Abstract
Lung function is an independent predictor of mortality and serves as an aging marker in never smokers. The protein sirtuin-1 of gene SIRT1 has profound anti-inflammatory effects and regulates metabolic pathways. Its suggested longevity effects on lower organisms remain poorly studied in humans. In 1132 never smokers of the population-based SAPALDIA cohort, we investigated associations between single nucleotide polymorphisms (SNPs; rs730821, rs10997868, rs10823116) of SIRT1 and aging-related lung function decline over 11 years in terms of change in forced expiratory volume in the first second (FEV1), forced vital capacity (FVC), FEV1/FVC ratio, and forced expiratory flow between 25 and 75 % of FVC (FEF25–75) using multiple linear regression models. Interactions between the SIRT1 SNPs and adiposity parameters (body mass index (BMI), its change and weight gain) were tested by including multiplicative interaction terms into the models. SIRT1 polymorphisms exhibited no main effects, but modified the association between obesity measures and FEV1/FVC and FEF25–75 decline (p = 0.009–0.046). Per risk allele, FEV1/FVC decline was accelerated up to −0.5 % (95 % CI −1.0 to 0 %) and −0.7 % (−1.3 to −0.2 %) over interquartile range increases in BMI (2.4 kg/m2) or weight (6.5 kg), respectively. For FEF25–75 decline, corresponding estimates were −57 mL/s (−117 to 4 mL/s) and −76 mL/s (−1429 to −9 mL/s). Interactions were not present in participants with genetically lowered C-reactive protein concentrations. Genetic variation in SIRT1 might therefore affect lung function and human longevity by modifying subclinical inflammation arising from abdominal adipose tissue.
Electronic supplementary material
The online version of this article (doi:10.1007/s11357-016-9917-y) contains supplementary material, which is available to authorized users.
Keywords: Sirtuin-1, Lung, Metabolism, Inflammation, Cohort study, Population-based
Introduction
The level of lung function is one of the most consistent and long-known predictors of survival (Burney and Hooper 2011; Knuiman et al. 1999; Schunemann et al. 2000). Lung function naturally starts to decline at the age of 30 years (Quanjer et al. 2012), and accelerated decline in adult life was repeatedly associated with increased mortality, not only due to cardiovascular and respiratory disease (Tockman et al. 1995) which could be attributable to smoking but also with all-cause mortality and in never smokers (Mannino et al. 2006; Ryan et al. 1999). According to recent findings from the British Whitehall study, the direct effects of smoking only explain 4.9 % of the association between lung function and mortality, whereas 21.3 % could be attributed to inflammatory processes in the body and a further 9.8 % to lifestyle factors including nutrition and body fitness (Sabia et al. 2010). Moreover, increases in inflammatory biomarkers were also longitudinally associated with accelerated lung function decline (Ahmadi-Abhari et al. 2014; Shaaban et al. 2006). The level of lung function thus not only reflects past pulmonary exposures to tobacco smoke or environmental hazards or presence of respiratory disease but also equally functions as a biomarker of aging processes in the body (Albrecht et al. 2014).
Biological processes related to aging include persistent low-grade inflammation, mitochondrial malfunctioning with reduced energy expenditure, reduced nutrient sensing, and metabolic disturbances (Franceschi et al. 2000; Lopez-Otin et al. 2013). The accumulation of intra-abdominal adipose tissue during normal aging (Picard and Guarente 2005) plays an important role in this respect, as it alters the endocrine profile of adipocytes towards inflammation, dyslipidemia, and insulin resistance (Berg and Scherer 2005; Calabro et al. 2009). Increased abdominal adiposity is associated with adverse cardiovascular outcomes (Berg and Scherer 2005) and was also repeatedly related to impaired lung function (Leone et al. 2009; Ochs-Balcom et al. 2006; Wehrmeister et al. 2012). In studies relying on waist-to-hip ratio, waist circumference, or imaging techniques as markers of intra-abdominal fat tissue, higher abdominal fat mass was most often associated with increased overall mortality (Bigaard et al. 2005; Reis et al. 2009; Zhang et al. 2008).
A central protein involved in the regulation of aging-related metabolic functions is sirtuin-1, which is encoded by the gene SIRT1. Sirtuin-1 is a class III histone deacetylase regulating nuclear transcription. It was first described in yeast Saccharomyces cerevisiae and suggested to extend lifespan under starving conditions (Burnett et al. 2011; Guarente 2011; Herranz et al. 2010; Herranz and Serrano 2010; Kennedy et al. 1997; Rizki et al. 2011; Viswanathan and Guarente 2011). Upregulation of sirtuin-1 reduces lipid storage, mobilizes fatty acids in white adipose tissue, and increases their β-oxidation in liver and muscle tissue. It activates biosynthesis of mitochondria and increases peripheral insulin sensitivity and central nutrient sensing in the hypothalamus (Guarente 2011). By modulating the expression of the cardinal pathways of forkhead transcription factors of class O (FoxOs), nuclear factor-kappa-B (NFκB), and p53, sirtuin-1 also plays a major role in cellular defense against oxidative stress and inflammation, and at higher stress levels, it protects cells from senescence and apoptosis (Chong et al. 2012; Radak et al. 2013). Likewise, experimental studies in rodents have shown that SIRT1 overexpression mitigates matrix metalloprotease imbalance, cell senescence, and autophagy in lungs upon cigarette smoke exposure (Hwang et al. 2010; Yao et al. 2013; Yao et al. 2014).
Because of the proposed longevity effects in several species, sirtuin-1 and other members of its enzyme family have been in the focus of aging research over recent years. Studies in humans have thereby focused on cardiovascular risk factors and health outcomes due to the anti-inflammatory and profound metabolic functions of sirtuin-1. Population-based cohort studies found significant associations between common single nucleotide polymorphisms (SNPs) in SIRT1 and increased glucose tolerance, body mass index, overweight, and atherosclerosis (Figarska et al. 2013; Kedenko et al. 2014; Zillikens et al. 2009a), but except for one study (Figarska et al. 2013), not with survival (Flachsbart et al. 2006; Kuningas et al. 2007; Zillikens et al. 2009b). Moreover, a Dutch study using data from two population-based cohorts assessed the relationship of SIRT1 SNPs with lung function levels but did not observe significant associations (Siedlinski et al. 2012). However, the diverse regulatory roles of sirtuin-1 in the body also warrant consideration of several pathways and potential interactions.
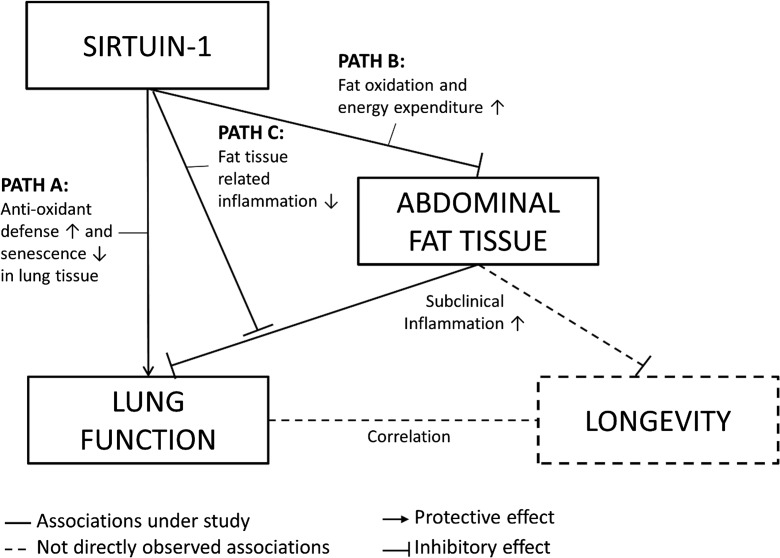
In the current study, we investigated age-related lung function decline in never smokers as a proxy for aging processes. We hypothesized that the level of SIRT1 expression could affect the course of lung function decline in different ways, as depicted in Fig. 1: first, high sirtuin-1 concentrations could boost anti-inflammatory and anti-oxidative defenses locally in the lungs and protect them from inflammation, apoptosis, and tissue remodeling (path A); second, it could reduce the amount of abdominal fat tissue and consequently the accompanying, subclinical inflammation (path B); third, sirtuin-1 could mitigate the systemic, inflammatory effects arising from abdominal fat accumulation (path C).
Fig. 1.
Hypothesized paths of sirtuin-1 effects on lung function decline as proxy measure of aging
Using data from the population-based Swiss SAPALDIA cohort study, we thus investigated possible associations and interactions between lung function decline, common SNPs in SIRT1, and several measures of body fat mass (including body mass index (BMI), its change and gain in weight) as potential evidence for the involvement of SIRT1 in human aging.
Methods
Design and study population
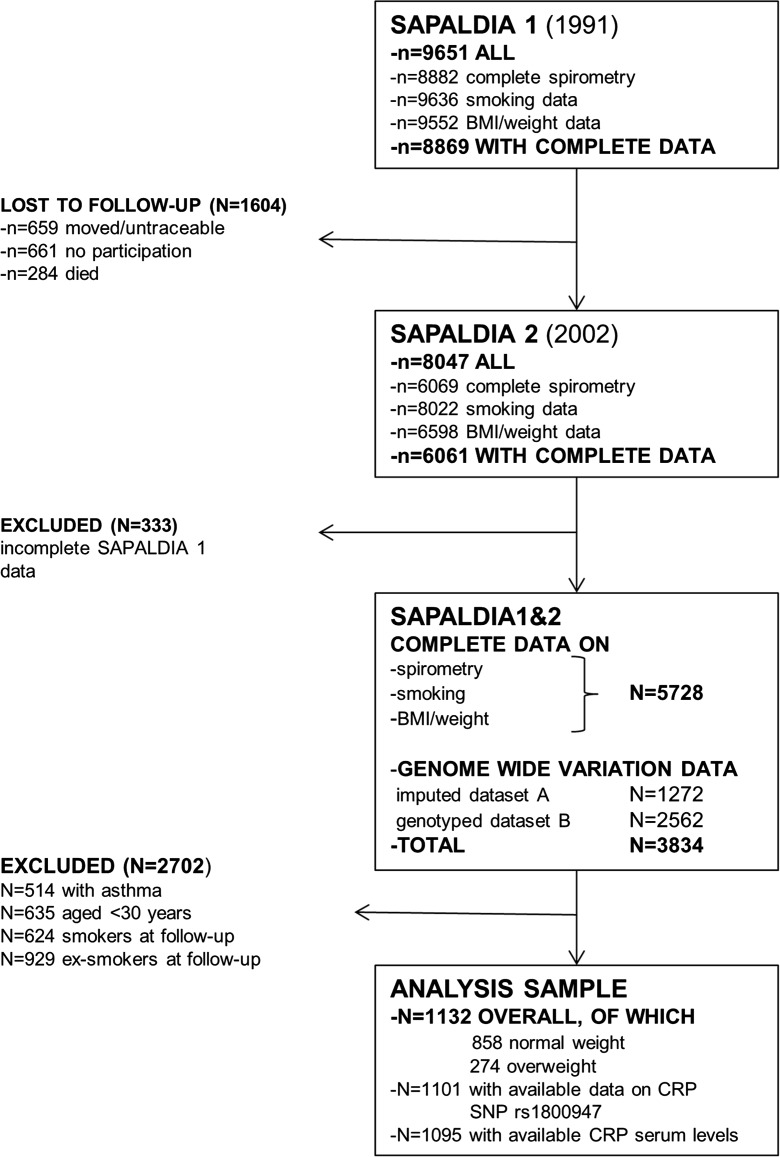
The Swiss cohort study on air pollution and lung and heart disease in adults (SAPALDIA) recruited adults aged 18–60 years from eight Swiss communities in 1991 and followed them up in the year 2002. A detailed overview of the study design and methods is given elsewhere (Ackermann-Liebrich et al. 2005). The current analysis comprised a study sample (Fig. 2) restricted to 1132 non-asthmatic, never smoking adults who attended the baseline and the first follow-up examination including spirometry, who had genotype data on common SIRT1 variants available, and who were aged at least 30 years at baseline. The restrictions were applied since our aim was to study the association of SIRT1 genetic variation with natural lung function decline as a marker of biological aging. Asthmatic participants were also excluded due to their known, differing genetic background affecting lung function (Imboden et al. 2012). Current smokers at follow-up were included in a sensitivity analysis to validate the functional impact of genetic associations.
Fig. 2.
Selection of study sample
All study participants gave written informed consent, and the study was approved by the Swiss Academy of Medical Sciences as well as the respective regional ethics committees.
Data collection
Spirometry
Spirometry was done with Sensormedics devices (Sensormedics model 2200, Yorba Linda, USA) following the protocol of the European Community Respiratory Health Survey. Devices were calibrated daily, and comparability between devices was checked. Participants performed three to eight forced expiratory maneuvers which were subjected to American Thoracic Society quality criteria (American Thoracic Society 1987). For the forced expiratory volume in the first second of exhalation (FEV1) and forced vital capacity (FVC), at least two acceptable values were recorded. The FEV1/FVC ratio (a marker of airway obstruction) and mean forced expiratory flow between 25 and 75 % of FVC (FEF25–75) were derived from the maneuver with the highest sum of FVC and FEV1. Longitudinal change was calculated as the difference between baseline and follow-up value. SAPALDIA-specific lung function equations were used for the calculation of percent predicted lung function values and lower limits of normal (Brandli et al. 2000).
Body measures
For the measurement of weight and height, participants wore no shoes or heavy clothes. BMI was calculated by dividing weight (in kilograms) by the square of height (in meters). Change in BMI and weight was calculated as the respective difference between follow-up and baseline value. Overweight was defined as BMI ≥ 75th percentile of the observed population distribution (corresponding to BMI ≥ 26.3 kg/m2). This definition was chosen because more suitable measures such as waist-to-hip ratio were not available. Moreover, the optimal BMI threshold to capture subclinical inflammation is unknown, and previous studies have shown that the WHO overweight definition (BMI ≥ 25 kg/m2) does not correlate well with the amount of abdominal adipose tissue (Goyal et al. 2014). Weight gain during 11 years of follow-up was used to approximate the increase in abdominal adipose tissue in our adult population. Given our interest in gain of abdominal adipose tissue, participants experiencing a weight loss of 2 kg (corresponding to the median of the observed weight loss distribution) or more were excluded from weight gain analyses.
Covariates
Questionnaires were applied to gather information on smoking behavior and socioeconomic factors. Education was categorized into low (primary school only), medium (high school), and high (university or college degree) depending on the highest attained degree at follow-up.
Never smokers were defined as having smoked less than 20 packs of cigarettes or 360 g of tobacco during their lifetime. Current smokers indicated active smoking within 30 days prior to the follow-up examination.
SIRT1 genotypes
Blood samples were collected at follow-up examination. Genomic DNA was extracted manually from EDTA-buffered whole blood using the Puregene™ DNA Isolation Kit (Gentra Systems, Plymouth, MN, USA).
The genotype data on SIRT1 SNPs used in this analysis consisted of three tagging SNPs (rs730821, rs10997868, rs10823116) obtained from two different genotyping platforms (Fig. 2). The three SNPs were selected to represent the common genetic variation in our study population in terms of the observed linkage disequilibrium in the SIRT1 region.
Genotype data A was typed on the Illumina Human 610Kquad BeadChip (Moffatt et al. 2010) and subsequently imputed to 2.5 Mio SNPs using the HapMap v22 CEPH reference panel of Utah residents with ancestry from northern and western Europe (International HapMap Consortium 2005) to fill in missing genotype values and improve genomic coverage. Genotype data B was typed on the Illumina Human OmniExpress-Exome BeadChip in a non-overlapping sample of the SAPALDIA population. Stringent quality control filters were applied to both platforms.
Details of the genotyping procedures and biomarker measurements are given in Online Resource 1.
Statistical analysis
The distributions of sex, age, educational level, lung function parameters FEV1, FVC, FEV1/FVC, FEF25–75, and their change during the first 11 years of follow-up, as well as those of adiposity measures and CRP levels, were tabulated for the whole study population and for normal and overweight subgroups.
Main effects of SIRT1 SNPs were modeled using multiple linear regression of change in FEV1, FVC, FEV1/FVC, and FEF25–75 adjusting for the percent predicted lung function value at baseline, sex, age, height, education, and study area. Age and height were centered at the median value of the population distribution for all regression analyses. SNPs were modeled under an additive genetic model. To account for uncertainty in imputation, observations with imputed SIRT1 genotypes (genotypes from genotyping platform A) were weighted with the imputation quality metric in regression analysis. Effect estimates were compared across models incrementally adjusting for BMI at baseline (centered at its median), its change, or weight change during follow-up.
Next, interaction analyses were performed by including multiplicative interaction terms between SIRT1 SNPs and change in BMI or weight gain during follow-up in multiple regression models adjusting for the same set of covariates. Estimates were scaled to represent effects over an increase of interquartile range (IQR) in BMI (2.42 units) and weight (6.5 kg). For weight gain, interaction analyses were additionally adjusted for baseline BMI.
To evaluate the possible impact of multiple testing on our results, interaction models with change in BMI and weight gain were re-specified as multivariate multiple regressions on all four outcomes (thereby taking account of the correlation between the lung function decline parameters). Interaction terms and main effects of all three SNPs were simultaneously included into the model, and the null hypothesis that none of the SNPs presented a statistically significant interaction was eventually tested using a joint Wald test on all interaction terms.
The interaction models were stratified by the genotypes of C-reactive protein (CRP) gene SNP rs1800947 to assess the influence of inflammatory pathways. rs1800947 is a functional SNP whose C-alleles have been associated with lower systemic levels of C-reactive protein (Kettunen et al. 2011). Due to the low minor allele frequency of 6.4 %, the rare allele genotypes GC and CC of rs1800947 were grouped together and compared to the common GG genotype.
To exclude undiagnosed cases of lung disease, interaction models were recalculated after excluding participants with spirometric obstruction at baseline (FEV1/FVC < 5th percentile of the population-specific distribution in never smokers, representing the lower limit of normal) or with wheezing without a cold in 12 months before examination in a sensitivity analysis.
Finally, SIRT1 SNP effects were reassessed in current smokers at follow-up regarding size and direction of genetic main effects on lung function to validate their functional impact in comparison to never smokers.
All analyses were conducted using STATA IC version 12.1 (StataCorp, College Station, TX, USA). Two-sided α-values of 0.05 were chosen as significance thresholds for main effects and interactions, respectively. Multivariate analyses were done using STATA module “mvreg.”
Results
Characteristics of study population
The basic characteristics of the whole study population and of normal and overweight subgroups are shown in Table 1. 60.7 % of our study population was female, and 12.3 % had only primary school education. Mean (standard deviation) age was 44.8 (8.7) years. Baseline weight was 67.4 (11.9) kilograms (kg) and BMI 23.8 (3.5) kg/meter2 (kg/m2). Mean lung function values were 3.44 (0.78) liters (L), 4.38 (1.00) L, 78.8 (6.7) %, and 3.27 (1.11) L/second (L/s) for FEV1, FVC, FEV1/FVC, and FEF25–75, respectively. These values declined by 0.38 (0.29) L, 0.30 (0.39) L, 3.7 (5.0) %, and 0.73 (0.67) L/s over 11 years of follow-up. During the same period, participants gained 6.1 (5.1) kg weight on average, and their BMI increased by 2.2 (2.0) units. Participants with overweight at baseline were on average older, had less education, and slightly worse FEV1/FVC and FEF25–75 values than normal weight participants. Of note, overweight participants had considerably higher C-reactive protein levels (median 1.7, IQR 0.9–3.7 mg/L) than those with normal weight (0.8, IQR 0.4–1.7 mg/L).
Table 1.
Baseline characteristics of whole study population and by strata of overweight
| Variable | All | Normal weight | Overweight | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Estimates | n | Estimates | n | Estimates | ||||
| Female sex | 1132 | 687 | /60.7 | 858 | 545 | /63.5 | 274 | 142 | /51.8 |
| Baseline age (years) | 1132 | 44.8 | (8.7) | 858 | 43.9 | (8.5) | 274 | 47.5 | (8.7) |
| Education primary only | 1132 | 139 | /12.3 | 858 | 83 | /9.7 | 274 | 56 | /20.4 |
| High school | 1132 | 782 | /69.1 | 858 | 599 | /69.8 | 274 | 183 | /66.8 |
| College/university | 1132 | 211 | /18.6 | 858 | 176 | /20.5 | 274 | 35 | /12.8 |
| Baseline FEV1 (L) | 1132 | 3.44 | (0.78) | 858 | 3.45 | (0.76) | 274 | 3.41 | (0.86) |
| FVC (L) | 1132 | 4.38 | (1.00) | 858 | 4.37 | (0.95) | 274 | 4.39 | (1.12) |
| FEV1/FVC (%) | 1132 | 78.8 | (6.7) | 858 | 79.1 | (6.8) | 274 | 77.9 | (6.2) |
| FEF25–75 (L/s) | 1132 | 3.27 | (1.11) | 858 | 3.30 | (1.11) | 274 | 3.16 | (1.10) |
| Change in FEV1 (L) | 1132 | -0.38 | (0.29) | 858 | -0.37 | (0.29) | 274 | -0.42 | (0.31) |
| FVC (L) | 1132 | -0.30 | (0.39) | 858 | -0.27 | (0.38) | 274 | -0.39 | (0.41) |
| FEV1/FVC (%) | 1132 | -3.7 | (5.0) | 858 | -3.9 | (4.8) | 274 | -3.1 | (5.3) |
| FEF25–75 (L/s) | 1132 | -0.73 | (0.67) | 858 | -0.74 | (0.64) | 274 | -0.69 | (0.75) |
| Baseline height (cm) | 1132 | 168 | (9) | 858 | 168 | (9) | 274 | 167 | (10) |
| Baseline weight (kg) | 1132 | 67.4 | (11.9) | 858 | 63.3 | (9.2) | 274 | 80.2 | (10.5) |
| Change in weight (kg)a | 1071 | 6.1 | (5.1) | 824 | 5.7 | (4.9) | 247 | 7.3 | (5.7) |
| Baseline BMI (kg/m2) | 1132 | 23.8 | (3.5) | 858 | 22.3 | (2.1) | 274 | 28.6 | (2.5) |
| Change in BMI (kg/m2) | 1132 | 2.2 | (2.0) | 858 | 2.1 | (1.9) | 274 | 2.4 | (2.5) |
| Follow-up CRP (mg/L) | 1095 | 1 | (0.5/2.1) | 842 | 0.8 | (0.4/1.7) | 253 | 1.7 | (0.9/3.7) |
Estimates are in numbers/% for count data, mean (standard deviation) for continuous data, and median (25th/75th percentile) for CRP
CRP C-reactive protein serum value, values > = 10 mg/L have been excluded, FEV 1 forced expiratory volume in 1 s of expiration, FVC forced vital capacity, FEF 25–75 forced expiratory flow between 25th and 75th percentile of FVC, L liters, s second
aValues ≤−2 kg (the median value of all participants with weight loss) have been excluded
Main effects of SIRT1 SNPs on lung function decline
No statistically significant associations of SIRT1 SNPs rs730821, rs10997868, and rs10823116 with change in lung function parameters were observed, and additional adjustment for baseline BMI value, change in BMI or weight during follow-up only slightly altered the effect estimates (Table 1 in Online Resource 2).
Main effects of SIRT1 SNPs stratified by overweight at baseline
Within strata of normal weight and overweight, no statistically significant main effects were observed (Table 2). However, allele effects on change in FEV1/FVC differed significantly between the strata for SNP rs10823116: 0.2 % (95 % confidence interval −0.6 to 1.1) in normal weight versus −1.8 % (−3.9 to 0.3) in overweight participants (p = 0.048 for interaction). Effect estimates also differed significantly for change in FEF25–75 (p = 0.022 for interaction) and amounted to 47 mL/s (−57 to 150) in normal weight versus −244 mL/s (−495 to 6) in overweight participants. No interactions were observed between SIRT1 SNPs and baseline BMI as continuous variable (data not shown).
Table 2.
SIRT1 SNP main effects on change in lung function stratified by overweight status
| Outcome | SNP | Eff. all. | Freq | Normal weight (n = 858) | Overweight (n = 274) | All (n = 1132) | |||
|---|---|---|---|---|---|---|---|---|---|
| β-coeff. (95 % CI) | p | β-coeff. (95 % CI) | p | β-coeff. (95 % CI) | p | ||||
| Change in FEV1 (in mL) | rs730821 a | C | 0.3 | 3 (−27 to 34) | 0.83 | -11 (−75 to 53) | 0.74 | 0 (−28 to 27) | 0.98 |
| rs10997868 | C | 0.68 | -11 (−35 to 14) | 0.41 | -21 (−77 to 35) | 0.46 | -12 (−35 to 11) | 0.30 | |
| rs10823116 | G | 0.13 | -20 (−73 to 33) | 0.46 | -117 (−242 to 9) | 0.068 | -38 (−88 to 12) | 0.13 | |
| Change in FVC (in mL) | rs730821 a | C | 0.38 | 0 (−43 to 44) | 0.99 | -4 (−85 to 78) | 0.93 | 1 (−38 to 39) | 0.97 |
| rs10997868 | C | 0.68 | -13 (−49 to 23) | 0.48 | -33 (−106 to 41) | 0.38 | -14 (−46 to 19) | 0.41 | |
| rs10823116 | G | 0.13 | -39.2 (−106 to 28) | 0.25 | -84 (−248 to 80) | 0.32 | -42 (−105 to 22) | 0.20 | |
| Change in FEV1/FVC (in percent) | rs730821 a | C | 0.38 | 0.1 (−0.5 to 0.6) | 0.80 | -0.2 (−1.2 to 0.9) | 0.75 | 0.0 (−0.5 to 0.4) | 0.85 |
| rs10997868 | C | 0.68 | 0.0 (−0.4 to 0.4) | 0.93 | 0.1 (−0.8 to 1.0) | 0.84 | 0.0 (−0.4 to 0.3) | 0.85 | |
| rs10823116 | G | 0.13 | 0.2 (−0.6 to 1.1)* | 0.64 | -1.8 (−3.9 to 0.3)* | 0.099 | -0.3 (−1.1 to 0.5) | 0.48 | |
| Change in FEF25–75 (in mL/s) | rs730821 a | C | 0.38 | 9 (−52 to 71) | 0.77 | -19 (−156 to 118) | 0.79 | -4 (−61 to 52) | 0.88 |
| rs10997868 | C | 0.68 | -13 (−62 to 37) | 0.62 | -10 (−128 to 108) | 0.87 | -18 (−65 to 28) | 0.45 | |
| rs10823116 | G | 0.13 | 47 (−57 to 150)* | 0.37 | -244 (−495 to 6)* | 0.056 | -20 (−117 to 78 | 0.69 | |
Estimates are given per effect allele. Negative values mean an acceleration and positive an attenuation of lung function decline. All models were adjusted for percent predicted baseline value of lung function, sex, age (centered to median), height (centered to median), educational level, and study area
FEV1 forced expiratory volume in 1 s of expiration, FVC forced vital capacity, FEF 25–75 forced expiratory flow between 25th and 75th percentile of FVC, overweight BMI ≥ 75th percentile of the observed distribution, Eff. all. effect allele, Freq effect allele frequency, β-coeff beta-coefficient, 95 % CI 95 % confidence interval, p p value
*Statistically significant interaction (p < 0.05)
a n = 1130 in whole study sample and n = 856 in normal weight due to missing genotype data
Interaction effects with change in BMI and weight
SNP rs730821 presented a significant interaction with change in BMI on the change in FEV1/FVC ratio, while corresponding estimates were marginally significant for rs10997868 and rs10823116 (p = 0.033 to p = 0.093; Table 3). Over an IQR increase in BMI, FEV1/FVC decline was accelerated by −0.5 % (−1.0 to 0.0; rs730821) per each effect allele compared to the homozygous reference group. The corresponding estimate for an IQR increase in weight was larger, amounting to −0.7 % (−1.3 to −0.2; rs730821), and interaction p values were stronger (p = 0.009, rs730821; p = 0.070, rs10997868; and p = 0.094, rs10823116).
Table 3.
Interaction effect estimates between SIRT1 SNPs and change in BMI or weight on change in lung function
| Outcome | SNP | Eff. all. | Freq | Effect estimates for interaction with | |||||
|---|---|---|---|---|---|---|---|---|---|
| change in BMI (n = 1132) | change in weightb (n = 1071) | ||||||||
| β-coeff. | 95 % CI | p | β-coeff | 95 % CI | p | ||||
| Change in FEV1 (in mL) | rs730821 a | C | 0.38 | 7 | (−27 to 41) | 0.68 | 5 | (−33 to 42) | 0.81 |
| rs10997868 | C | 0.68 | -3 | (−29 to 23) | 0.82 | -10 | (−38 to 18) | 0.47 | |
| rs10823116 | G | 0.13 | -33 | (−95 to 29) | 0.29 | -41 | (−111 to 29) | 0.25 | |
| Change in FVC (in mL) | rs730821 a | C | 0.38 | 43 | (−7 to 92) | 0.091 | 49 | (−7 to 105) | 0.087 |
| rs10997868 | C | 0.68 | 18 | (−21 to 56) | 0.36 | 11 | (−29 to 51) | 0.60 | |
| rs10823116 | G | 0.13 | -7 | (−83 to 70) | 0.87 | -10 | (−97 to 77) | 0.82 | |
| Change in FEV1/FVC (in percent) | rs730821 a | C | 0.38 | -0.5 | (−1.0 to 0.0) | 0.033 * | -0.7 | (−1.3 to −0.2) | 0.009 * |
| rs10997868 | C | 0.68 | -0.4 | (−0.8 to 0.1) | 0.085 | -0.4 | (−0.9 to 0.0) | 0.070 | |
| rs10823116 | G | 0.13 | -0.8 | (−1.6 to 0.1) | 0.093 | -0.9 | (−1.9 to 0.2) | 0.094 | |
| Change in FEF25–75 (in mL/s) | rs730821 a | C | 0.38 | -57 | (−117 to 4) | 0.066 | -76 | (−142 to −9) | 0.025 * |
| rs10997868 | C | 0.68 | -41 | (−90 to 9) | 0.10 | -56 | (−111 to −1) | 0.046 * | |
| rs10823116 | G | 0.13 | -95 | (−204 to 13) | 0.086 | -112 | (−239 to 15) | 0.084 | |
Estimates for the multiplicative interaction term are given per effect allele and interquartile range increase in BMI (2.42 units) and weight (6.5 kg). Negative values mean an acceleration and positive an attenuation of lung function decline. All models were adjusted for percent predicted baseline value of lung function, sex, age (centered to median), height (centered to median), educational level, and study area
FEV 1 forced expiratory volume in 1 s of expiration, FVC forced vital capacity, FEF 25–75 forced expiratory flow between 25th and 75th percentile of FVC, BMI body mass index, Eff. all. effect allele, Freq effect allele frequency, β-coeff beta-coefficient, 95 % CI 95 % confidence interval, p p value of interaction
*Statistically significant interaction (p < 0.05)
a n = 1130 for change in BMI and n = 1069 for weight change analyses due to missing genotype data
bFor analyses with change in weight, participants with change ≤−2 kg (median of observed weight loss distribution) were excluded
Similar interaction patterns were observed on change in FEF25–75: interactions with an IQR increase in BMI were marginally significant (p = 0.66 to p = 0.10 for interaction), and FEF25–75 decline was accelerated up to −95 mL/s (−204 to 13; rs10823116) per each effect allele. Significant interactions with an IQR increase in weight were observed for SNPs rs730821 and rs10997868 (p = 0.025 and p = 0.046, respectively), and corresponding interaction estimates were −76 mL/s (−142 to −9; rs730821) and -56 mL/s (−111 to −1; rs10997868).
In multivariate multiple regression models, the joined Wald test of the null hypothesis that none of the SNPs presented a statistically significant interaction yielded p values of p = 0.086 and p = 0.032 for the model with change in BMI and weight gain, respectively.
Interaction analyses stratified by CRP rs1800947 genotype
When stratifying the interaction analyses by GC/CC versus GG genotype of CRP SNP rs1008947, the interaction effects of SIRT1 variation only persisted on the common GG stratum (Table 4). The GG stratum had 0.2 mg/L higher average C-reactive protein levels than the GC/CC stratum within the normal serum range up to 10 mg/L (p = 0.060 for the Mann-Whitney test of difference in medians).
Table 4.
Interaction effect estimates of SIRT1 SNPs with change in BMI and weight gain stratified by CRP SNP rs1800947
| Outcome | SNP | Effect allele | Freq | CRP SNP rs1800947 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Wild type GG (n = 964b) | Mutant alleles GC/CC (n = 137b) | ||||||||
| β-coeff. | 95 % CI | p | β-coeff. | 95 % CI | p | ||||
| Interaction effect estimates for change in BMI | |||||||||
| Change in FEV1/FVC | rs730821 a | C | 0.38 | -0.7 | (−1.2 to −0.2) | 0.012* | 0.3 | −1.5 to 2.1 | 0.76 |
| rs10997868 | C | 0.68 | -0.4 | (−0.8 to 0.1) | 0.12 | -0.5 | −1.8 to 0.8 | 0.45 | |
| rs10823116 | G | 0.13 | -0.7 | (−1.6 to 0.3) | 0.19 | -2.4 | −4.4 to −0.4 | 0.021* | |
| change in FEF25–75 (in L/s) | rs730821 a | C | 0.38 | -73 | (−138 to −9) | 0.025* | 62 | −149 to 273 | 0.57 |
| rs10997868 | C | 0.68 | -43 | (−96 to 11) | 0.12 | -22 | −191 to 146 | 0.80 | |
| rs10823116 | G | 0.13 | -100 | (−219 to 19) | 0.099 | -74 | −292 to 145 | 0.51 | |
| Interaction effect estimates for weight gain | |||||||||
| Change in FEV1/FVC (in percent) | rs730821 a | C | 0.38 | -0.9 | (−1.5 to −0.3) | 0.003* | 0.4 | −1.1 to 1.9 | 0.62 |
| rs10997868 | C | 0.68 | -0.5 | (−1.0 to 0.1) | 0.076 | -0.5 | −1.7 to 0.8 | 0.47 | |
| rs10823116 | G | 0.13 | -0.8 | (−1.9 to 0.4) | 0.19 | -2.3 | −4.5 to 0.0 | 0.054 | |
| Change in FEF25–75 (in mL/s) | rs730821 a | C | 0.38 | -94 | (−165 to −24) | 0.009* | 96 | −114 to 306 | 0.37 |
| rs10997868 | C | 0.68 | -63 | (−123 to −4) | 0.037* | 7 | −166 to 180 | 0.94 | |
| rs10823116 | G | 0.13 | -111 | (−252 to 31) | 0.13 | -89 | −357 to 179 | 0.52 | |
Estimates for the interaction term are given per effect allele and interquartile range increase in BMI (2.42 units) and weight (6.5 kg). Negative values mean an acceleration and positive an attenuation of lung function decline. All models were adjusted for percent predicted baseline value of lung function, sex, age (centered to median), height (centered to median), educational level, and study area
FEV 1 forced expiratory volume in 1 s of expiration, FVC forced vital capacity, FEF 25–75 forced expiratory flow between 25th and 75th percentile of FVC, BMI body mass index, Freq effect allele frequency, β-coeff. beta-coefficient, 95 % CI 95 % confidence interval, p p value of interaction
*Statistically significant interaction (p < 0.05)
a n = 963 in wild type stratum due to missing genotype data
b n = 911 for wild type and n = 131 for mutant stratum after excluding participants with weight change ≤−2 kg
Sensitivity analyses
Excluding participants with obstructive lung function at baseline or wheezing without a cold at either examination did not substantially alter the observed interaction patterns (Tables 2 and 3 in Online Resource 2).
SIRT1 SNP effects on smokers
In current smokers at follow-up, significant main effects were observed for SNP rs730821, accelerating the decline in FEV1 by −59 mL (−106 to −13) per each effect allele and in FVC by −77 mL (−135 to −18) (Table 4 in Online Resource 2). A marginally significant main effect was observed for rs10997868 (p = 0.051) with an estimated accelerated FVC decline of −46 mL (−93 to 0) per allele.
Discussion
Using data from a general population sample of non-smoking, non-asthmatic adults aged 30 years and older, the present study investigated long-term effects of genetic variation in SIRT1 on the age-related decline in lung function as a marker of biological aging. SIRT1 SNPs rs730821, rs10997868, and rs10823116 were not associated directly with lung function decline, and adjustments for body weight measures hardly affected the respective main effect estimates. However, SNPs rs730821 and rs10997868 showed significant evidence of interaction with increases in BMI or weight, while for rs10823116, estimates were marginally significant. The interaction was restricted to participants with normal genetic regulation of C-reactive protein serum levels.
The absence of genetic main effects on our never smoking study population is in line with previous findings from a Dutch population-based study, where no significant associations between SIRT1 tagging SNPs and lung function level were observed (but partial evidence was found for interactions with smoking) (Siedlinski et al. 2012). In terms of our hypothesized pathways of sirtuin-1 effects, our results do not support direct effects on oxidative stress defense in the lungs of never smokers (corresponding to path A in Fig. 1). However, the observed significant genetic main effects on smokers point to a potential, protective role in high-level oxidative stress exposures. Estimates for SNP main effects on lung function decline were only slightly affected by the inclusion of body weight measures. Regulation of body fat mass by sirtuin-1 (corresponding to path B in Fig. 1) is thus less likely to underlie effects on lung function decline. The observed interactions between SIRT1 SNPs and increased body weight measures rather suggest that differences in SIRT1 expression modify the deleterious effects of abdominal adipose tissue on lung function decline (path C in Fig. 1). This effect modification has to the best of our knowledge not been described before. The patterns of interaction suggest that sirtuin-1 might modulate systemic low-grade inflammation arising from increased abdominal fat tissue. BMI only insufficiently captures the amount of central body fat relevant for subclinical inflammation (Goyal et al. 2014). Accordingly, we did not observe interaction effects with baseline BMI centered at 23.5 kg/m2, but the interaction became notable in subjects with a BMI > 26.3 kg/m2 (associated with higher serum C-reactive protein concentrations). Furthermore, stronger SIRT1 interaction effects were observed with change in BMI and particularly with weight gain, which more likely reflect increases in abdominal fat tissue in adult populations. Finally, the interaction effects were restricted to participants with normal genetic CRP expression.
Limitations of our study were that only proxy measures of visceral fat mass were available. Moreover, the genetic effects under study were small and comprehensive assessment of potential interactions required multiple tests to be performed, all factors affecting the statistical power of our analysis. Nevertheless, the joined tests performed in multivariate, multiple regression models provide evidence that some of the observed SNP interactions are true findings. Furthermore, our definitions of overweight at baseline, BMI increase, and weight gain were strongly and positively associated with higher C-reactive protein levels at follow-up (all p < 0.0001, data not shown) and thus captured subclinical inflammatory states. Further, the functional impacts of the studied SNPs are not exactly known, and depending on the position, the numerous biological functions of sirtuin-1 can differently be affected. The variants were selected to represent common genetic variation in the Swiss general population, and all three were in non-coding gene regions. Queries in the HaploReg database yielded evidence from different, independent studies that SNPs rs730821 and rs10997868 are associated with SIRT1 expression in blood (GTEx Consortium. Ardlie KG et al. 2015), and rs10823116 is located in a DNAse-sensitive site in lung cells (Ward and Kellis 2012). Further, all three SNPs tag several variants that potentially affect SIRT1 expression via enhancer and promoter histone marks in lung and adipose tissue. Thus, our studied SNPs might directly affect gene expression or likely tag such regulatory variants in the region. Finally, the main results in our never smoking population were observed with decline in FEF25–75 and not FEV1 or FVC, which were the classical correlates of future mortality in previous studies. Due to their large surface area, the small airways are known to be an important compartment of early respiratory disease processes (Burgel et al. 2011; Hamid 2012) were subclinical changes often manifest first. Accordingly, in our study sample of never smokers from the general population, inflammation-related aging processes presented most strongly in FEF25–75 decline.
Strengths of our study include the population-based design and the availability of high-quality spirometry and genome-wide genotyping data for a large part of our cohort participants. This enabled us to study aging processes in a representative group of healthy, non-smoking adults using a lung function-based model of aging.
In conclusion, our study observed that genetic variation in SIRT1 affects age-related lung function decline in never smoking adults from the general population by modifying the deleterious effects of weight gain, likely related to systemic low-grade inflammation from visceral adipose tissue. These results suggest that differences in SIRT1 expression might play a role in human aging via modulation of adipose tissue-related inflammation. In light of the increasing obesity epidemic and the availability of pharmacological substances and dietary constituents that affect the biological availability and activity of sirtuin-1, these findings could be relevant to public health considerations.
Electronic supplementary material
(DOCX 30.7 kb)
(DOCX 65.8 kb)
Acknowledgments
The authors acknowledge the work of the whole SAPALDIA team: Study directorate: NM Probst-Hensch (PI; e/g); T Rochat (p), C Schindler (s), N Künzli (e/exp), JM Gaspoz (c); the scientific team: JC Barthélémy (c), W Berger (g), R Bettschart (p), A Bircher (a), C Brombach (n), PO Bridevaux (p), L Burdet (p), Felber Dietrich D (e), M Frey (p), U Frey (pd), MW Gerbase (p), D Gold (e), E de Groot (c), W Karrer (p), F Kronenberg (g), B Martin (pa), A Mehta (e), D Miedinger (o), M Pons (p), F Roche (c), T Rothe (p), P Schmid-Grendelmeyer (a), D Stolz (p), A Schmidt-Trucksäss (pa), J Schwartz (e), A Turk (p), A von Eckardstein (cc), E Zemp Stutz (e); and the scientific team at coordinating centers: M Adam (e), I Aguilera (exp), S Brunner (s), D Carballo (c), S Caviezel (pa), I Curjuric (e), A Di Pascale (s), J Dratva (e), R Ducret (s), E Dupuis Lozeron (s), M Eeftens (exp), I Eze (e), E Fischer (g), M Foraster (e), M Germond (s), L Grize (s), S Hansen (e), A Hensel (s), M Imboden (g), A Ineichen (exp), A Jeong (g), D Keidel (s), A Kumar (g), N Maire (s), A Mehta (e), R Meier (exp), E Schaffner (s), T Schikowski (e), M Tsai (exp).
The study could not have been done without the help of the study participants, technical and administrative support, and the medical teams and field workers at the local study sites. Local fieldworkers: Aarau: S Brun, G Giger, M Sperisen, M Stahel, Basel: C Bürli, C Dahler, N Oertli, I Harreh, F Karrer, G Novicic, N Wyttenbacher, Davos: A Saner, P Senn, R Winzeler, Geneva: F Bonfils, B Blicharz, C Landolt, J Rochat, Lugano: S Boccia, E Gehrig, MT Mandia, G Solari, B Viscardi, Montana: AP Bieri, C Darioly, M Maire, Payerne: F Ding, P Danieli A Vonnez, Wald: D Bodmer, E Hochstrasser, R Kunz, C Meier, J Rakic, U Schafroth, and A Walder; administrative staff: N Bauer Ott, C Gabriel, and R Gutknecht.
(a) corresponds to allergology, (c) cardiology, (cc) clinical chemistry, (e) epidemiology, (exp) exposure, (g) genetic and molecular biology, (m) meteorology, (n) nutrition, (o) occupational health, (p) pneumology, (pa) physical activity, (pd) pediatrics, (s) statistics.
Compliance with ethical standards
All study participants gave written informed consent, and the study was approved by the Swiss Academy of Medical Sciences as well as the respective regional ethics committees.
Funding
This work was supported by the Swiss National Science Foundation (grant nos. 33CS30-148470/1, 33CSCO-134276/1, 33CSCO-108796, 324730_135673, 3247BO-104283, 3247BO-104288, 3247BO-104284, 3247-065896, 3100-059302, 3200-052720, 3200-042532, 4026-028099, PMPDP3_129021/1, PMPDP3_141671/1); the Federal Office for the Environment; the Federal Office of Public Health; the Federal Office of Roads and Transport; the canton’s government of Aargau, Basel-Stadt, Basel-Land, Geneva, Luzern, Ticino, Valais, and Zürich; the Swiss Lung League; the canton’s Lung League of Basel Stadt/ Basel Landschaft, Geneva, Ticino, Valais, Graubünden, and Zurich; Stiftung ehemals Bündner Heilstätten; SUVA; Freiwillige Akademische Gesellschaft; UBS Wealth Foundation; Talecris Biotherapeutics GmbH; Abbott Diagnostics; European Commission 018996 (GABRIEL), and Wellcome Trust WT 084703MA.
References
- Ackermann-Liebrich U, et al. Follow-up of the Swiss Cohort Study on Air Pollution and Lung Diseases in Adults (SAPALDIA 2) 1991–2003: methods and characterization of participants. Soz Praventivmed. 2005;50:245–263. doi: 10.1007/s00038-005-4075-5. [DOI] [PubMed] [Google Scholar]
- Ahmadi-Abhari S, Kaptoge S, Luben RN, Wareham NJ, Khaw KT. Longitudinal association of C-reactive protein and lung function over 13 years: The EPIC-Norfolk study. Am J Epidemiol. 2014;179:48–56. doi: 10.1093/aje/kwt208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht E, et al. Telomere length in circulating leukocytes is associated with lung function and disease. Eur Respir J. 2014;43:983–992. doi: 10.1183/09031936.00046213. [DOI] [PubMed] [Google Scholar]
- American Thoracic Society (1987) Standardization of spirometry—1987 update. Statement of the American Thoracic Society. Am Rev Respir Dis 136:1285–1298 [DOI] [PubMed]
- Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005;96:939–949. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- Bigaard J, Frederiksen K, Tjonneland A, Thomsen BL, Overvad K, Heitmann BL, TI Sorensen (2005) Waist circumference and body composition in relation to all-cause mortality in middle-aged men and women. Int J Obes 29:778–784 doi:10.1038/sj.ijo.0802976. [DOI] [PubMed]
- Brandli O, et al. Re-estimated equations for 5th percentiles of lung function variables. Thorax. 2000;55:173–174. doi: 10.1136/thorax.55.2.172a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgel PR, et al. Update on the roles of distal airways in COPD. Eur Respir Rev. 2011;20:7–22. doi: 10.1183/09059180.10010610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett C, et al. Absence of effects of Sir2 overexpression on lifespan in C. elegans and Drosophila. Nature. 2011;477:482–485. doi: 10.1038/nature10296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burney PG, Hooper R. Forced vital capacity, airway obstruction and survival in a general population sample from the USA. Thorax. 2011;66:49–54. doi: 10.1136/thx.2010.147041. [DOI] [PubMed] [Google Scholar]
- Calabro P, et al. Adipose tissue-mediated inflammation: the missing link between obesity and cardiovascular disease? Intern Emerg Med. 2009;4:25–34. doi: 10.1007/s11739-008-0207-2. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Shang YC, Wang S, Maiese K. SIRT1: new avenues of discovery for disorders of oxidative stress. Expert Opin Ther Targets. 2012;16:167–178. doi: 10.1517/14728222.2012.648926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figarska SM, Vonk JM, Boezen HM. SIRT1 polymorphism, long-term survival and glucose tolerance in the general population. PLoS One. 2013;8:e58636. doi: 10.1371/journal.pone.0058636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flachsbart F, Croucher PJ, Nikolaus S, Hampe J, Cordes C, Schreiber S, Nebel A. Sirtuin 1 (SIRT1) sequence variation is not associated with exceptional human longevity. Exp Gerontol. 2006;41:98–102. doi: 10.1016/j.exger.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G. Inflamm-aging: an evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- Goyal A, Nimmakayala KR, Zonszein J. Is there a paradox in obesity? Cardiol Rev. 2014;22:163–170. doi: 10.1097/CRD.0000000000000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GTEx Consortium. Ardlie KGDD, Segrè AV, Sullivan TJ, Young TR, Gelfand ET, et al. Human genomics. The Genotype-Tissue Expression (GTEx) pilot analysis: multitissue gene regulation in humans. Science. 2015;348:648–660. doi: 10.1126/science.1262110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarente L. Franklin H. Epstein lecture: sirtuins, aging, and medicine. N Engl J Med. 2011;364:2235–2244. doi: 10.1056/NEJMra1100831. [DOI] [PubMed] [Google Scholar]
- Hamid Q. Pathogenesis of small airways in asthma. Respiration. 2012;84:4–11. doi: 10.1159/000339550. [DOI] [PubMed] [Google Scholar]
- Herranz D, Munoz-Martin M, Canamero M, Mulero F, Martinez-Pastor B, Fernandez-Capetillo O, Serrano M. Sirt1 improves healthy ageing and protects from metabolic syndrome-associated cancer. Nat Commun. 2010;1:3. doi: 10.1038/ncomms1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herranz D, Serrano M. SIRT1: recent lessons from mouse models. Nat Rev Cancer. 2010;10:819–823. doi: 10.1038/nrc2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang JW, Chung S, Sundar IK, Yao H, Arunachalam G, McBurney MW, Rahman I. Cigarette smoke-induced autophagy is regulated by SIRT1-PARP-1-dependent mechanism: implication in pathogenesis of COPD. Arch Biochem Biophys. 2010;500:203–209. doi: 10.1016/j.abb.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imboden M, et al. Genome-wide association study of lung function decline in adults with and without asthma. J Allergy Clinical Immunol. 2012;129:1218–1228. doi: 10.1016/j.jaci.2012.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International HapMap Consortium (2005) A haplotype map of the human genome. Nature 437:1299–1320. doi:10.1038/nature04226 [DOI] [PMC free article] [PubMed]
- Kedenko L, et al. Genetic polymorphisms at SIRT1 and FOXO1 are associated with carotid atherosclerosis in the SAPHIR cohort. BMC Med Genet. 2014;15:112. doi: 10.1186/s12881-014-0112-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy BK, et al. Redistribution of silencing proteins from telomeres to the nucleolus is associated with extension of life span in S. cerevisiae. Cell. 1997;89:381–391. doi: 10.1016/S0092-8674(00)80219-6. [DOI] [PubMed] [Google Scholar]
- Kettunen T, et al. Polymorphism in the C-reactive protein (CRP) gene affects CRP levels in plasma and one early marker of atherosclerosis in men: The Health 2000 Survey. Scand J Clin Lab Invest. 2011;71:353–361. doi: 10.3109/00365513.2011.568123. [DOI] [PubMed] [Google Scholar]
- Knuiman MW, James AL, Divitini ML, Ryan G, Bartholomew HC, Musk AW. Lung function, respiratory symptoms, and mortality: results from the Busselton Health Study. Ann Epidemiol. 1999;9:297–306. doi: 10.1016/S1047-2797(98)00066-0. [DOI] [PubMed] [Google Scholar]
- Kuningas M, Putters M, Westendorp RG, Slagboom PE, van Heemst D. SIRT1 gene, age-related diseases, and mortality: the Leiden 85-plus study. J Gerontol A Biol Sci Med Sci. 2007;62:960–965. doi: 10.1093/gerona/62.9.960. [DOI] [PubMed] [Google Scholar]
- Leone N, et al. Lung function impairment and metabolic syndrome: the critical role of abdominal obesity. Am J Respir Crit Care Med. 2009;179:509–516. doi: 10.1164/rccm.200807-1195OC. [DOI] [PubMed] [Google Scholar]
- Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannino DM, Reichert MM, Davis KJ. Lung function decline and outcomes in an adult population. Am J Respir Crit Care Med. 2006;173:985–990. doi: 10.1164/rccm.200508-1344OC. [DOI] [PubMed] [Google Scholar]
- Moffatt MF, et al. A large-scale, consortium-based genomewide association study of asthma. N Engl J Med. 2010;363:1211–1221. doi: 10.1056/NEJMoa0906312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochs-Balcom HM, et al. Pulmonary function and abdominal adiposity in the general population. Chest. 2006;129:853–862. doi: 10.1378/chest.129.4.853. [DOI] [PubMed] [Google Scholar]
- Picard F, Guarente L. Molecular links between aging and adipose tissue. Int J Obes. 2005;29(Suppl 1):S36–S39. doi: 10.1038/sj.ijo.0802912. [DOI] [PubMed] [Google Scholar]
- Quanjer PH, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40:1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radak Z, et al. Redox-regulating sirtuins in aging, caloric restriction, and exercise. Free Radic Biol Med. 2013;58:87–97. doi: 10.1016/j.freeradbiomed.2013.01.004. [DOI] [PubMed] [Google Scholar]
- Reis JP, Macera CA, Araneta MR, Lindsay SP, Marshall SJ, Wingard DL. Comparison of overall obesity and body fat distribution in predicting risk of mortality. Obesity. 2009;17:1232–1239. doi: 10.1038/oby.2008.664. [DOI] [PubMed] [Google Scholar]
- Rizki G, et al. The evolutionarily conserved longevity determinants HCF-1 and SIR-2.1/SIRT1 collaborate to regulate DAF-16/FOXO. PLoS Genet. 2011;7:e1002235. doi: 10.1371/journal.pgen.1002235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan G, Knuiman MW, Divitini ML, James A, Musk AW, Bartholomew HC. Decline in lung function and mortality: the Busselton Health Study. J Epidemiol Community Health. 1999;53:230–234. doi: 10.1136/jech.53.4.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabia S, Shipley M, Elbaz A, Marmot M, Kivimaki M, Kauffmann F, Singh-Manoux A. Why does lung function predict mortality? Results from the Whitehall II Cohort Study. Am J Epidemiol. 2010;172:1415–1423. doi: 10.1093/aje/kwq294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schunemann HJ, Dorn J, Grant BJ, W Winkelstein. Jr., Trevisan M (2000) Pulmonary function is a long-term predictor of mortality in the general population: 29-year follow-up of the Buffalo Health Study. Chest 118:656–664. [DOI] [PubMed]
- Shaaban R, et al. Change in C-reactive protein levels and FEV1 decline: a longitudinal population-based study. Respir Med. 2006;100:2112–2120. doi: 10.1016/j.rmed.2006.03.027. [DOI] [PubMed] [Google Scholar]
- Siedlinski M, Boer JM, Smit HA, Postma DS, Boezen HM. Dietary factors and lung function in the general population: wine and resveratrol intake. Eur Respir J. 2012;39:385–391. doi: 10.1183/09031936.00184110. [DOI] [PubMed] [Google Scholar]
- Tockman MS, et al. Rapid decline in FEV1. A new risk factor for coronary heart disease mortality. Am J Respir Crit Care Med. 1995;151:390–398. doi: 10.1164/ajrccm.151.2.7842197. [DOI] [PubMed] [Google Scholar]
- Viswanathan M, Guarente L. Regulation of Caenorhabditis elegans lifespan by sir-2.1 transgenes. Nature. 2011;477:E1–E2. doi: 10.1038/nature10440. [DOI] [PubMed] [Google Scholar]
- Ward LD, Kellis M. HaploReg: a resource for exploring chromatin states, conservation, and regulatory motif alterations within sets of genetically linked variants. Nucleic Acids Res. 2012;40:D930–D934. doi: 10.1093/nar/gkr917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrmeister FC, Menezes AM, Muniz LC, Martinez-Mesa J, Domingues MR, Horta BL. Waist circumference and pulmonary function: a systematic review and meta-analysis. Syst Rev. 2012;1:55. doi: 10.1186/2046-4053-1-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H, et al. SIRT1 redresses the imbalance of tissue inhibitor of matrix metalloproteinase-1 and matrix metalloproteinase-9 in the development of mouse emphysema and human COPD. Am J Phys Lung Cell Mol Phys. 2013;305:L615–L624. doi: 10.1152/ajplung.00249.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H, et al. SIRT1 protects against cigarette smoke-induced lung oxidative stress via a FOXO3-dependent mechanism. Am J Phys Lung Cell Mol Phys. 2014;306:L816–L828. doi: 10.1152/ajplung.00323.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Rexrode KM, van Dam RM, Li TY, Hu FB. Abdominal obesity and the risk of all-cause, cardiovascular, and cancer mortality: sixteen years of follow-up in US women. Circulation. 2008;117:1658–1667. doi: 10.1161/CIRCULATIONAHA.107.739714. [DOI] [PubMed] [Google Scholar]
- Zillikens MC, et al. SIRT1 genetic variation is related to BMI and risk of obesity. Diabetes. 2009;58:2828–2834. doi: 10.2337/db09-0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zillikens MC, et al. SIRT1 genetic variation and mortality in type 2 diabetes: interaction with smoking and dietary niacin. Free Radic Biol Med. 2009;46:836–841. doi: 10.1016/j.freeradbiomed.2008.12.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 30.7 kb)
(DOCX 65.8 kb)