Abstract
The calcium-sensing receptor (CaSR) was cloned over 20 years ago and functionally demonstrated to regulate circulating levels of parathyroid hormone by maintaining physiological serum ionized calcium concentration ([Ca2+]). The receptor is highly expressed in the kidney; however, intrarenal and intraspecies distribution remains controversial. Recently, additional functions of the CaSR receptor in the kidney have emerged, including parathyroid hormone-independent effects. It is therefore critical to establish unequivocally the localization of the CaSR in the kidney to relate this to its proposed physiological roles. In this study, we determined CaSR expression in mouse, rat, and human kidneys using in situ hybridization, immunohistochemistry (using 8 different commercially available and custom-made antibodies), and proximity ligation assays. Negative results in mice with kidney-specific CaSR ablation confirmed the specificity of the immunohistochemistry signal. Both in situ hybridization and immunohistochemistry showed CaSR expression in the thick ascending limb, distal tubule, and collecting duct of all species, with the thick ascending limb showing the highest levels. Within the collecting ducts, there was significant heterogeneity of expression between cell types. In the proximal tubule, lower levels of immunoreactivity were detected by immunohistochemistry and proximity ligation assays. Proximity ligation assays were the only technique to demonstrate expression within glomeruli. This study demonstrated CaSR expression throughout the kidney with minimal discrepancy between species but with significant variation in the levels of expression between cell and tubule types. These findings clarify the intrarenal distribution of the CaSR and enable elucidation of the full physiological roles of the receptor within this organ.
Keywords: calcium-sensing receptor, kidney, localization, proximity ligation assay, antibody
the calcium-sensing receptor (CaSR) is a G protein-coupled receptor with a key role in extracellular free ionized calcium (Cao2+) homeostasis. The CaSR was first cloned and characterized in the parathyroid by Brown and coworkers in 1993 (7) and subsequently identified in other organs involved in the control of Cao2+, namely, the kidney (37), gastrointestinal tract (15), and bone (18). In addition, CaSR expression has been reported in tissues outside the Cao2+ homeostatic system such as the blood vessels (8), thyroid (16), nerve (38), and heart (42). In the kidney, the CaSR regulates calcium excretion by modulating the actions of parathyroid hormone (PTH) (26). However, the CaSR also regulates calcium excretion in a PTH-independent way, by controlling paracellular movement of Ca2+ in the thick ascending limb (TAL) through modulation of both the transepithelial potential difference and paracellular permeability (17, 40). Additional functions of the renal CaSR include renin release (23, 29), acidification (13, 33), and concentration of urine (39). The distribution of intrarenal CaSR is critical to elucidating the full functional role of the receptor. The initial characterization of CaSR expression in the rat kidney assessed the distribution of CaSR mRNA by Northern blotting and in situ hybridization (ISH) (36, 37). Transcripts were detected predominantly in outer medulla and cortex, with stronger expression in the cortical medullary rays, indicating a predominant expression of CaSR in the TAL (37). More detailed analysis of transcripts in the rat kidney, using ISH and RT-PCR in dissected rat nephron segments, demonstrated expression in glomeruli, proximal tubules (PT), medullary (mTAL) and cortical (cTAL) TAL, distal tubules (DT), and collecting ducts (CD) (36). Comparable protein distribution was subsequently described in the rat kidney using immunofluorescence; expression was observed in PT, mTAL, cTAL, macula densa cells, DT, and type A intercalated cells in cortical CDs (CCDs). In addition, the authors observed different cellular polarity of the receptor in different cell types along the nephron (35). Over the last decade, more detailed studies have been undertaken to address the precise localization of the CaSR within the glomerulus. Expression has been demonstrated in juxtaglomerular cells (23, 29), activation of which inhibits renin release via an indirect effect on enzymes (adenylyl cyclase 5, phosphodiesterase 1C) that are the targets of classic renin stimuli (30). Furthermore, calcimimetics inhibit plasma renin activity in a PTH-independent fashion both in vitro and in vivo (1, 20, 23). Consistent with CaSR expression in glomerular podocytes, calcimimetics ameliorate toxin-induced glomerulosclerosis via antiapoptotic and cytoskeleton-stabilizing effects (28). CaSR expression has also been demonstrated in mouse (20) and human (25) mesangial cells and has been shown to mediate Ca2+ influx and cell proliferation via the canonical transient receptor potential channels 3 and 6.
Since the initial characterization of CaSR expression in the kidney, the majority of studies have supported expression of the CaSR in rat TAL, DT, and CD (11, 14, 39, 45). However, these studies have failed to agree on the distribution of the CaSR within the CD and also whether expression is present in the PT and glomerulus. RT-PCR on dissected rat nephron segments led to discrepant results, with some groups reporting CaSR mRNA expression in the PT (36) and in the glomeruli (11) while others failed to demonstrate CaSR expression in these nephron segments (11, 45). CaSR protein expression is also controversial, with some authors reporting CaSR expression in the PT (notably at the apical membrane) using immunofluorescence on sections of frozen rat (34) and mouse kidney (3) while recent studies using human, mouse, and rat kidney tissue failed to observe CaSR expression throughout the kidney with the exception of the TAL (22).
The main problems associated with these seemingly contradictory results is that the studies describing the expression of the CaSR have differed in whether they have investigated mRNA or protein expression, used different fixation and/or immunostaining methodologies, and utilized different species. It is plausible that discrepancies between studies have arisen as a consequence of a lack of sensitivity with certain methods or nonspecific detection of the CaSR. This study aimed to utilize technical advances in detection methods to gain greater sensitivity and specificity in determining CaSR expression in mouse, rat, and human kidneys. Using a combination of a branched DNA ISH, immunohistochemistry (using antibodies raised against different epitopes of the CaSR), and a highly sensitive and specific chromogenic in situ proximity ligation assay (PLA) method, we have demonstrated CaSR expression throughout the kidney. These observations will help to describe the full physiological role of the CaSR in the kidney.
METHODS
Tissue Samples
Animal kidney tissue was obtained from adult CD-1 mice or Han Wistar rats, supplied by Harlan (Hillcrest, Leicestershire, UK) or Charles River (Harlow, Essex, UK). Tissue from kidney-specific CaSR-deficient mice was obtained as previously described utilizing transgenic animals expressing Cre recombinase under the Sine oculis homeobox homolog 2 promoter (Six2-Cre Casr floxed mice) (40). Animals were euthanized by schedule 1 methods under the UK Animals (Scientific Procedures) Act (1986). Adult normal human kidney cortex tissue used for immunohistochemistry was obtained from the AstraZeneca Global Tissue Bank, compliant with the UK Human Tissue Act (HTA) and AstraZeneca global policies. Adult human kidney tissue used for Western blotting was obtained from human kidneys surgically resected at The University Hospital Wales due to renal cell carcinoma (RCC) (Research Ethics Committee approval reference number 07/WSE04/53) in collaboration with the Wales Cancer Bank. Macroscopically normal kidney cortex tissue was taken from a region separate from the carcinoma.
ISH
Mouse, rat, and human kidneys were fixed in 10% neutral-buffered formalin for 24–48 h and embedded in paraffin. Five-micrometer-thick sections were cut, and ISH was performed using a QuantiGene ViewRNA ISH tissue assay (Affymetrix, Santa Clara, CA) according to the manufacturer's instructions, with the following modifications: incubation times were 5 min for pretreatment, 20 min for protease digestion, 40 min for PreAmp Hybridization, and 30 min for Amp Hybridization and Label Probe (alkaline phosphatase-conjugated probe) incubation. Rat- and human-specific CaSR probes were obtained from Affymetrix and used at a dilution of 1:40 in the probe diluents provided in the kit. For mouse tissue, a rat probe was used since preliminary experiments showed good species cross-reactivity (rat and mouse CaSR sequences share 94% homology). Sections were mounted using Immu-mount (Thermo Scientific, Waltham, MA). Negative controls were performed using both a scrambled CaSR probe and an antisense probe complementary to the CaSR sense probe.
Immunofluorescence
The antibodies used in this study were screened by immunofluorescence patterns in HEK293 cells stably transfected with the human CaSR (CaSR-HEK), as described previously (24). Cells were fixed in 4% paraformaldehyde in PBS, washed, and incubated in PBS containing 50 mM NH4Cl for 10 min. Blocking was performed using 1% bovine serum albumin/0.05% Triton X-100 in PBS. Primary antibodies (Table 1: Antibodies 1–8) were incubated overnight at 4°C at a dilution of 1:100 in blocking buffer. Alexa Fluor 488 or Alexa Fluor 594 fluorescence dye-coupled secondary anti-IgG antibodies (Life Technologies, Paisley, UK) were incubated at a dilution of 1:500 in blocking buffer to visualize primary antibody binding. Hoechst 34580 (Life Technologies) was used to stain the nuclei.
Table 1.
Details of antibodies used in the study
| Epitope |
|||||||
|---|---|---|---|---|---|---|---|
| Antibody | Manufacturer | Reactivity | Species | Region | Host | Clone | IHC Dilution |
| N-term1 | Abcam (ab19347)/Thermo (MA1-934)* | Mouse, rat, human | Human | N-term (ADD) N'-ADDDYGRPGIEKFREEAEERDI-C' | Mouse | Mono | 1:500 |
| N-term2 | Anaspec† (53286) | Mouse, rat, human | Rat | N-term | Rabbit | Poly | 1:500 |
| N-term3 | W. Chang (custom made) | Mouse, rat, human | Human | N-term (ADD) N'-DYGRPGIEKFREEAEE-C' | Mouse | Mono | Not tested |
| N-term4 | W. Chang (custom made) | Mouse, rat, human | Human | N-term (ADD) N'-DYGRPGIEKFREEAEE-C' | Mouse | Mono | Not tested |
| N-term5 | W. Chang (custom made) | Mouse, rat, human | Human | N-term (ADD) N'-ADDDYGRPGIEKFREEAEERDI-C' | Rabbit | Poly | Not tested |
| N-term6 | Alomone (ACR-004) | Mouse, rat, human | Human | N-term (ADD) | Rabbit | Poly | 1:500 |
| C-term1 | W. Chang (custom made) | Mouse, rat | Mouse | C-term N'-NSEDRFPQPERQKQ-C' | Mouse | Mono | 1:150 |
| C-term2 | LS Bio‡ (LS-C117834) | Mouse, rat, human | Human | C-term aa854-903 | Rabbit | Poly | Not tested |
| Full length | Novus Bio (H00000846-B01P) | Human | Human | Full length | Mouse | Poly | 1:75 |
N-term1 is a monoclonal antibody produced using the 5C10, ADD clone and commercialized by different companies, including Abcam, Thermo-Scientific, Novus Bio, Genetex (Irvine, CA), and Acris (Herford, Germany).
Anaspec, Fremont, CA.
LS Bio, Seattle, WA.
Western Blotting
Mouse, rat, and human kidney samples and CaSR-HEK or untransfected HEK293 cells were homogenized in modified RIPA buffer (25 mM Tris·HCl, pH 7.6, 150 mM NaCl, 1% NP-40, 0.1% SDS, 1% sodium deoxycholate, 1 mM n-ethylmaleimide, 1 mM PMSF) containing Halt protease and phosphatase inhibitors (Thermo Scientific). Cell lysis was carried out using a Polytron (Kinematica, Bohemia, NY) homogenizer. Following lysis, homogenates were centrifuged at 10,000 g for 5 min to remove insoluble debris. Protein extracts were quantified using a BCA protein assay (Thermo Scientific). Twenty micrograms of each extract were subjected to electrophoresis on NuPage 10% BisTris polyacrylamide gels (Life Technologies). Gels were transferred to nitrocellulose membranes and stained with Ponceau S to confirm even protein loading. Nonspecific protein binding was prevented using 5% low-fat dry milk in Tris-buffered saline containing 0.1% Tween (TBST) for 1 h at room temperature. Primary antibodies (Table 1: N-term1, N-term2, C-term1, and full length) were added at a dilution of 1:2,000 in 5% milk/TBST overnight at 4°C. A horseradish peroxidase (HRP)-conjugated anti-mouse or anti-rabbit secondary antibody (Promega, Madison, WI) was added at a dilution of 1:20,000 or 1:6,000, respectively, in 5% milk/TBST before detection of immunoreactivity with ECL prime using a ChemiDoc MP (Bio-Rad, Hercules, CA).
Immunohistochemistry
Mouse, rat, and human kidneys were fixed in 10% neutral-buffered formalin for 24–48 h and embedded in paraffin. Four-micrometer-thick sections were cut, dewaxed in xylene, and rehydrated in ethanol. Antigen retrieval was performed in a Milestone RHS-2 microwave (Milestone, Sorisole, Italy) at 110°C for 2 min in 1 mM EDTA buffer, pH 8. Endogenous peroxidase activity was blocked with 3% aqueous hydrogen peroxide for 10 min. Immunostaining was carried out using a Labvision autostainer (Labvision, Fremont, CA). Nonspecific binding of the antibody was prevented by incubating slides with background blocker with casein (Menarini, Florence, Italy) for 20 min. Slides were incubated with primary antibodies (Table 1: N-term1, N-term2, full length, and C-term1) for 1 h at room temperature. Primary antibodies were detected with X-Cell Plus HRP (Menarini), Envision anti-mouse labeled polymer (Dako, Glostrup, Denmark), or Ultravision Quanto Mouse on Mouse (Thermo Scientific), and peroxidase was visualized with diaminobenzidine (Menarini). Double labeling was performed by incubating tissue sections with antibodies against aquaporin-2 (AQP2; 1:4,000, Sigma, St. Louis, MO), Tamm-Horsfall (1:100, Santa Cruz Biotechnology, Santa Cruz, CA), or the thiazide-sensitive NaCl cotransporter (NCC; 1:500, Millipore, Billerica, MA) as nephron segment markers for 1 h at room temperature. AQP2, Tamm-Horsfall, and NCC were detected by 30-min incubation with a goat anti-rabbit antibody conjugated with alkaline phosphatase (Life Technologies), and visualized using Quanto Fast Red Permanent (Thermo Scientific). Sections were counterstained with hematoxylin before dehydration in ethanol, clearing in xylene, and mounting using Hystomount (TAAB Labs, Aldermaston, UK). Negative controls were performed using the appropriate isotype or no primary antibody controls.
PLA
PLA were performed using a Duolink assay with brightfield detection (Sigma) according to the manufacturer's instructions. Briefly, 5-μm-thick sections were cut and antigen retrieval and peroxidase quenching were carried out as described for IHC. Sections were incubated with primary antibody pairs (N-term1/N-term2, N-term1/N-term6, full length/N-term6, C-term1/N-term2, C-term1/N-term6) for 1 h at room temperature. Primary antibody pairs were detected by secondary antibodies conjugated with oligonucleotide probes (anti-Rabbit PLA probe Plus and anti-Mouse PLA probe Minus), incubated for 1 h at 37°C. Ligation of the probes was performed by adding oligonucleotides capable of hybridizing to the two probes and a ligase enzyme for 30 min at 37°C. Amplification was carried out by adding nucleotides and the polymerase enzyme and incubating for 2 h at 37°C. Detection was performed by incubation with HRP-conjugated oligonucleotide probes for 1 h at room temperature. Peroxidase was then visualized with diaminobenzidine (Menarini). Sections were counterstained with hematoxylin before dehydration in ethanol, clearing in xylene, and mounting using Hystomount (TAAB Labs). Negative controls were performed using the appropriate isotype or no primary antibody controls. Additional controls were performed by incubating rat sections with C-term1 and ki67 (1:200, Novus, Littleton, CO) or N-term6 and α-smooth muscle actin (SMA, 1:3,000, Sigma).
Immunoprecipitation
Rat kidney samples were prepared as described for Western blotting. Immunoprecipitation was performed using μMACS Protein G MicroBeads and μ Columns (Miltenyi Biotec, Cologne, Germany) according to the manufacturer's instructions. Immunoprecipitation was performed to pull down CaSR (3 μg, Thermo Scientific), SMA (3 μg, Sigma), and IgG2a (3 μg, Isotype control, Abcam, Cambridge, UK). Twenty microliters of each immunoprecipitated sample, the lysate preclearing flowthrough (negative control), and rat kidney extract (positive control) were subjected to electrophoresis on NuPage 4–12% BisTris polyacrylamide gels (Life Technologies). Western blotting was performed as described above for SMA (1:2,000, Sigma). Controls for Western blotting were performed by omission of a primary antibody.
RESULTS
CaSR mRNA Expression
Using ISH, all species showed a high expression of CaSR mRNA in cortical medullary rays (Fig. 1, A–C) and in the outer medulla (Fig. 1, D–F), consistent with CaSR expression in the TAL. Lower levels of CaSR mRNA were also detected in the DCT and cortical and medullary CDs, identified by distribution and morphology (Fig. 1, A–F). There was no signal detected in the rat and mouse papilla (this region was not present in the human kidney samples). There was also no signal detected in the proximal tubules. Negative controls using either an antisense probe or a scrambled probe did not detect any positive signal (Fig. 1, G–I).
Fig. 1.
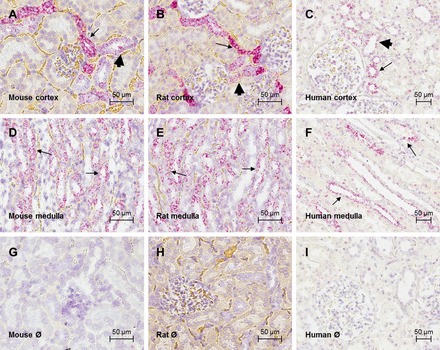
Localization of calcium-sensing receptor (CaSR) mRNA in mouse, rat, and human kidney. Photomicrographs of in situ hybridization using probes directed against CaSR in cortical sections of mouse (A), rat (B), and human (C) kidney and in medullary sections of mouse (D), rat (E), and human (F) kidney are shown. CaSR signal (in red) corresponding to fast red staining and nuclei are counterstained with hematoxylin (blue). Photomicrographs of negative controls for the in situ hybridization in mouse (G), rat (H), and human (I) cortical sections are also shown. Negative controls were performed by incubation with antisense probes (rat) or omission of CaSR probe (mouse and human). Scale bar = 50 μm. Arrows indicate stronger staining, consistent with thick ascending limb (TAL) expression; arrowheads indicate weaker expression, consistent with the distal tubule (DT) and collecting duct (CD). Pictures are representative of 3 (mouse and rat) or 2 (human) independent experiments.
CaSR Protein Expression
Selection of antibodies.
To determine whether the discrepancy in expression patterns obtained in previously published studies could be ascribed to the use of different CaSR antibodies, nine antibodies raised against different regions of the CaSR protein were investigated in this study (Table 1). All antibodies, except N-term6, were initially screened using immunofluorescence on CaSR-HEK cells. For all antibodies, except C-term2, immunofluorescence was detected predominantly at the plasma membrane and in the cytoplasm of the cells (Fig. 2). C-term2 showed strong nuclear immunoreactivity, which was considered to be nonspecific, particularly as this has not been reported previously, and therefore was excluded from further experiments.
Fig. 2.
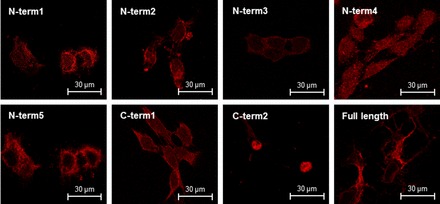
CaSR immunolocalization in HEK293 cells stably expressing the human CaSR. Immunofluorescence using 8 different anti-CaSR antibodies: N-term1 (Abcam), N-term2 (Anaspec), N-term3 (W. Chang), N-term4 (W. Chang), N-term5 (W. Chang), C-term1 (W. Chang), C-term2 (Lifespan), and full length (Novus). Scale bar = 30 μm.
Western blotting.
From the initial immunofluorescence screening process, four antibodies were selected to carry out further studies, based on their recognition of different CaSR epitopes. Antibodies N-term1, N-term3, N-term4, and N-term5 all recognize the ADD amino acid sequence within the N terminal of the CaSR and showed comparable immunofluorescence patterns; therefore, only one was selected for further investigation. Of these, N-term1 was chosen since it has been used most extensively in published studies. N-term2 was selected because it was the only antibody raised against a different epitope within the N terminal, according to the manufacturer's information; full-length and C-term1 antibodies were selected as they were the only available antibodies raised against the full-length protein and against the CaSR C-terminal domain, respectively.
To further confirm the specificity of these antibodies, Western blot profiles were analyzed using mouse, rat, and human kidneys and CaSR-HEK cell extracts (Fig. 3). Immunostaining carried out using N-term1 and N-term2 exhibited CaSR-like immunoreactivity in all 3 species. Conversely, no specific CaSR immunoreactivity was observed in untransfected HEK293 cells immunostained with N-term1 and N-term2. The full-length antibody only recognized the CaSR in human tissue and CaSR-HEK cells, consistent with this antibody being raised against a human antigen. C-term1 only cross-reacted with the mouse and rat tissue, consistent with this antibody being raised against a mouse antigen. Unfortunately, the antibodies that had been raised against the full-length protein did not cross-react with mouse or rat kidney tissue, while the antibody against the C-terminal region of the CaSR protein did not recognize human kidney tissue. Immunoreactivity of the expected size, that is 140–160 kDa for the glycosylated monomer and/or 260–300 kDa for the mature, fully glycosylated CaSR dimer (43), were observed with all the antibodies. The Western blotting profiles revealed some differences between antibodies. Using N-term1, strong immunoreactivity was detected for all the samples; in mouse and rat kidneys, immunoreactivity corresponding to the CaSR monomer was detected more strongly than the dimer whereas in human and CaSR-HEK samples the signal distribution was more homogeneous. The N-term2 antibody detected preferentially the dimeric form of the CaSR compared with the monomer in all three species. Full length detected very weak CaSR immunoreactivity in the human kidney lysate, but a stronger signal was obtained in the CaSR-HEK lysate. This antibody appeared to detect the monomer and dimer equally well. C-term1 preferentially detected the monomeric form of the CaSR compared with the dimer in both the mouse and rat, but it did not cross-react with human or CaSR-HEK samples. Thus further immunohistochemistry studies for each species utilized the following antibodies: N-term1, N-term2 and C-term1 for mouse and rat kidneys and N-term1, N-term2, and whole for human kidneys.
Fig. 3.
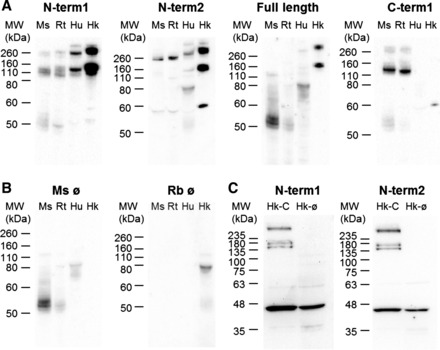
Western blotting profile for the different CaSR antibodies used in this study. A: Western blotting using N-term1 (Thermo), N-term2 (Anaspec), full length (Novus), and C-term1 (W. Chang) antibodies in mouse (Ms), rat (Rt), and human (Hu) kidney and CaSR-HEK (Hk) extracts. CaSR monomers and dimers are detected at 140–160 kDa and 260–300 kDa, respectively. B: Western blotting negative controls performed by omitting the primary antibodies. C: Western blotting using N-term1 and N-term2 antibodies in CaSR-HEK (Hk) and in untransfected HEK (Hk-Ø) cell extracts.
CaSR protein localization.
Having selected suitable antibodies, the CaSR intrarenal distribution was investigated in sections of kidney tissue. Generally, the strongest immunoreactivity was detected in the outer medulla and medullary rays and weak immunoreactivity was detected in the cortex for all the species (Figs. 4–6, A–F). CaSR immunoreactivity in the different nephron segments was assessed by analyzing cellular morphology and costaining using the CaSR antibodies in combination with established segment-specific markers [Fig. 4, G–R (mouse), Fig. 5, G–R (rat), and Fig. 6, G–R (human)]. In general, CaSR immunoreactivity was found all along the nephron, with a comparable expression pattern across the different species. Specifically, all antibodies detected CaSR immunoreactivity at the basolateral membrane and cytoplasm of the TAL, identified by colocalization with Tamm-Horsfall protein (Figs. 4–6 H, L, and P).
Fig. 4.

CaSR immunolocalization in mouse kidney sections. Photomicrographs of mouse cortical kidney sections immunostained with the CaSR antibodies N-term1 (Thermo; A), N-term2 (Anaspec; B), and C-term1 (W. Chang; C) are shown. Photomicrographs of mouse medullary kidney sections immunostained with the CaSR antibodies N-term1 (D), N-term2 (E), and C-term1 (F) are also shown. Positive CaSR signal corresponds to immunoperoxidase staining (brown), and nuclei are counterstained with hematoxylin (blue). Arrows indicate stronger signal consistent with TAL, and arrowheads indicate weaker signal consistent with DCT and CD. Also shown are photomicrographs showing CaSR immunoreactivity in the PT, identified by the presence of brush border in kidney sections immunostained with N-term1 (G), N-term2 (K), and C-term1 (O). Asterisk indicates the PT. Photomicrographs appear showing CaSR immunoreactivity in the TAL, identified by dual staining with Tamm-Horsfall protein in kidney sections immunostained with N-term1 (H), N-term2 (L), and C-term1 (P). Photomicrographs show CaSR immunoreactivity in the DCT, identified by dual staining with the thiazide-sensitive Na-Cl cotransporter (NCC) in kidney sections immunostained with N-term1 (I), N-term2 (M), and C-term1 (Q). Photomicrographs show CaSR immunoreactivity in the CD, identified by dual staining with aquaporin-2 (AQP2) in kidney sections immunostained with N-term1 (J), N-term2 (N), and C-term1 (R). Nephron segment marker signal corresponds to (red) fast red staining. Photomicrographs for the negative controls for N-term1 (S), N-term2 (T), and C-term1 (U) were performed by omitting the primary antibody. Scale bar = 100 μm for pictures in A–F and S–U, and 50 μm for pictures in G–R.
Fig. 6.
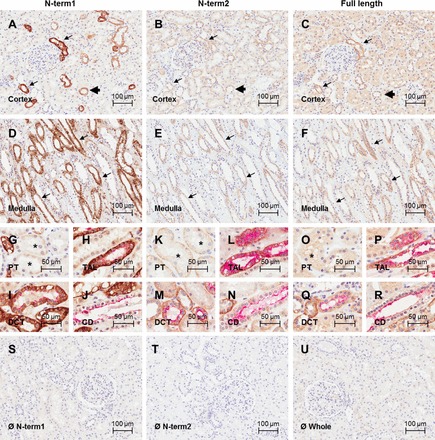
CaSR immunolocalization in human kidney sections. Photomicrographs shown of human cortical kidney sections immunostained with the CaSR antibodies N-term1 (Thermo; A), N-term2 (Anaspec; B), and full length (Novus; C). Photomicrographs of human medullary kidney sections immunostained with the CaSR antibodies N-term1 (D), N-term2 (E), and full length (F). Positive CaSR signal corresponds to immunoperoxidase staining (brown), and nuclei are counterstained with hematoxylin (blue). Arrows indicate stronger signal consistent with TAL, and arrowheads indicate weaker signal consistent with DCT and CD. Photomicrographs show CaSR immunoreactivity in the PT, identified by the presence of brush border in kidney sections immunostained with N-term1 (G), N-term2 (K), and full length (O). Asterisk indicates the PT. Photomicrographs show CaSR immunoreactivity in the TAL, identified by dual staining with Tamm-Horsfall protein in kidney sections immunostained with N-term1 (H), N-term2 (L), and full length (P). Photomicrographs show CaSR immunoreactivity in the DCT, identified by dual staining with NCC in kidney sections immunostained with N-term1 (I), N-term2 (M), and full length (Q). Photomicrographs show CaSR immunoreactivity in the CD, identified by dual staining with AQP2 in kidney sections immunostained with N-term1 (J), N-term2 (N), and full length (R). Nephron segment marker signal corresponds to (red) fast red staining. Photomicrographs are also shown for the negative controls for N-term1 (S), N-term2 (T), and full length (U) performed by omitting the primary antibody. Scale bar = 100 μm for pictures in A–F and S–U and 50 μm for pictures G–R.
Fig. 5.
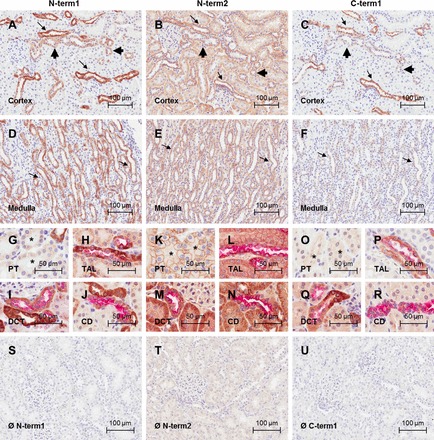
CaSR immunolocalization in rat kidney sections. Photomicrographs of rat cortical kidney sections immunostained with the CaSR antibodies N-term1 (Thermo; A), N-term2 (Anaspec; B), and C-term1 (W. Chang; C) are shown. Photomicrographs of rat medullary kidney sections immunostained with the CaSR antibodies N-term1 (D), N-term2 (E), and C-term1 (F) are also shown. Positive CaSR signal corresponds to immunoperoxidase staining (brown), and nuclei are counterstained with hematoxylin (blue). Arrows indicate stronger signal consistent with TAL, and arrowheads indicate weaker signal consistent with DCT and CD. Photomicrographs show CaSR immunoreactivity in the PT, identified by the presence of brush border in kidney sections immunostained with N-term1 (G), N-term2 (K), and C-term1 (O). Asterisk indicates the PT. Photomicrographs show CaSR immunoreactivity in the TAL, identified by dual staining with Tamm-Horsfall protein in kidney sections immunostained with N-term1 (H), N-term2 (L), and C-term1 (P). Photomicrographs show CaSR immunoreactivity in the DCT, identified by dual staining with the thiazide-sensitive Na-Cl cotransporter (NCC) in kidney sections immunostained with N-term1 (I), N-term2 (M), and C-term1 (Q). Photomicrographs show CaSR immunoreactivity in the CD, identified by dual staining with AQP2 in kidney sections immunostained with N-term1 (J), N-term2 (N), and C-term1 (R). Nephron segment marker signal corresponds to (red) fast red staining. Photomicrographs for the negative controls are shown for N-term1 (S), N-term2 (T), and C-term1 (U) performed by incubation with the corresponding isotype controls. Scale bar = 100 μm for pictures in A–F and S–U and 50 μm for pictures in G–R.
Consistent with the mRNA distribution by ISH, IHC showed that CaSR immunoreactivity was strongest in the TAL. In addition, CaSR immunoreactivity was consistently detected by all antibodies in the DCT, identified by colocalization with antibodies directed against NCC (Figs. 4–6, I, M, and Q). In this segment, immunoreactivity was detected apically, basolaterally, and within the cytoplasm. CaSR was also present in the connecting tubule (CNT), identified by a combination of morphology and NCC-negative, AQP2-positive cells. In the CDs, identified by a combination of colocalization with AQP2 and morphology (Figs. 4–6, J, N, and R), CaSR expression was heterogeneous. In mouse and rat, CaSR immunoreactivity was found at the basolateral membrane of some cortical CD cells and in the apical membrane and cytoplasm of other cortical CD cells. In CDs in the medulla and papilla, CaSR expression was only observed with N-term2, which showed apical and basolateral immunoreactivity. In human tissue, CaSR immunoreactivity was detected in the cytoplasm and apical and basolateral membranes of most cortical CD cells. Stronger immunoreactivity was detected in a population of CD cells when antibodies N-term1 or N-term2 were used. With N-term2, CaSR immunoreactivity was observed within all cells in the CCDs and therefore all Aqp2-positive cells were also positive for the CaSR. In the inner and outer medullary CDs (MCDs) and also for all the remaining antibodies, some cells were CaSR+/Aqp2+, some CaSR+/Aqp2−, some CaSR−/Aqp2+, and some CaSR−/Aqp2−. CaSR immunoreactivity was very weak in the PT (identified by the presence of brush border) with N-term1, full length, and C-term1 (Figs. 4–6, I and Q). In this segment, the intensity of immunostaining signal varied between repeat experiments and in some cases was difficult to distinguish from background levels obtained with isotype controls. However, N-term2 (Figs. 4–6 M) consistently detected relatively strong immunoreactivity in the cytoplasm and apical membrane of PT cells, which generally increased from the S1 segment down to the S3 segment of the PT. CaSR immunoreactivity was absent or very weak (indistinguishable from background) in the glomeruli and JGA. No specific immunoreactivity was detected in kidney sections from mice deficient in the renal CaSR immunostained with N-term1 or C-term1, or in the corresponding isotype controls (Fig. 7). However, N-term2 displayed positive signal throughout the kidney in the knockout mice. Some signal was observed in the isotype control for this antibody; however, the signal with N-term2 appeared to be of greater intensity (data not shown).
Fig. 7.

CaSR immunostaining in mice lacking the renal CaSR. Photomicrographs are shown of cortical kidney sections from wild-type (WT) mice immunostained with the CaSR antibodies N-term1 (A) and C-term1 (B) or with the corresponding isotype control (C). Photomicrographs are also shown of cortical kidney sections from mice lacking the renal CaSR (D–F) immunostained with the CaSR antibodies N-term1 (D) and C-term1 (E) or with the corresponding isotype control (F). Positive signal corresponds to immunoperoxidase staining (brown), and nuclei are counterstained with hematoxylin (blue). Scale bar = 100 μm.
PLA.
Antibody pairs were initially selected for PLA on their ability to recognize different regions of the CaSR protein, to maximize the chance of signal being observed from the same protein molecule. PLA on mouse and rat kidney using the C-term1/N-term6 antibody pair or in human kidney using the full length/N-term6 antibody pair indicated that, similar to the ISH and IHC results, CaSR expression was greatest in the outer medulla and medullary rays and weaker in the cortex (Fig. 8). This distribution clearly demonstrates stronger expression of the CaSR in the TAL and weaker expression in the DT and CD. Consistent with the ISH and conventional IHC, both positive and negative cells were observed within the CD (Fig. 9). Importantly, PLA signal was detected in the PT, with S3 showing higher expression than S1, consistent with conventional IHC using N-term2. In the glomeruli, a positive signal was detected but at a low level, comparable to the proximal tubule expression. The low level of signal combined with the cytoplasmic distribution precluded any determination of positive cell types within the glomerulus. Negative controls showed only the occasional dot of false-positive signal, but these were generally associated with areas of the section containing no tissue (i.e., lumen of tubules or blood vessels). PLA in rat kidney using other antibody pairs, including N-term1/N-term2, N-term1/N-term6, and N-term2/C-term1, showed similar results to the C-term1/N-term6 antibody pair (Fig. 10). Incubation of rat kidney sections with a C-term1/anti-ki67 antibody pair (used as an antibody to an unrelated protein) also displayed only the occasional dot of positive signal (Fig. 11, A and B). A further control was conducted using the N-term6 and an anti-SMA antibody pair on rat kidney sections. The CaSR and SMA may interact at a molecular level since both proteins have been demonstrated to interact with filamin (2, 21) and so should result in a positive PLA signal. However, in the kidney, SMA is only expressed in the blood vessels and not in epithelial cells. Accordingly, PLA signal was not observed within cells of the nephron but only in blood vessels (Fig. 11C). The specificity of the signal was confirmed by analyzing the interaction between the CaSR and SMA with a coimmunoprecipitation assay: SMA expression was observed by Western blotting using rat kidney extracts immunoprecipitated with the N-term1 antibody, suggesting that there is an interaction of the CaSR and SMA in vascular smooth muscle cells (Fig. 11D). The results from the ISH, IHC, and PLA experiments are summarized in Table 2.
Fig. 8.

CaSR detection by proximity ligation assay in mouse, rat, and human kidney. Photomicrographs are shown of CaSR immunolocalization by proximity ligation assay in mouse (A), rat (C), and human (E) cortical kidney sections. Immunoperoxidase staining (brown dots) indicates the proximity between the epitopes detected by the C-term1/N-term6 antibody pair in mouse and rat sections and by the full length/N-term6 antibody pair in human sections. Photomicrographs are also shown of the negative controls performed by incubation with isotype control or omission of a primary antibody (mouse, B; rat, D; human, F). Arrow indicates TAL, and asterisk indicates PT. Scale bar = 50 μm.
Fig. 9.

CaSR expression in rat kidney CD. Photomicrographs are shown of CaSR mRNA expression in the cortical CD detected by in situ hybridization (ISH; A). Positive signal (as detected by ISH) corresponds to fast red staining. Photomicrographs of CaSR protein expression in the cortical CD detected by IHC with N-term1 (Thermo; B), N-term2 (Anaspec; C), C-term1 (W. Chang; D) or proximity ligation assay with C-term1/N-term6 (Alomone; E) are also shown. F: photomicrographs of CaSR protein expression in outer medullary CD detected by PLA with C-term1/N-term6. Positive signal corresponds to immunoperoxidase staining (brown), and nuclei are counterstained with hematoxylin (blue) in IHC and PLA images. Scale bar = 50 μm.
Fig. 10.
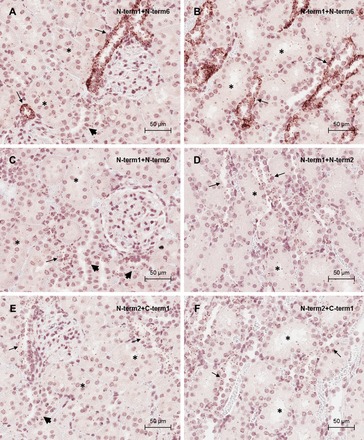
CaSR detection by proximity ligation assay in rat kidney. Photomicrographs of proximity ligation assay using the CaSR antibody pair N-term1/N-term6 in outer (A) and inner (B) cortical kidney sections. Photomicrographs of proximity ligation assay using the CaSR antibody pair N-term1/N-term2 in outer (C) and inner (D) cortical kidney sections are shown. Photomicrographs are also shown of proximity ligation assay using the CaSR antibody pair N-term2/C-term1 in outer (E) and inner (F) cortical kidney sections. Positive staining corresponds to immunoperoxidase staining (brown dots). Asterisks indicate PT. Scale bar = 50 μm.
Fig. 11.
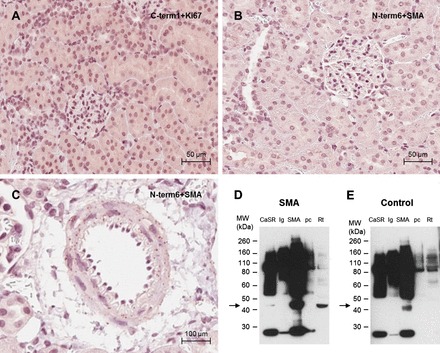
Controls for CaSR detection by proximity ligation assay in rat kidney. A: photomicrograph of the negative control for the proximity ligation assay using the C-term1/anti-ki67 antibody pair in an outer cortical kidney section. B: photomicrograph of the negative control for the proximity ligation assay using the N-term6/anti-α-smooth muscle actin (SMA) antibody pair in an outer cortical kidney section. C: photomicrograph of the proximity ligation assay signal observed using the N-term6/anti-SMA antibody pair in a renal blood vessel. Positive staining corresponds to immunoperoxidase staining (brown dots). Scale bar = 50 μm for the outer cortex pictures and 100 μm for the magnified blood vessel. The interaction between SMA and CaSR in blood vessels was confirmed by coimmunoprecipitation: proteins from rat kidney extracts were immunoprecipitated with N-term1, anti-SMA, or an IgG control. The immunoprecipitated proteins, together with the buffer used to preclear the assay columns (pc) and total protein extracts (Rt) were analyzed by Western blotting with an anti-SMA antibody (D). Control for the Western blotting was performed by omitting the primary antibody (E). SMA MW = 42 kDa.
Table 2.
Summary of the localization of the CaSR observed by ISH, IHC, and PLA
| Glomerulus | PT | TAL | DCT | CNT | CCD | MCD | ||
|---|---|---|---|---|---|---|---|---|
| ISH | − | − | ++++ | ++ | + | ++ | + | |
| IHC | N-term1 | − | ± | ++++ | ++ | + | ++ | − |
| N-term2 | − | ++ | ++++ | ++ | ++ | ++ | ++ | |
| Full length | − | ± | ++++ | ++ | + | ++ | − | |
| C-term1 | − | ± | ++++ | ++ | + | ++ | − | |
| PLA | C-term1/N-term6 | + | + | ++++ | ++ | + | ++ | + |
| Full length/N-term6 | + | + | ++++ | ++ | + | ++ | + | |
PT, proximal tubule; TAL, thick ascending limb; DCT, distal convoluted tubule; CNT, connecting tubule; CCD and MCD, cortical and medullary collecting duct, respectively; ISH, in situ hybridization; IHC, immunohistochemistry; PLA, proximity ligation assay.
DISCUSSION
Recent accumulating evidence supports a direct role for the CaSR in the kidney, independent of its indirect, PTH-mediated actions. Yet, its intrarenal distribution is still controversial. Pharmacological CaSR modulators are either in use in the clinic (calcimimetics) (6) or are currently being developed for the treatment of genetic hypocalcemia with hypercalciuria (autosomal dominant hypocalcemia, calcilytics) (27). Therefore, the aim of the current study was to determine, conclusively and unequivocally, the intrarenal distribution of the CaSR using the most sensitive and specific detection methods available for examining large numbers of each renal cell type across different species. This knowledge is essential to fully elucidate the role of the CaSR in the kidney and key to discriminating between CaSR-dependent and CaSR-independent roles of physiological and pharmacological CaSR modulators in the kidney. Because some of the discrepancies were previously ascribed to species differences, we assessed both CaSR mRNA and protein localization in mouse, rat, and human kidneys. For protein localization, we tested a wide variety of commercially and noncommercially available antibodies, raised against different regions of the CaSR protein. The initial screening of antibodies using immunofluorescence indicated that seven of the eight investigated antibodies specifically detected the CaSR in positively transfected cells, but not in sham-transfected cells. This result suggests that false positive protein detection is unlikely to be a major cause of discrepancies between studies. One antibody, C-term2, did fail this initial screen although this could be due to an inability of this antibody to detect the correct antigen following paraformaldehyde fixation.
Consistent with previous reports, Western blotting showed the expected heterogeneity of CaSR species present in the kidney given by the combination of the nude protein, the high-mannose, and fully mature monomeric and dimeric CaSR (4, 5, 43). Interestingly, the battery of antibodies tested showed different profiles for the detection of the different molecular species. Despite the different Western blotting profiles, there was generally a consistent pattern of protein expression by immunohistochemistry, regardless of the antibody used. N-term2 displayed a slightly different profile by immunohistochemistry compared with N-term1 and C-term1, notably a more intense expression of the CaSR in proximal tubules. By Western blotting, this antibody was the only antibody to predominantly detect dimeric forms of the CaSR. Since the dimerization is an essential step in the maturation of the CaSR(31), it is possible that N-term2 has a greater sensitivity to detect more mature forms of this receptor than the remaining antibodies. Alternatively, this antibody may display some nonspecific binding in FFPE tissue in addition to CaSR binding. The lack of specificity of this antibody in FFPE tissue is supported by the positive signal obtained in the CaSR-deficient kidney tissue. In contrast, the specificity of the signal detected by the N-term1 and N-term 2 antibodies was confirmed in mice with selective CaSR ablation from the kidney.
Loop of Henle and Distal Tubule
The highest CaSR mRNA and protein expression were observed basolaterally in the TAL, the only segment unanimously recognized to express the CaSR (3, 10, 22, 34, 40, 45). Functional studies have shown that the CaSR inhibits PTH-induced transcellular divalent cation reabsorption in this nephron segment (26). Also, in this nephron segment CaSR activation directly inhibits paracellular divalent cation reabsorption through PTH-independent processes (22, 40). These processes are associated with the modulation of transepithelial potential difference through the inhibition of Na-K-Cl cotransporter 2 (NKCC2) activity (40) and with the epigenetic regulation of micro-RNA controlling claudin 14 expression (17, 40). Expression of both CaSR mRNA and protein was also observed in the DCT, where functional expression of CaSR in the DCT has been previously linked to regulation of Ca reabsorption from the pro-urine via TRPV5 (41) and to regulation of Kir4.1 (19).
CDs
Within the CD, all techniques demonstrated greatest CaSR expression in CCD compared with outer and inner MCDs. IHC with N-term2 and PLA demonstrated a lower expression of CaSR in MCDs that was not observed with other techniques. With the exception of IHC with N-term2, all methods (including PLA) revealed that only a proportion of CCDs were positive for CaSR expression (∼30%). While it cannot be ruled out that N-term2 shows greater sensitivity than all the other techniques, it is more likely that this antibody is either recognizing a nonspecific signal in addition to the CaSR in FFPE tissue, or it is the only method for detecting a specific form of the receptor. Given the significantly greater signal for the dimer observed by Western blotting with this antibody (compared with the others), the latter explanation is a possibility. CaSR-positive CD cells could not be attributed to being either principal or intercalated cells since populations of CaSR+/AQP2+, CaSR+/Aqp2−, CaSR−/Aqp2+, and CaSR−/Aqp2− were clearly observed. In agreement with the original description of CaSR localization in CCDs (34), heterogeneous expression within the cytoplasm, apical, and/or basolateral membranes was observed in the current study. Characterization of the cell types in the study by Riccardi et al. (34) demonstrated CaSR expression in some, but not all, type A intercalated cells. Functionally, this is supported by studies in TRPV5−/− mice, where the CaSR is activated by increased urinary Ca2+ levels, which trigger urinary acidification by increasing H+-ATPase activity (33). Principal cell expression is also suggested by studies demonstrating that activation of an apical CaSR expression in CDs is specifically involved in reduction of AVP-elicited osmotic water permeability via reduction of Aqp2 (9, 32, 39). Recently, pH-sensitive CaSR expression was described as being restricted to the basolateral membrane of type B intercalated cells (46), although the images presented appeared to show weaker staining in N-term2- and anion exchanger 1-positive cells. Our data support the presence of the CaSR in both principal and intercalated cells, with significant heterogeneity in expression levels and also polarity. Further work is needed to elucidate the reasons behind the restricted expression of the CaSR to subpopulations of these different cell types.
PT
The PT is the nephron segment where greatest discrepancies in CaSR expression have been reported between studies. Our data with IHC and ISH was inconclusive since only one antibody, N-term2, convincingly showed PT expression. Since this antibody also showed signal in the CaSR-deficient mice, this staining was likely to be nonspecific. The level of staining with the other antibodies and also with ISH varied slightly between repeat experiments and was difficult to distinguish above possible background staining. However, using PLA we were specifically able to detect a low level of CaSR expression in the PT. This method confers greater specificity than conventional IHC since specific detection of the same molecule by a pair of antibodies is necessary to produce a positive signal. In addition, the method includes an amplification step, further increasing the sensitivity over conventional methods. This finding supports functional studies demonstrating that, in mice, activation of the CaSR by increased luminal gadolinium blocks the inhibitory effect of PTH in phosphate absorption in the proximal tubule (3) and also that in both rats and mice, activation of the CaSR by increased luminal calcium concentration enhances fluid reabsorption in the PT (10). Finally, a recent study has demonstrated expression of a functional CaSR at the apical membrane of conditionally immortalized PT epithelial cells obtained from healthy urine (12).
Glomerulus
The majority of studies addressing CaSR expression in the kidney have failed to observe localization within the glomerulus. In the current study, PLA was also the only technique to convincingly demonstrate CaSR expression within glomeruli. Functional effects of the CaSR have been described in both podocytes (28) and in cultured mesangial cells (20, 25). Interestingly, our results showed considerable variation in CaSR expression within glomeruli from the same animal, even between adjacent glomeruli. The reason for this is not currently understood but could reflect differences in functional activity of individual glomeruli at a given time.
In conclusion, this study has demonstrated CaSR expression throughout the nephron and in glomeruli, although the level of expression varied considerably between different cell types. A summary of the distribution and roles of the CaSR in the kidney is shown in Fig. 12. In contrast, there was little variation in the expression pattern between mouse, rat, and human, suggesting conserved functions of the receptor between these species. The highest CaSR expression was detected in the TAL, where this receptor regulates Ca2+ reabsorption. Besides, the CaSR was detected in different cell types from other nephron segments with significantly lower expression compared with the abundantly expressing cells of the TAL. These data corroborate previous functional studies showing CaSR expression in cell types where functional effects have been demonstrated specifically in the PT, DCT, and CD. Furthermore, this information will prove valuable for studies aimed at testing the ability of calcimimetics in transplant patients (44) and of calcilytics for the treatment of hypercalciuria and nephrocalcinosis (27).
Fig. 12.
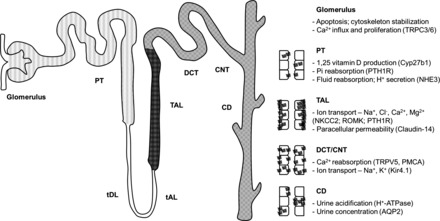
Distribution and roles of the CaSR in the kidney. Diagrams are shown representing the intrarenal distribution (left) and polarity (right) of the CaSR observed in this study, which is comparable in mouse, rat, and human kidney. The strongest CaSR expression was observed in the TAL (dark grey) using in situ hybridization, immunohistochemistry, and proximity ligation assay; however, the CaSR was also consistently detected in the DCT/CNT and CD by these 3 techniques (medium grey). Additionally, weak CaSR expression was detected in the glomerulus and PT using a proximity ligation assay (light gray). The diagrams also summarize functional roles previously reported for the CaSR in the different nephron segments. tDL, thin descending limb; tAL, thin ascending limb; DCT, distal convoluted tubule; CNT connecting tubule; TRPC 3/6, transient receptor potential cation channel 3/6; PTH1R, parathyroid hormone 1 receptor; NHE3, sodium-hydrogen exchanger 3; NKCC2, Na-K-Cl cotransporter 2; ROMK, renal outer medullary K+ channel; TRPV5, transient receptor potential vanilloid 5; PMCA, plasma membrane Ca2+-ATPase; Kir 4.1, inward rectifying potassium channel 4.1.
GRANTS
This work was supported by a Marie Curie Initial Training Network (“Multifaceted CaSR,” to S. A. Price and D. Riccardi).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: J.G., D.R., and S.A.P. provided conception and design of research; J.G., M.S., S.C.B., J.R., W.C., and P.Y. performed experiments; J.G., M.S., S.C.B., J.R., D.R., and S.A.P. analyzed data; J.G., M.S., S.C.B., J.R., H.T., D.R., and S.A.P. interpreted results of experiments; J.G. prepared figures; J.G., M.S., S.C.B., W.C., P.Y., H.T., D.R., and S.A.P. edited and revised manuscript; J.G., M.S., S.C.B., J.R., W.C., P.Y., H.T., D.R., and S.A.P. approved final version of manuscript; D.R. and S.A.P. drafted manuscript.
ACKNOWLEDGMENTS
The authors thank Sonia Eckersley for technical assistance with proximity ligation assays, Roy Milner for assistance with the Co-IPs, and Martin Pollak for the KO mouse sections.
REFERENCES
- 1.Atchison DK, Ortiz-Capisano MC, Beierwaltes WH. Acute activation of the calcium-sensing receptor inhibits plasma renin activity in vivo. Am J Physiol Regul Integr Comp Physiol 299: R1020–R1026, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Awata H, Huang C, Handlogten ME, Miller RT. Interaction of the calcium-sensing receptor and filamin, a potential scaffolding protein. J Biol Chem 276: 34871–34879, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Ba J, Brown D, Friedman PA. Calcium-sensing receptor regulation of PTH-inhibitable proximal tubule phosphate transport. Am J Physiol Renal Physiol 285: F1233–F1243, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Bai M, Quinn S, Trivedi S, Kifor O, Pearce SH, Pollak MR, Krapcho K, Hebert SC, Brown EM. Expression and characterization of inactivating and activating mutations in the human Cao2+-sensing receptor. J Biol Chem 271: 19537–19545, 1996. [DOI] [PubMed] [Google Scholar]
- 5.Bai M, Trivedi S, Brown EM. Dimerization of the extracellular calcium-sensing receptor (CaR) on the cell surface of CaR-transfected HEK293 cells. J Biol Chem 273: 23605–23610, 1998. [DOI] [PubMed] [Google Scholar]
- 6.Block GA, Martin KJ, de Francisco AL, Turner SA, Avram MM, Suranyi MG, Hercz G, Cunningham J, Abu-Alfa AK, Messa P, Coyne DW, Locatelli F, Cohen RM, Evenepoel P, Moe SM, Fournier A, Braun J, McCary LC, Zani VJ, Olson KA, Drueke TB, Goodman WG. Cinacalcet for secondary hyperparathyroidism in patients receiving hemodialysis. N Engl J Med 350: 1516–1525, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Brown EM, Gamba G, Riccardi D, Lombardi M, Butters R, Kifor O, Sun A, Hediger MA, Lytton J, Hebert SC. Cloning and characterization of an extracellular Ca2+-sensing receptor from bovine parathyroid. Nature 366: 575–580, 1993. [DOI] [PubMed] [Google Scholar]
- 8.Bukoski RD, Bian K, Wang Y, Mupanomunda M. Perivascular sensory nerve Ca2+ receptor and Ca2+-induced relaxation of isolated arteries. Hypertension 30: 1431–1439, 1997. [DOI] [PubMed] [Google Scholar]
- 9.Bustamante M, Hasler U, Leroy V, de Seigneux S, Dimitrov M, Mordasini D, Rousselot M, Martin PY, Féraille E. Calcium-sensing receptor attenuates AVP-induced aquaporin-2 expression via a calmodulin-dependent mechanism. J Am Soc Nephrol 19: 109–116, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Capasso G, Geibel PJ, Damiano S, Jaeger P, Richards WG, Geibel JP. The calcium sensing receptor modulates fluid reabsorption and acid secretion in the proximal tubule. Kidney Int 84: 277–284, 2013. [DOI] [PubMed] [Google Scholar]
- 11.Caride AJ, Chini EN, Homma S, Dousa TP, Penniston JT. mRNAs coding for the calcium-sensing receptor along the rat nephron: effect of a low-phosphate diet. Kidney Blood Press Res 21: 305–309, 1998. [DOI] [PubMed] [Google Scholar]
- 12.Di Mise A, Tamma G, Ranieri M, Svelto M, van den Heuvel B, Levtchenko EN, Valenti G. Conditionally immortalized human proximal tubular epithelial cells isolated from the urine of a healthy subject express functional calcium sensing receptor. Am J Physiol Renal Physiol 308: F1200–F1206, 2015. [DOI] [PubMed] [Google Scholar]
- 13.Farajov EI, Morimoto T, Aslanova UF, Kumagai N, Sugawara N, Kondo Y. Calcium-sensing receptor stimulates luminal K+-dependent H+ excretion in medullary thick ascending limbs of Henle's loop of mouse kidney. Tohoku J Exp Med 216: 7–15, 2008. [DOI] [PubMed] [Google Scholar]
- 14.Ferreira MCdJ, Héliès-Toussaint C, Imbert-Teboul M, Bailly C, Verbavatz JM, Bellanger AC, Chabardès D. Coexpression of a Ca2+-inhibitable adenylyl cyclase and of a Ca2+-sensing receptor in the cortical thick ascending limb cell of the rat kidney: inhibition of hormone-dependent cAMP accumulation by extracellular Ca2+. J Biol Chem 273: 15192–15202, 1998. [DOI] [PubMed] [Google Scholar]
- 15.Gama L, Baxendale-Cox LM, Breitwieser GE. Ca2+-sensing receptors in intestinal epithelium. Am J Physiol Cell Physiol 273: C1168–C1175, 1997. [DOI] [PubMed] [Google Scholar]
- 16.Garrett JE, Tamir H, Kifor O, Simin RT, Rogers KV, Mithal A, Gagel RF, Brown EM. Calcitonin-secreting cells of the thyroid express an extracellular calcium receptor gene. Endocrinology 136: 5202–5211, 1995. [DOI] [PubMed] [Google Scholar]
- 17.Gong Y, Renigunta V, Himmerkus N, Zhang J, Renigunta A, Bleich M, Hou J. Claudin-14 regulates renal Ca++ transport in response to CaSR signalling via a novel microRNA pathway. EMBO J 31: 1999–2012, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.House MG, Kohlmeier L, Chattopadhyay N, Kifor O, Yamaguchi T, Leboff MS, Glowacki J, Brown EM. Expression of an extracellular calcium-sensing receptor in human and mouse bone marrow cells. J Bone Miner Res 12: 1959–1970, 1997. [DOI] [PubMed] [Google Scholar]
- 19.Huang C, Sindic A, Hill CE, Hujer KM, Chan KW, Sassen M, Wu Z, Kurachi Y, Nielsen S, Romero MF, Miller RT. Interaction of the Ca2+-sensing receptor with the inwardly rectifying potassium channels Kir4.1 and Kir42 results in inhibition of channel function. Am J Physiol Renal Physiol 292: F1073–F1081, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Kwak JO, Kwak J, Kim HW, Oh KJ, Kim YT, Jung SM, Cha SH. The extracellular calcium sensing receptor is expressed in mouse mesangial cells and modulates cell proliferation. Exp Mol Med 37: 457–465, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Lebart MC, Mejean C, Casanova D, Audemard E, Derancourt J, Roustan C, Benyamin Y. Characterization of the actin binding site on smooth muscle filamin. J Biol Chem 269: 4279–4284, 1994. [PubMed] [Google Scholar]
- 22.Loupy A, Ramakrishnan SK, Wootla B, Chambrey R, de la Faille R, Bourgeois S, Bruneval P, Mandet C, Christensen EI, Faure H, Cheval L, Laghmani K, Collet C, Eladari D, Dodd RH, Ruat M, Houillier P. PTH-independent regulation of blood calcium concentration by the calcium-sensing receptor. J Clin Invest 122: 3355–3367, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maillard MP, Tedjani A, Perregaux C, Burnier M. Calcium-sensing receptors modulate renin release in vivo and in vitro in the rat. J Hypertens 27: 1980–1987, 2009. [DOI] [PubMed] [Google Scholar]
- 24.Maldonado-Perez D, Breitwieser GE, Gama L, Elliott AC, Ward DT, Riccardi D. Human calcium-sensing receptor can be suppressed by antisense sequences. Biochem Biophys Res Commun 311: 610–617, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Meng K, Xu J, Zhang C, Zhang R, Yang H, Liao C, Jiao J. Calcium sensing receptor modulates extracellular calcium entry and proliferation via TRPC3/6 channels in cultured human mesangial cells. PLoS One 9: e98777, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Motoyama HI, Friedman PA. Calcium-sensing receptor regulation of PTH-dependent calcium absorption by mouse cortical ascending limbs. Am J Physiol Renal Physiol 283: F399–F406, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Nemeth EF, Shoback D. Calcimimetic and calcilytic drugs for treating bone and mineral-related disorders. Best Pract Res Clin Endocrinol Metab 27: 373–384, 2013. [DOI] [PubMed] [Google Scholar]
- 28.Oh J, Beckmann J, Bloch J, Hettgen V, Mueller J, Li L, Hoemme M, Gross ML, Penzel R, Mundel P, Schaefer F, Schmitt CP. Stimulation of the calcium-sensing receptor stabilizes the podocyte cytoskeleton, improves cell survival, and reduces toxin-induced glomerulosclerosis. Kidney Int 80: 483–492, 2011. [DOI] [PubMed] [Google Scholar]
- 29.Ortiz-Capisano MC, Ortiz PA, Garvin JL, Harding P, Beierwaltes WH. Expression and function of the calcium-sensing receptor in juxtaglomerular cells. Hypertension 50: 737–743, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Ortiz-Capisano MC, Reddy M, Mendez M, Garvin JL, Beierwaltes WH. Juxtaglomerular cell CaSR stimulation decreases renin release via activation of the PLC/IP3 pathway and the ryanodine receptor. Am J Physiol Renal Physiol 304: F248–F256, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pidasheva S, Grant M, Canaff L, Ercan O, Kumar U, Hendy GN. Calcium-sensing receptor dimerizes in the endoplasmic reticulum: biochemical and biophysical characterization of CASR mutants retained intracellularly. Hum Mol Genet 15: 2200–2209, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Procino G, Mastrofrancesco L, Tamma G, Lasorsa DR, Ranieri M, Stringini G, Emma F, Svelto M, Valenti G. Calcium-sensing receptor and aquaporin 2 interplay in hypercalciuria-associated renal concentrating defect in humans. An in vivo and in vitro study. PLoS One 7: e33145, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Renkema KY, Velic A, Dijkman HB, Verkaart S, van der Kemp AW, Nowik M, Timmermans K, Doucet A, Wagner CA, Bindels RJ, Hoenderop JG. The calcium-sensing receptor promotes urinary acidification to prevent nephrolithiasis. J Am Soc Nephrol 20: 1705–1713, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Riccardi D, Hall AE, Chattopadhyay N, Xu JZ, Brown EM, Hebert SC. Localization of the extracellular Ca2+/polyvalent cation-sensing protein in rat kidney. Am J Physiol Renal Physiol 274: F611–F622, 1998. [DOI] [PubMed] [Google Scholar]
- 35.Riccardi D, Hall AE, Chattopadhyay N, Xu JZ, Brown EM, Hebert SC. Localization of the extracellular Ca2+/polyvalent cation-sensing protein in rat kidney. Am J Physiol Renal Physiol 274: F611–F622, 1998. [DOI] [PubMed] [Google Scholar]
- 36.Riccardi D, Lee WS, Lee K, Segre GV, Brown EM, Hebert SC. Localization of the extracellular Ca2+-sensing receptor and PTH/PTHrP receptor in rat kidney. Am J Physiol Renal Fluid Electrolyte Physiol 271: F951–F956, 1996. [DOI] [PubMed] [Google Scholar]
- 37.Riccardi D, Park J, Lee WS, Gamba G, Brown EM, Hebert SC. Cloning and functional expression of a rat kidney extracellular calcium/polyvalent cation-sensing receptor. Proc Natl Acad Sci USA 92: 131–135, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruat M, Molliver ME, Snowman AM, Snyder SH. Calcium sensing receptor: molecular cloning in rat and localization to nerve terminals. Proc Natl Acad Sci USA 92: 3161–3165, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sands JM, Naruse M, Baum M, Jo I, Hebert SC, Brown EM, Harris HW. Apical extracellular calcium/polyvalent cation-sensing receptor regulates vasopressin-elicited water permeability in rat kidney inner medullary collecting duct. J Clin Invest 99: 1399–1405, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Toka HR, Al-Romaih K, Koshy JM, DiBartolo S, Kos CH, Quinn SJ, Curhan GC, Mount DB, Brown EM, Pollak MR. Deficiency of the calcium-sensing receptor in the kidney causes parathyroid hormone-independent hypocalciuria. J Am Soc Nephrol 23: 1879–1890, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Topala CN, Schoeber JPH, Searchfield LE, Riccardi D, Hoenderop JGJ, Bindels RJM. Activation of the Ca2+-sensing receptor stimulates the activity of the epithelial Ca2+ channel TRPV5. Cell Calcium 45: 331–339, 2009. [DOI] [PubMed] [Google Scholar]
- 42.Wang R, Xu C, Zhao W, Zhang J, Cao K, Yang B, Wu L. Calcium and polyamine regulated calcium-sensing receptors in cardiac tissues. Eur J Biochem 270: 2680–2688, 2003. [DOI] [PubMed] [Google Scholar]
- 43.Ward DT, Brown EM, Harris HW. Disulfide bonds in the extracellular calcium-polyvalent cation-sensing receptor correlate with dimer formation and its response to divalent cations in vitro. J Biol Chem 273: 14476–14483, 1998. [DOI] [PubMed] [Google Scholar]
- 44.Weekers L, de Tullio P, Bovy C, Poma L, Maree R, Bonvoisin C, Defraigne JO, Krzesinski JM, Jouret F. Activation of the calcium-sensing receptor before renal ischemia/reperfusion exacerbates kidney injury. Am J Transl Res 7: 128–138, 2015. [PMC free article] [PubMed] [Google Scholar]
- 45.Yang T, Hassan S, Huang YG, Smart AM, Briggs JP, Schnermann JB. Expression of PTHrP, PTH/PTHrP receptor, and Ca2+-sensing receptor mRNAs along the rat nephron. Am J Physiol Renal Physiol 272: F751–F758, 1997. [DOI] [PubMed] [Google Scholar]
- 46.Yasuoka Y, Sato Y, Healy J, Nonoguchi H, Kawahara K. pH-sensitive expression of calcium-sensing receptor (CaSR) in type-B intercalated cells of the cortical collecting ducts (CCD) in mouse kidney. Clin Exp Nephrol 1–12, 2014. [DOI] [PubMed] [Google Scholar]


