Abstract
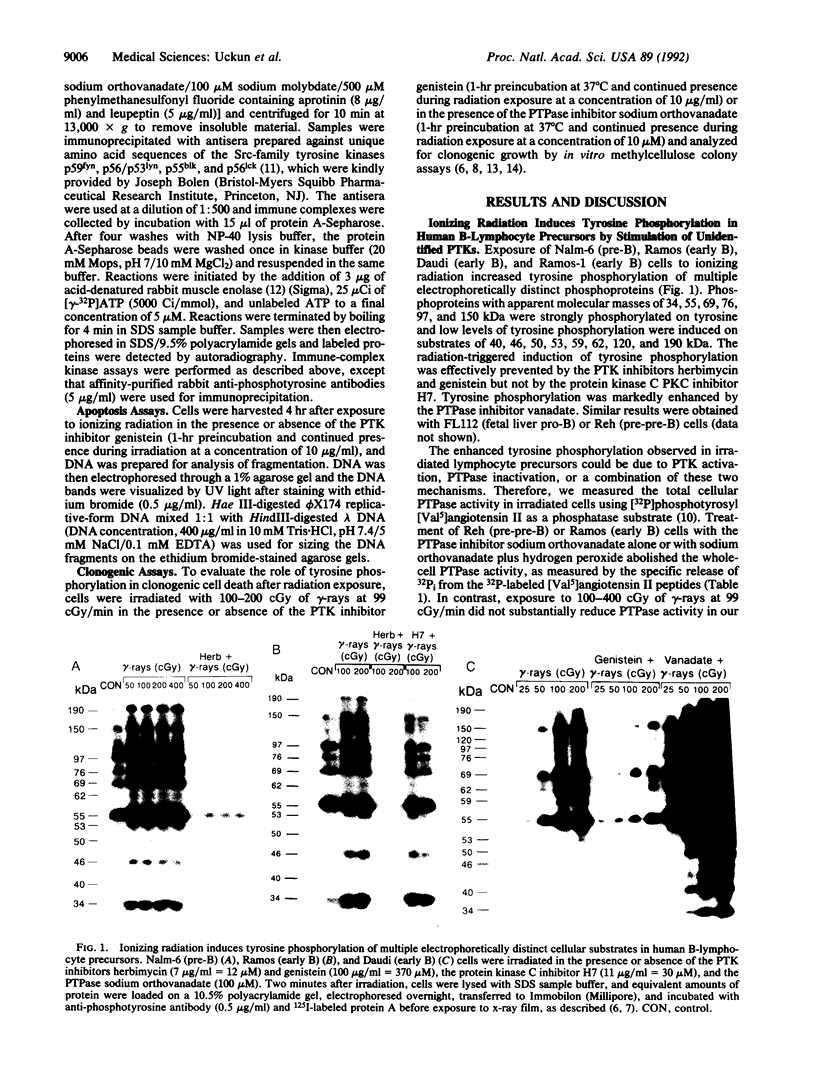
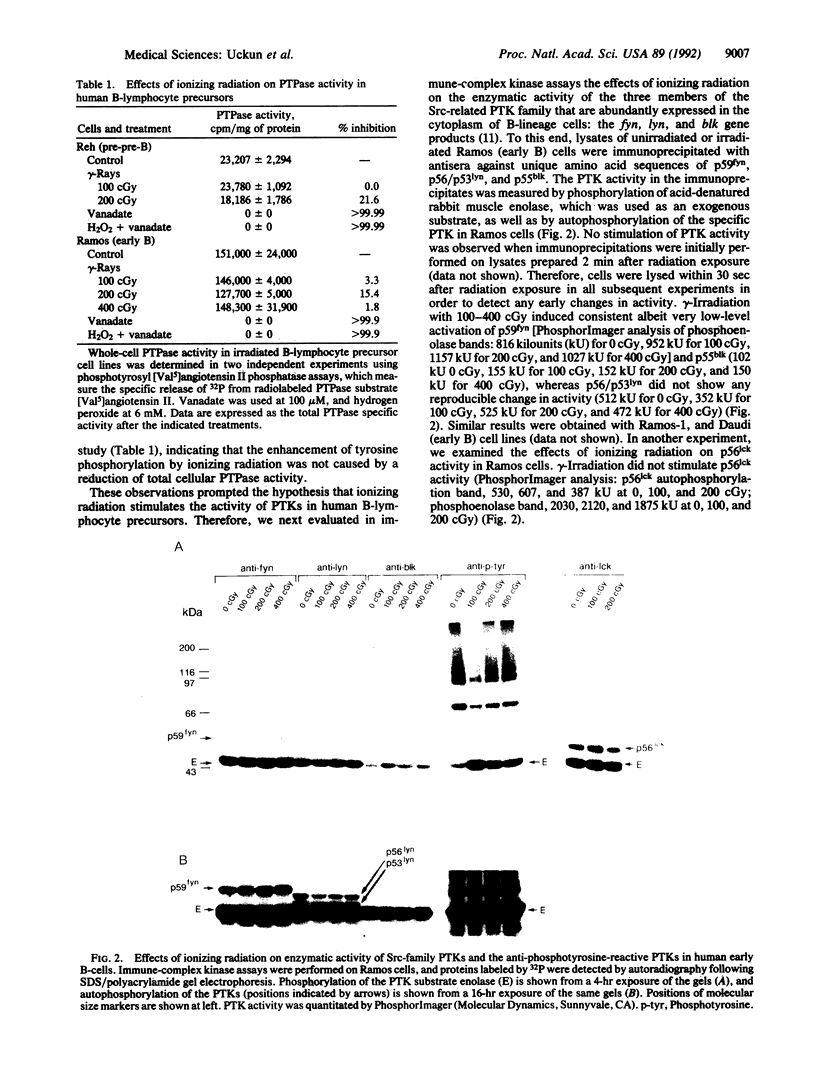
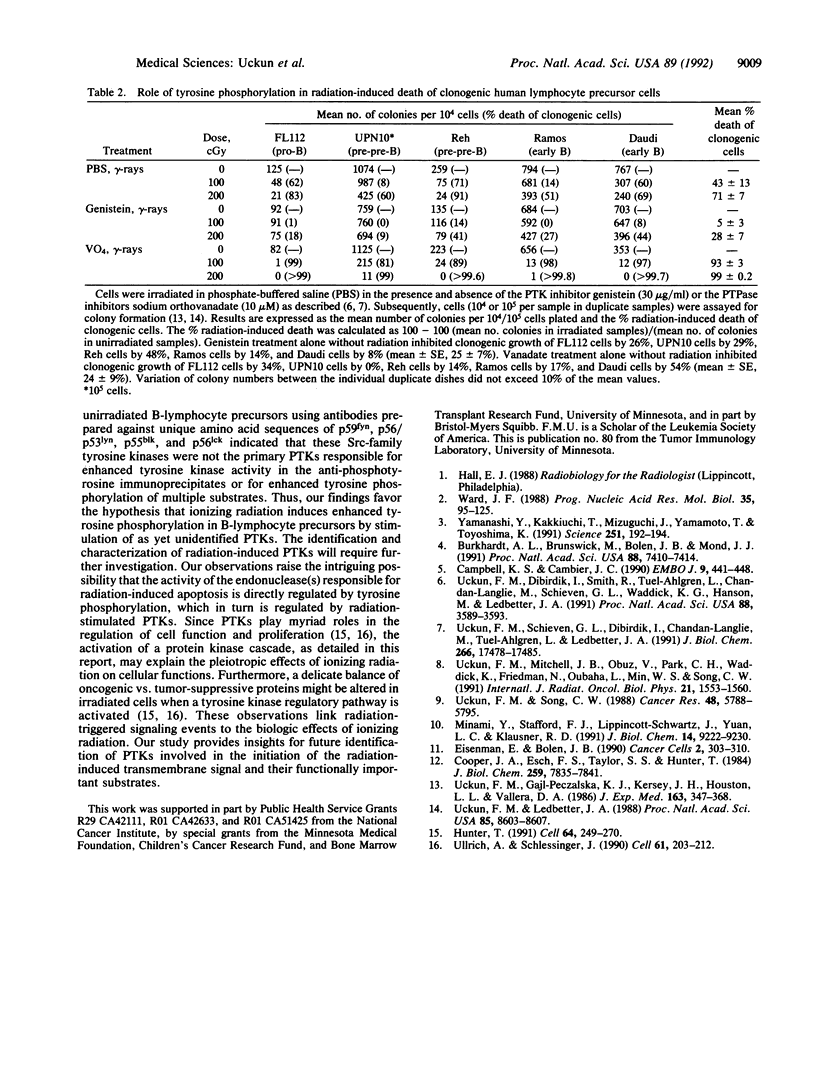
Very little is known regarding the effects of ionizing radiation on cytoplasmic signal transduction pathways. Here, we show that ionizing radiation induces enhanced tyrosine phosphorylation of multiple substrates in human B-lymphocyte precursors. This response to ionizing radiation was also observed in cells pretreated with vanadate, a potent protein-tyrosine-phosphatase (PTPase) inhibitor, and phosphotyrosyl [Val5]angiotensin II phosphatase assays showed no decreased PTPase activity in irradiated cells. Thus, enhanced tyrosine phosphorylation in irradiated B-lymphocyte precursors is not triggered by inhibition of total cellular PTPase activity. Immune-complex kinase assays using anti-phosphotyrosine antibodies demonstrated enhanced protein-tyrosine kinase (PTK) activity in the immunoprecipitates from irradiated cells, and the PTK inhibitors genistein and herbimycin effectively prevented radiation-induced tyrosine phosphorylation. Immune-complex kinase assays on irradiated and unirradiated B-lymphocyte precursors using antibodies prepared against unique amino acid sequences of p59fyn, p56/p53lyn, p55blk, and p56lck demonstrated that these Src-family tyrosine kinases were not the primary PTKs responsible for enhanced tyrosine kinase activity in the anti-phosphotyrosine antibody immunoprecipitates or for enhanced tyrosine phosphorylation of multiple substrates. Thus, our findings favor the hypothesis that ionizing radiation induces enhanced tyrosine phosphorylation in B-lymphocyte precursors by stimulation of as yet unidentified PTKs. Tyrosine phosphorylation appears to be an important proximal step in radiation-induced apoptosis and clonogenic cell death because inhibition of PTK prevents DNA fragmentation and loss of clonogenicity of irradiated B-lymphocyte precursors. Since PTKs play myriad roles in the regulation of cell function and proliferation, the activation of a PTK cascade, as detailed in this report, may explain some of the pleiotropic effects of ionizing radiation on cellular functions of B-lymphocytes and their precursors.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burkhardt A. L., Brunswick M., Bolen J. B., Mond J. J. Anti-immunoglobulin stimulation of B lymphocytes activates src-related protein-tyrosine kinases. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7410–7414. doi: 10.1073/pnas.88.16.7410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell K. S., Cambier J. C. B lymphocyte antigen receptors (mIg) are non-covalently associated with a disulfide linked, inducibly phosphorylated glycoprotein complex. EMBO J. 1990 Feb;9(2):441–448. doi: 10.1002/j.1460-2075.1990.tb08129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Esch F. S., Taylor S. S., Hunter T. Phosphorylation sites in enolase and lactate dehydrogenase utilized by tyrosine protein kinases in vivo and in vitro. J Biol Chem. 1984 Jun 25;259(12):7835–7841. [PubMed] [Google Scholar]
- Eiseman E., Bolen J. B. src-related tyrosine protein kinases as signaling components in hematopoietic cells. Cancer Cells. 1990 Oct;2(10):303–310. [PubMed] [Google Scholar]
- Hunter T. Cooperation between oncogenes. Cell. 1991 Jan 25;64(2):249–270. doi: 10.1016/0092-8674(91)90637-e. [DOI] [PubMed] [Google Scholar]
- Minami Y., Stafford F. J., Lippincott-Schwartz J., Yuan L. C., Klausner R. D. Novel redistribution of an intracellular pool of CD45 accompanies T cell activation. J Biol Chem. 1991 May 15;266(14):9222–9230. [PubMed] [Google Scholar]
- Uckun F. M., Dibirdik I., Smith R., Tuel-Ahlgren L., Chandan-Langlie M., Schieven G. L., Waddick K. G., Hanson M., Ledbetter J. A. Interleukin 7 receptor ligation stimulates tyrosine phosphorylation, inositol phospholipid turnover, and clonal proliferation of human B-cell precursors. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3589–3593. doi: 10.1073/pnas.88.9.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uckun F. M., Gajl-Peczalska K. J., Kersey J. H., Houston L. L., Vallera D. A. Use of a novel colony assay to evaluate the cytotoxicity of an immunotoxin containing pokeweed antiviral protein against blast progenitor cells freshly obtained from patients with common B-lineage acute lymphoblastic leukemia. J Exp Med. 1986 Feb 1;163(2):347–368. doi: 10.1084/jem.163.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uckun F. M., Ledbetter J. A. Immunobiologic differences between normal and leukemic human B-cell precursors. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8603–8607. doi: 10.1073/pnas.85.22.8603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uckun F. M., Mitchell J. B., Obuz V., Park C. H., Waddick K., Friedman N., Oubaha L., Min W. S., Song C. W. Radiation sensitivity of human B-lineage lymphoid precursor cells. Int J Radiat Oncol Biol Phys. 1991 Nov;21(6):1553–1560. doi: 10.1016/0360-3016(91)90332-x. [DOI] [PubMed] [Google Scholar]
- Uckun F. M., Schieven G. L., Dibirdik I., Chandan-Langlie M., Tuel-Ahlgren L., Ledbetter J. A. Stimulation of protein tyrosine phosphorylation, phosphoinositide turnover, and multiple previously unidentified serine/threonine-specific protein kinases by the Pan-B-cell receptor CD40/Bp50 at discrete developmental stages of human B-cell ontogeny. J Biol Chem. 1991 Sep 15;266(26):17478–17485. [PubMed] [Google Scholar]
- Uckun F. M., Song C. W. Radiobiological features of fresh leukemic bone marrow progenitor cells in acute lymphoblastic leukemia. Cancer Res. 1988 Oct 15;48(20):5788–5795. [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Ward J. F. DNA damage produced by ionizing radiation in mammalian cells: identities, mechanisms of formation, and reparability. Prog Nucleic Acid Res Mol Biol. 1988;35:95–125. doi: 10.1016/s0079-6603(08)60611-x. [DOI] [PubMed] [Google Scholar]
- Yamanashi Y., Kakiuchi T., Mizuguchi J., Yamamoto T., Toyoshima K. Association of B cell antigen receptor with protein tyrosine kinase Lyn. Science. 1991 Jan 11;251(4990):192–194. doi: 10.1126/science.1702903. [DOI] [PubMed] [Google Scholar]