PubMed] [Google Scholar]
- Wu W. X., Brooks J., Millar M. R., Ledger W. L., Glasier A. F., McNeilly A. S. Immunolocalization of oestrogen and progesterone receptors in the human decidua in relation to prolactin production. Hum Reprod. 1993 Jul;8(7):1129–1135. doi: 10.1093/oxfordjournals.humrep.a138206. [DOI] [PubMed] [Google Scholar]