Abstract
Curcumin, a major ingredient in turmeric, has a long history of medicinal applications in a wide array of maladies including treatment for diabetes and cancer. Seemingly counterintuitive to the documented hypoglycemic effects of curcumin, however, a recent report indicates that curcumin directly inhibits glucose uptake in adipocytes. The major glucose transporter in adipocytes is GLUT4. Therefore, this study investigates the effects of curcumin in cell lines where the major transporter is GLUT1. We report that curcumin has an immediate inhibitory effect on basal glucose uptake in L929 fibroblast cells with a maximum inhibition of 80% achieved at 75 μM curcumin. Curcumin also blocks activation of glucose uptake by azide, glucose deprivation, hydroxylamine, or phenylarsine oxide. Inhibition does not increase with exposure time and the inhibitory effects reverse within an hour. Inhibition does not appear to involve a reaction between curcumin and the thiol side chain of a cysteine residue since neither prior treatment of cells with iodoacetamide nor curcumin with cysteine alters curcumin’s inhibitory effects. Curcumin is a mixed inhibitor reducing the Vmax of 2DG transport by about half with little effect on the Km. The inhibitory effects of curcumin are not additive to the effects of cytochalasin B and 75 μM curcumin actually reduces specific cytochalasin B binding by 80%. Taken together, the data suggest that curcumin binds directly to GLUT1 at a site that overlaps with the cytochalasin B binding site and thereby inhibits glucose transport. A direct inhibition of GLUT proteins in intestinal epithelial cells would likely reduce absorption of dietary glucose and contribute to a hypoglycemic effect of curcumin. Also, inhibition of GLUT1 activity might compromise cancer cells that overexpress GLUT1 and be another possible mechanism for the documented anticancer effects of curcumin.
Keywords: Curcumin, GLUT1, L929 fibroblast cells, Glucose uptake, Cytochalasin B, Inhibition of glucose transport
1. Introduction
Curcumin is a yellow-colored polyphenol found in Curcuma longa, commonly known as turmeric, with a long history of medicinal applications in China and Southeast Asia. A wide variety of physiological properties for curcumin have been documented over the last 20 years. This research record includes over 7000 individual studies exploring the molecular basis for curcumin’s antioxidant, antiplatelet, anti-inflammatory, anti-apoptosis, antibacterial, anti-fungal, antiviral, anticancer, antidiabetic properties and over 100 clinical trials exploring the efficacy of this compound in the treatment of chronic disease such as diabetes, cancer, autoimmune, cardiovascular, neurological and psychological [1–4]. Much of the recent work summarized in over 400 review articles has focused on curcumin’s efficacy as an anticancer agent in a wide variety of cancers [3,5,6] including gastrointestinal, colon [7,8], and glioblastoma [9] as examples. The mechanisms of action are diverse ranging from inhibition of several common cancer promoting pathways such as MAPK, Akt, HF-kB, and Cox-2 [10,11] to decreasing microRNA, miR-21 [12].
The beneficial effects of curcumin on diabetes have also been noted [2,13–16] citing an enhancement of insulin sensitivity and lowering of blood glucose levels in multiple animal diabetic models [17–22]. Recently, however, curcumin has been reported to block the stimulatory effect of insulin in 3T3-L1 adipocytes [23] and shown to have an immediate, irreversible inhibitory effect in primary rat adipocytes suggesting a more direct interaction of curcumin with the glucose transporter [24]. This inhibition likely represents an interaction of curcumin with the major transporter in cells, GLUT4, however, little is known about the potential interaction of curcumin with the more widely expressed glucose transporter isoform, GLUT1. Therefore, in this study we investigate the effects of curcumin on glucose uptake in L929 fibroblast cells, which express exclusively GLUT1 [25] and other GLUT1-expressing cell lines. Given the similar structures of the GLUT proteins, we hypothesized that curcumin would inhibit the transport activity of GLUT1. In addition, the previously reported irreversible inhibitory effect of curcumin in adipocytes [24] might imply the formation of a covalent adduct. The chemical structure of curcumin contains two α-β unsaturated carbonyl motifs, which are excellent Michele acceptors of thiols in a 1–4 nucleophilic addition reaction. We have previously shown in a number of studies that thiol chemistry (cysteine residue) is likely involved in the acute regulation of GLUT1 activity in L929 cells [26–29]. A chemical reaction between a cysteine residue and curcumin might explain the irreversible effects of curcumin on GLUT4. If a covalent adduct is indeed required for curcumin’s inhibitory effects, we would predict that either prior treatment of cells with iodoacetamide (IA) to react with free thiols, or pretreatment of curcumin with cysteine should block the inhibitory effects of curcumin.
2. Materials and methods
2.1. Chemicals
Curcumin (Cur), hydroxylamine (HA), iodoacetamide (IA), phenylarsine oxide (PAO), and sodium azide (Az) were purchased from the Sigma-Aldrich Chemical Company (St. Louis, MO, USA) and 2-deoxy-D-glucose- [1,2-3H] (2DG) and D-mannitol-1-14C were purchased from Moravek Biochemicals (Brea, CA, USA). 3H-cytochalasin B was purchased from Amersham Biosciences.
2.2. Cell culture
L929 mouse fibroblast cells and HK2 (human kidney) cells were obtained from the American Type Culture Collection. The immortalized human corneal–limbal epithelial (HCLE) cell line was obtained from Dr. Ilene Gipson (Department of Ophthalmology, Harvard Medical School) and maintained as monolayer cultures in Keratinocyte-Serum Free medium (K-SFM) (Invitrogen, Carlsbad, CA), as previously described [30]. To initiate each experiment, a 24-well plate was seeded either 1 or 2 days prior to experimentation. The cells were grown at 37 °C in an incubator supplied with humidified room air with 5% CO2. Experiments were done with cells near confluency, which is about 1.0 × 105 cells per well for HCLE and HK2 cells and 3.0 × 105 for L929 fibroblast cells.
2.3. General experimental design
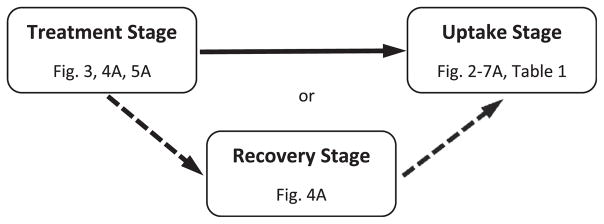
As shown in Fig. 1, various experiments had up to three stages: a treatment stage, a recovery stage, and a glucose uptake measurement stage. Cells were maintained at 37 °C throughout the experiment and conditions and lengths of time for each stage are indicated in the figure legends. To initiate the treatment stage, the media from cells in 24-well plates were removed and then cells were incubated in 0.4 mL of fresh treatment media consisting of either low-glucose DMEM alone (0% FBS) or low-glucose DMEM plus the chemical of interest (see figure legends) or glucose-free DMEM (activation by glucose deprivation). Media was then removed and replaced either with media only for a recovery stage, or with buffered solution for the measurement of uptake stage (see below for details). Reagents were added to the treatment media or to the uptake solution from 100–200× aqueous (Az, HA), or DMSO (Cur, PAO) stock solutions. DMSO has no effect on glucose uptake at the concentrations added [28].
Fig. 1.
Experimental design. Each experiment had up to three stages; treatment, recovery, or glucose uptake measurement. Experiments utilizing each stage are indicated and the details concerning concentrations and exposure times will be indicated in each figure legend.
2.4. Glucose uptake assay
Glucose uptake was measured using the radiolabeled glucose analog 2-deoxyglucose (2DG) as previously described [31]. Briefly, the media was replaced with 0.2 mL of glucose-free HEPES buffer (140 mM NaCl, 5 mM KCl, 20 mM HEPES/Na pH = 7.4, 2.5 mM MgSO4, 1 mM CaCl2, 2 mM NaPyruvate, 1 mM mannitol) supplemented with 1.0 mM (0.3 μCi/mL) 2-DG (1,2-3H) and 1.0 mM (0.02 μCi/mL) mannitol (1-14C). 1.0 mM 2-DG is below the Km of transport, 6–8 mm, and allows us to monitor linear uptake for longer times. Uptake media was supplemented with additional compounds, such as Cur, PAO, or HA, as indicated in the figure or table legends. For the kinetics experiment, the concentration of 2DG was varied as indicated in the figure legend. After a 10-min incubation, cells were washed twice with cold glucose-free HEPES. The cells were digested in 0.25 mL of 0.3 M NaOH and the 3H-2 DG and 14C-mannitol were measured using scintillation spectrometry. Mannitol, similar in structure to glucose, is not normally taken up by cells, therefore the inclusion of 14C-mannitol in the uptake media allows us to correct for any surface binding or trapping of radioactivity in extracellular spaces, to monitor potential toxic effects of the experimental treatments that would compromise the cell membrane, and to account for excess radioactivity that might remain after the washes.
2.5. Measurement of specific 3H-cytochalasin B binding
Specific binding of cytochalasin B was measured in the presence and absence of 75 μM curcumin. HCLE cells were cooled to 4 °C and the media were replaced with 0.3 mL of cold HEPES buffer containing 0.3 μCi/mL cytochalasin B (1-3H) plus 25 mM of either L-glucose or D-glucose. After a 40-min incubation, the cells were washed with cold media and the cells were digested and the radioactivity was measured. Specific D-glucose-inhibited cytochalasin B binding was determined as the difference in the binding of 3H-cytochalasin B in the presence of D-glucose and L-glucose.
2.6. Statistical analysis
Each experiment with quadruplicate samples was repeated a minimum of three times to insure that results could be replicated. 2DG uptake data were measured as nmol/10 min/well ± standard error, normalized to control conditions and reported as relative 2DG uptake. Statistical significance was determined by a two-tailed t-test. Statistical significance is reported at P < 0.05 or P < 0.01.
3. Results
3.1. Curcumin inhibits glucose uptake in a dose dependent manner
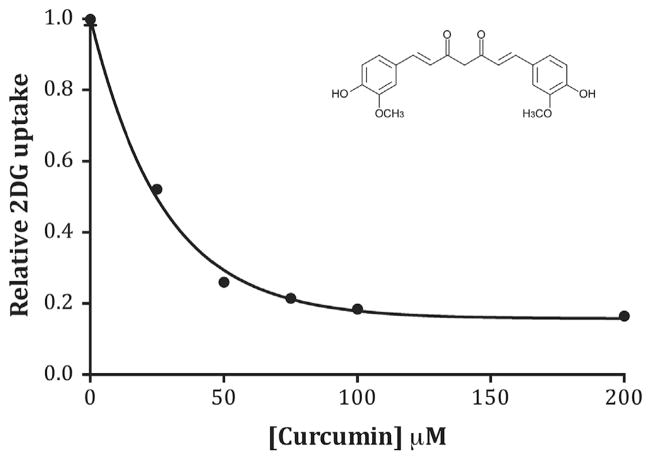
Previous work has demonstrated that curcumin is a direct inhibitor of glucose transport activity in primary rat adipocytes [24]. The primary transporter in adipocytes is the insulin-regulated transporter, GLUT4, but the effects of curcumin on GLUT1 transport activity are not known. Therefore, we measured glucose uptake in the presence of increasing concentrations of curcumin (0–200 μM) in L929 fibroblast cells, where glucose uptake is mediated exclusively by GLUT1 [25]. Results, shown in Fig. 2, indicate that curcumin inhibits 2DG uptake in a dose dependent manner with a maximum of 84% inhibition at 70–100 μM with an IC50 = 19 μM. We confirmed this inhibitory effect of curcumin in HCLE and HK2 cells, two other cell lines where GLUT1 is the primary transporter responsible for 2DG uptake [32,33]. The effects of a submax (25 μM) and maximum (75 μM) effective concentrations of curcumin were measured and reported on Table 1. Results indicate a similar pattern of inhibition as observed in L929 cells.
Fig. 2.
Dose dependent effects of curcumin. 2DG uptake was measured at various concentrations of curcumin ranging from 25 to 200 μM. Data (n = 10) were normalized to basal uptake (0 mM curcumin) and the relative uptakes are expressed as means ± S.E with a best-fit line to simple decay. All curcumin treatments were significantly lower at P < 0.01. Inset shows structure of curcumin (keto form).
Table 1.
Comparative effects of curcumin in GLUT1-expressing cell lines.
| Cell type | Normalized 2DG uptake
|
||
|---|---|---|---|
| Control | +Cur (25 μM) | +Cur (75 μM) | |
| L929 | 1.00 ± 0.02 | 0.52 ± 0.01* | 0.21 ± 0.01* |
| HCLE | 1.00 ± 0.06 | 0.65 ± 0.02* | 0.12 ± 0.02* |
| HK2 | 1.00 ± 0.03 | 0.58 ± 0.02* | 0.16 ± 0.02* |
2DG uptake was measured in each cell line in the absence (control) or presence of 25 or 75 μM. Uptakes were measure as nmol/10 min/well and are normalized to its respective control. Data are presented as means ± S.E.
Significantly different than its respective control (no curcumin) at P < 0.01.
3.2. Curcumin blocks activation of 2DG uptake in L929 cells
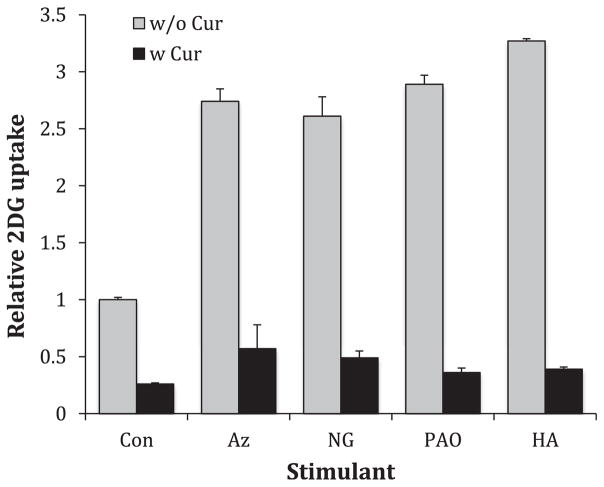
To determine the effects of curcumin on the effects of acute glucose uptake activators we measured 2DG in activating conditions in the absence and presence of 50 μM curcumin. We investigated two acute activators that require a 20-min preincubation to maximize activation; azide treatment and glucose deprivation [34,35], where the activator plus or minus curcumin was present only during a 20-min pretreatment. We also measured 2DG uptake in the presence of two activators that act immediately; PAO and HA [28,36], where the activator with or without curcumin was present only during the measurement of 2DG uptake. The results, shown on Fig. 3, reveal that in all cases curcumin effectively blocks acute activation of glucose uptake.
Fig. 3.
Effects of curcumin on various activators of glucose uptake. L929 cells were incubated in activating conditions with or without 50 μM curcumin. Activation with sodium azide (Az) (5 mM), or glucose deprivation (NG) (0 mM glucose) required a 20min-treatment stage, but phenylarsine oxide (PAO) (10 μM) and hydroxylamine (HA) (5 mM) are fast activating reagents and were present only during the measurement of 2DG uptake. 2DG uptakes were measured as nmol/10min/well, normalized to control and reported as relative uptake. Data are presented as means ± S.E. All curcumin treated cells were significantly lower than their respective controls at P < 0.01.
3.3. Inhibitory effect of curcumin is reversible and does not increase with time
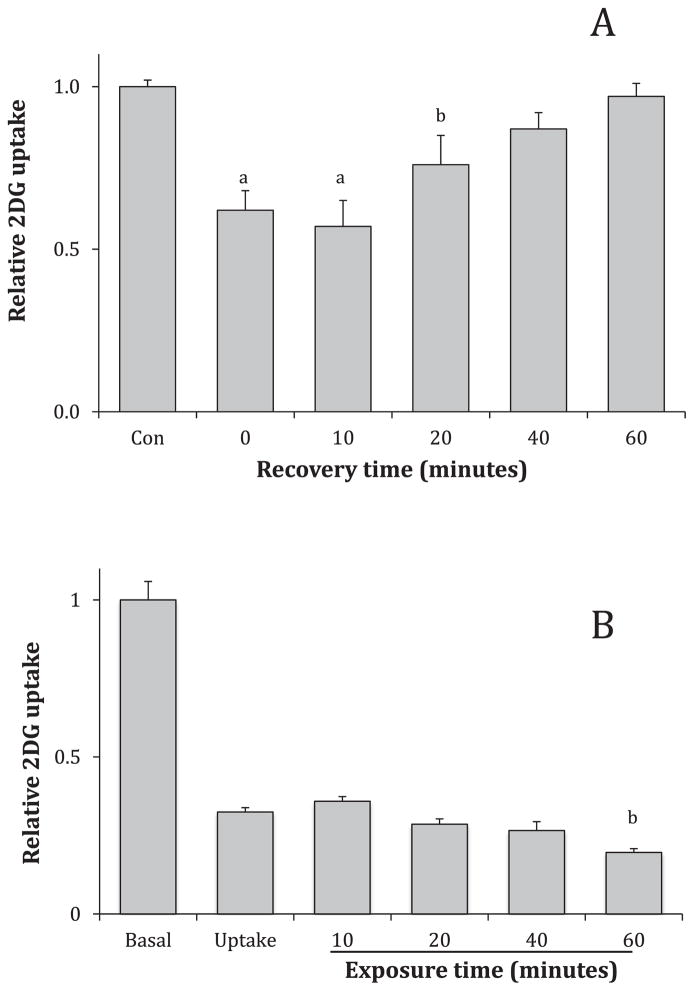
The effect of curcumin in adipocytes was not reversible in adipocytes within the timeframe of one hour [24]. To determine the reversibility of the effects on GLUT1, L929 fibroblasts cells were incubated with 75 μM curcumin for 20 min, the media were then replaced with media without curcumin for 0,10, 20, 40, or 60 min to allow recovery. Results, shown in Fig. 4A, indicate glucose transport activity is recovered within 40 min.
Fig. 4.
Relationship of time and inhibitory effects of curcumin. Panel A: Recovery from curcumin effects. Cells were treated with media containing 75 μM curcumin for 20 min. The media was replaced with curcumin-free media to recover for 0, 10, 20, 40 or 60 min. Panel B: Exposure time to curcumin. L929 fibroblast cells were treated with 75 μM curcumin during 2 DG uptake only or prior to 2DG uptake for 10, 20, 40 or 60 min. In both experiments, 2DG uptakes were measured, normalized to control and expressed as means of relative uptake ± S.E. aSignificant from control at P < 0.01. bSignificant from control at P < 0.05.
The inhibitory effects of curcumin are immediate, but we were curious if additional inhibition would occur with longer exposure times. L929 cells were exposed to 75 μM during uptake only or just prior to uptake for 10, 20, 40 or 60 min. The results, shown in Fig. 4B, demonstrate that the inhibitory effects are virtually identical with a small, enhanced inhibition after a 60-min exposure. Given the plethora of activities attributed to curcumin, we did not pursue longer exposure times, which would unduly complicate analysis. As monitored by changes in cell morphology or by abnormally high mannitol uptakes, curcumin does not demonstrate any toxic effects within the hour exposure.
3.4. Effects of curcumin does not depend on thiol chemistry
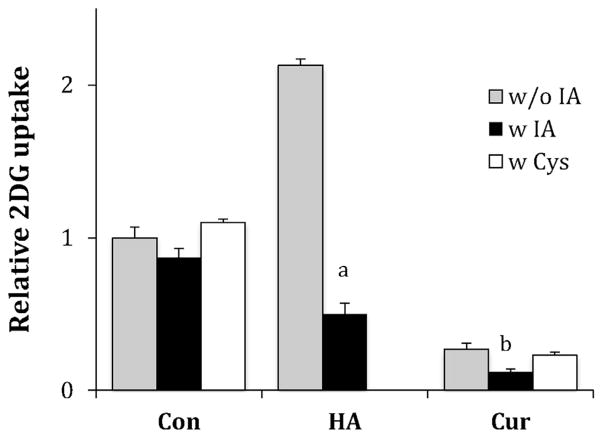
Curcumin has an α-β unsaturated carbonyl structure that is known to react with thiols via nucleophilic 1–4 addition. Thiol reactive compounds, such as cinnamaldehyde, phenylarsine oxide, hydroxylamine, and Angeli’s salt, have previously been shown to alter GLUT1 activity, suggesting a potential mode of action for curcumin [26–28,36]. To determine if curcumin requires free thiols to exert its inhibitory effects, we performed two experiments with results shown in Fig. 5. First, prior to the measurement of 2DG uptake, L929 cells were exposed to 0.75 mM iodoacetamide (IA) for 30 min to react with free thiols. This treatment tended to reduce basal uptake, but not significantly (P = 0.19) and curcumin’s inhibitory effects were not curtailed, but enhanced. As a positive control, we show that IA treatment was able block the activation of hydroxylamine (HA), a compound whose activation has been previously shown to be dependent upon the availability of cysteine side chains [36]. In the second experiment, 75 μM curcumin was incubated with excess cysteine (2.0 mM) for an hour, to potentially react with curcumin, before its incubation with cells. This treatment did not alter curcumin’s inhibitory effects (see Fig. 5).
Fig. 5.
Effects iodoacetamide and cysteine on curcumin’s inhibitory effects. L929 fibroblast cells were treated with or without 0.3 mM IA for 30 min 2DG uptakes were measured in the presence of either no additions or 2.0 mM cysteine (Con), or addition of 5 mM hydroxylamine (HA), or addition of 75 μM curcumin (Cur). Data are means of 2DG uptakes normalized to untreated control cells. aSignificant from control at P < 0.01. bSignificant from control at P < 0.05.
3.5. Curcumin is a mixed inhibitor of 2DG uptake
The apparent lack of thiol reactivity prompted us to ask if curcumin is simply a competitive inhibitor of 2DG uptake. We measured the kinetics of uptake in the absence and presence of curcumin. As seen in Fig. 6, high concentration of 2DG did not counter curcumin’s inhibitory effect as expected of a competitive inhibitor, rather curcumin appears to be a mixed inhibitor significantly reducing the Vmax of uptake from 7.53 ± 0.54 to 4.29 ± 0.42 nmol/10 min/well with little change in the Km (10.6 ± 2.0 and 13.0 ± 3.1 mM respectively).
Fig. 6.
Kinetics of 2DG uptake. 2DG uptake was measured at 0.5, 1.0, 5.0, 10, 20 and 40 mM 2DG in the absence or presence of 100 μM curcumin and reported as nmol/10 min/well. Data are means ± S.E. of quadruplicate samples from a representative experiment with a best-fit line to Michaelis-Menten kinetics.
3.6. Curcumin inhibition is not additive to effects of cytochalasin B and reduces specific cytochalasin B binding
Cytochalasin B is a classic inhibitor of GLUT proteins and is known to bind near the interior glucose exit site of GLUT proteins. If curcumin inhibits by a different mechanism we would predict that the effects of curcumin would be additive to the effects of cytochalasin B. We measured 2DG uptakes at the submax and maximally effective concentrations of cytochalasin B (2 μM, 50 μM) and curcumin (30 μM, 100 μM) both alone and in combination. The results, shown in Fig. 7A, reveal that curcumin does not add to the inhibitory effects of cytochalasin B at either concentration. This suggests a similar mechanism of action.
Fig. 7.
Combined effects of curcumin and cytochalasin B. Panel A: Effects on 2DG uptake. Uptake was measured in L929 cells at sub-maximally or maximally effective concentrations of curcumin (30 μM and 100 μM) or cytochalasin B (2 μM and 50 μM) or both. Data were normalized to untreated cells and reported as means ± S.E. of relative uptake. Panel B: Effects of curcumin on cytochalasin B binding. HCLE cells were incubated with HEPES buffer containing 3H-cytochalasin B for 40 min at 4 °C in the presence or absence of 75 μM curcumin with either 25 mM D or L-glucose. Specific cytochalasin B binding was determined by subtracting the isolated radioactivity of samples containing D-glucose from those containing L-glucose.
If curcumin shares a similar inhibitory mechanism with cytochalasin B, it may inhibit the binding of cytochalasin B. We tested this in HCLE cells, which have a much higher concentration of GLUT1 than L929 cells [32]. The results shown in Fig. 7B reveal that 75 μM curcumin reduces specific 3H-cytochalasin B binding by 80%.
4. Discussion
Curcumin, a major component of turmeric, appears to have numerous physiological effects and has received significant attention as a potential intervention in the treatment of multiple diseases. Over 860 reviews have been published over the last 25 years, many of them focusing on the medicinal benefits of this compound. While it has been well documented that curcumin lowers blood glucose in animal models of diabetes, there are few studies investigating the effects of curcumin on glucose uptake in tissue cell models. Curcumin has been shown to decrease the translocation of GLUT4 in both leptin-treated hepatic stellate cells and 3T3-L1 adipocytes suggesting inhibitory effects of curcumin on insulin signaling [23,37]. However, work from Green et al. indicated that the inhibitory effects of curcumin on glucose uptake in rat adipocytes were immediate, independent of a signaling system. These effects were not reversible within an hour of recovery [24]. The major glucose transporter in adipocyte cells is GLUT4 and, therefore, the purpose of this study was to determine the effects of curcumin on glucose uptake in L929 fibroblast cells, which exclusively express GLUT1.
The results reported in this study indicate that curcumin inhibits basal glucose uptake of GLUT1 in L929 fibroblasts, HCLE cells, and HK2 cells in a dose dependent manner (Fig. 2, Table 1). Curcumin also blocks acute activation of glucose uptake in L929 cells (Fig. 3). These results mimic the effects of curcumin on GLUT4 in adipocytes [24]. The effective concentrations of curcumin in L929 and adipocytes are very similar, but the inhibition is more robust in L929 cells (84% inhibition compared to 40%) (Fig. 1 and [24]). Curcumin is a mixed inhibitor and cannot be overcome by higher concentrations of 2DG (glucose) (Fig. 6). The inhibition is immediate, reversible, and does not increase with longer exposure times (Fig. 4).
Curcumin contains two α-β unsaturated carbonyl motifs and the observation that the inhibitory effect of curcumin in adipocytes is irreversible [24] suggested to us that curcumin might be forming a covalent bond with a key cysteine residue, perhaps within GLUT1 itself. Carruthers has shown that GLUT1 in erythrocytes forms homotetramers, which are more active than the monomer or dimer forms. Stabilization of the tetramer depends on the formation of a disulfide between residue C347 and C421 within each monomer [38,39]. We have previously shown the importance of thiol chemistry in the regulation of GLUT1 in L929 cells. Nitroxyl, which stimulates disulfide bond formation, activates uptake within 2 min [27], while cinnamaldehyde partially activates glucose uptake but also blocks full activation by nitroxyl [26]. Interestingly, the cinnamaldehyde effects appear to depend on a reaction between the thiol of a cysteine residue and the α-β unsaturated aldehyde of cinnamaldehyde. However, this same reaction between a cysteine and a α-β unsaturated carbonyl does not appear to be the mechanism of action for curcumin. In contrast to the effects of nitroxyl and cinnamaldehyde [26,27], which could be blocked by either the pretreatment of cells with IA or a prior incubation of the stimulant with cysteine, the inhibitory effects of curcumin were not blocked by either of these treatments (Fig. 5). In addition, unlike the curcumin’s effects in adipocytes, we find that the inhibitory effects are lost within an hour (Fig. 4A). This recovery time is more consistent with the time required for this lipophilic compound to be lost from membranes than for the reversal of a covalent bond. We would suggest that the previously reported irreversibility effect of curcumin in adipocytes [24] may result from a greater accumulation of lipophilic curcumin in adipocytes and 60 min is not enough time for curcumin to be lost from adipocytes.
The most likely mechanism for this inhibitory effect of curcumin on GLUT1 is a direct binding to the transporter, likely at an interior site on the transporter that overlaps with the binding site of cytochalasin B. This is based on several observations. 1) The effect is immediate both at basal and activating conditions and requires no preincubation. 2) Inhibition of curcumin is not additive to the effects of cytochalasin B either at submax or max concentrations (Fig. 7A), suggesting similar modes of action for the two inhibitors. 3) Curcumin (75 μM) reduces specific cytochalasin B binding by 80% (Fig. 7B). Interestingly, while glucose is competitive with cytochalasin B binding [40] it is not competitive with curcumin (Fig. 6). This suggests that the binding sites for curcumin and cytochalasin B overlap, but are not identical. This result parallels a recent report of the inhibitory effects of caffeine on GLUT1 activity. The binding of caffeine was mapped primarily to the nucleotide-binding site, but overlapping slightly with the cytochalasin B binding site. Thus, like curcumin, caffeine did not compete with glucose, but did reduce cytochalasin B binding [41]. Our results are consistent with curcumin binding to the nucleotide binding site, but with higher affinity than caffeine: IC50 = 3.5 mM for caffeine compared to 19 μM for curcumin.
We cannot eliminate the possibility that the inhibition is an indirect membrane effect caused by the accumulation of curcumin within the plasma membrane causing conformational changes within the transporter. However, this seems less likely because as more curcumin accumulates in membranes over time the inhibitory effects should increase. We do not observe any increase in curcumin inhibition over time (Fig. 4B).
The direct inhibitory effects of curcumin on glucose uptake initially appear to conflict with the strong evidence that curcumin lowers blood glucose levels and has beneficial effects in the treatment of diabetes [15,16]. It is difficult to compare tissue culture studies to whole animal studies largely due to the low bioavailability of dietary curcumin caused by low levels of uptake and a fast metabolism [42]. The oral bioavailability is only 0.47% and i.v. administration of 10 mg/kg leads to a maximum plasma concentration of 8.5 μM [43], lower than the concentrations used in this study. However, the concentration of curcumin within specific individual tissues is difficult to predict given the lipophilic nature of curcumin. Clearance of plasma curcumin is likely a combination of accumulation within lipid environments of tissues and metabolism by the liver.
One plausible mechanism for lowering blood glucose based on this data would be reduced absorption of glucose in the gut caused by a direct inhibition of GLUT2 on the basal surface of intestinal epithelial cells. Currently there is no direct evidence that curcumin inhibits GLUT2, but given the similarities of the GLUTs and evidence of direct inhibition of both GLUT1 and GLUT4, it seems likely that curcumin would also inhibit GLUT2. At the surface, this inhibition would appear to conflict with data suggesting that curcumin enhances intestinal transport of glucose [44]. However, in that study rats were fed curcumin for 5 days and curcumin was not present during the measurement of glucose transport. It seems reasonable that chronic inhibition by curcumin would lead to enhance cellular expression of GLUT2 as a mechanism of compensation. It has been reported that chronic curcumin treatment up-regulates GLUT protein expression [17].
The observation that curcumin directly inhibits the transport activity of GLUT1 is also potentially an additional mechanism for the documented anticancer effects of curcumin [3,5]. Many cancers are highly glycolytic and overexpress GLUT1 [45,46]. A direct inhibition of GLUT1 would be expected to slow glucose uptake and compromise growth.
5. Conclusions
This study reports that curcumin directly inhibits the GLUT1 activity in multiple cell lines in a dose dependent manner. Curcumin inhibition is immediate, reversible, and reduces the Vmax of 2DG uptake. Curcumin does not require a reaction with cysteine residues, but rather appears to bind at a site either near or overlapping with the cytochalasin B binding site on the interior surface of GLUT1, possibly at the nucleotide binding site.
Acknowledgments
This research was supported by a NIH R15 grant (DK08193-1A1). Special thanks go to the Ubels lab for supplying the HCLE cells, and to Brendan Looyenga for his critique of this manuscript.
Footnotes
Author agreement
The data and all materials submitted are original and all authors are in agreement to have the article published.
References
- 1.Chen J, He ZM, Wang FL, Zhang ZS, Liu XZ, Zhai DD, Chen WD. Curcumin and its promise as an anticancer drug: an analysis of its anticancer and antifungal effects in cancer and associated complications from invasive fungal infections. Eur J Pharmacol. 2015;772:33–42. doi: 10.1016/j.ejphar.2015.12.038. [DOI] [PubMed] [Google Scholar]
- 2.He Y, Yue Y, Zheng X, Zhang K, Chen S, Du Z. Curcumin, inflammation, and chronic diseases: how are they linked? Molecules. 2015;20:9183–9213. doi: 10.3390/molecules20059183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perrone D, Ardito F, Giannatempo G, Dioguardi M, Troiano G, Lo Russo L, DE Lillo A, Laino L, Lo Muzio L. Biological and therapeutic activities, and anticancer properties of curcumin. Exp Ther Med. 2015;10:1615–1623. doi: 10.3892/etm.2015.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kutluay SB, Doroghazi J, Roemer ME, Triezenberg SJ. Curcumin inhibits herpes simplex virus immediate-early gene expression by a mechanism independent of p300/CBP histone acetyltransferase activity. Virology. 2008;373:239–247. doi: 10.1016/j.virol.2007.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vallianou NG, Evangelopoulos A, Schizas N, Kazazis C. Potential anticancer properties and mechanisms of action of curcumin. Anticancer Res. 2015;35:645–651. [PubMed] [Google Scholar]
- 6.Hosseini A, Ghorbani A. Cancer therapy with phytochemicals: evidence from clinical studies. Avicenna J Phytomed. 2015;5:84–97. [PMC free article] [PubMed] [Google Scholar]
- 7.Park JM, Lee HJ, Yoo JH, Ko WJ, Cho JY, Hahm KB. Overview of gastrointestinal cancer prevention in Asia. Best Pract Res Clin Gastroenterol. 2015;29:855–867. doi: 10.1016/j.bpg.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 8.Pabla B, Bissonnette M, Konda VJ. Colon cancer and the epidermal growth factor receptor: current treatment paradigms, the importance of diet, and the role of chemoprevention. World J Clin Oncol. 2015;6:133–141. doi: 10.5306/wjco.v6.i5.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sordillo LA, Sordillo PP, Helson L. Curcumin for the treatment of glioblastoma. Anticancer Res. 2015;35:6373–6378. [PubMed] [Google Scholar]
- 10.Shehzad A, Lee YS. Molecular mechanisms of curcumin action: signal transduction. BioFactors. 2013;39:27–36. doi: 10.1002/biof.1065. [DOI] [PubMed] [Google Scholar]
- 11.Gupta RA, Dubois RN. Colorectal cancer prevention and treatment by inhibition of cyclooxygenase-2. Nat Rev Cancer. 2001;1:11–21. doi: 10.1038/35094017. [DOI] [PubMed] [Google Scholar]
- 12.Chen J, Xu T, Chen C. The critical roles of miR-21 in anti-cancer effects of curcumin. Ann Transl Med. 2015;3:330. doi: 10.3978/j.issn.2305-5839.2015.09.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rivera-Mancia S, Lozada-Garcia MC, Pedraza-Chaverri J. Experimental evidence for curcumin and its analogs for management of diabetes mellitus and its associated complications. Eur J Pharmacol. 2015;756:30–37. doi: 10.1016/j.ejphar.2015.02.045. [DOI] [PubMed] [Google Scholar]
- 14.Maradana MR, Thomas R, O’Sullivan BJ. Targeted delivery of curcumin for treating type 2 diabetes. Mol Nutr Food Res. 2013;57:1550–1556. doi: 10.1002/mnfr.201200791. [DOI] [PubMed] [Google Scholar]
- 15.Meng B, Li J, Cao H. Antioxidant and antiinflammatory activities of curcumin on diabetes mellitus and its complications. Curr Pharm Des. 2013;19:2101–2113. [PubMed] [Google Scholar]
- 16.Zhang DW, Fu M, Gao SH, Liu JL. Curcumin and diabetes: a systematic review. Evid Based Complement Altern Med eCAM. 2013;2013:636053. doi: 10.1155/2013/636053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghorbani Z, Hekmatdoost A, Mirmiran P. Anti-hyperglycemic and insulin sensitizer effects of turmeric and its principle constituent curcumin. Int J Endocrinol Metab. 2014;12:e18081. doi: 10.5812/ijem.18081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahesh T, Balasubashini MS, Menon VP. Effect of photo-irradiated curcumin treatment against oxidative stress in streptozotocin-induced diabetic rats. J Med Food. 2005;8:251–255. doi: 10.1089/jmf.2005.8.251. [DOI] [PubMed] [Google Scholar]
- 19.Weisberg SP, Leibel R, Tortoriello DV. Dietary curcumin significantly improves obesity-associated inflammation and diabetes in mouse models of diabesity. Endocrinology. 2008;149:3549–3558. doi: 10.1210/en.2008-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abd Allah ES, Gomaa AM. Effects of curcumin and captopril on the functions of kidney and nerve in streptozotocin-induced diabetic rats: role of angiotensin converting enzyme 1. Appl Physiol Nutr Metab Physiol Appl Nutr Metab. 2015;40:1061–1067. doi: 10.1139/apnm-2015-0145. [DOI] [PubMed] [Google Scholar]
- 21.Tian L, Zeng K, Shao W, Yang BB, Fantus IG, Weng J, Jin T. Short-term curcumin gavage sensitizes insulin signaling in dexamethasone-treated C57BL/6 mice. J Nutr. 2015;145:2300–2307. doi: 10.3945/jn.115.216853. [DOI] [PubMed] [Google Scholar]
- 22.Naijil G, Anju TR, Jayanarayanan S, Paulose CS. Curcumin pretreatment mediates antidiabetogenesis via functional regulation of adrenergic receptor subtypes in the pancreas of multiple low-dose streptozotocin-induced diabetic rats. Nutr Res. 2015;35:823–833. doi: 10.1016/j.nutres.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 23.Ikonomov OC, Sbrissa D, Mlak K, Shisheva A. Requirement for PIKfyve enzymatic activity in acute and long-term insulin cellular effects. Endocrinology. 2002;143:4742–4754. doi: 10.1210/en.2002-220615. [DOI] [PubMed] [Google Scholar]
- 24.Green A, Krause J, Rumberger JM. Curcumin is a direct inhibitor of glucose transport in adipocytes. Phytomed Int J Phytother Phytopharmacol. 2014;21:118–122. doi: 10.1016/j.phymed.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 25.Liong E, Kong SK, Au KK, Li JY, Xu GY, Lee YL, Kwok TT, Choy YM, Lee CY, Fung KP. Inhibition of glucose uptake and suppression of glucose transporter 1 mRNA expression in L929 cells by tumour necrosis factor-alpha. Life Sci. 1999;65:PL215–PL220. doi: 10.1016/s0024-3205(99)00408-7. [DOI] [PubMed] [Google Scholar]
- 26.Plaisier C, Cok A, Scott J, Opejin A, Bushhouse KT, Salie MJ, Louters LL. Effects of cinnamaldehyde on the glucose transport activity of GLUT1. Biochimie. 2011;93:339–344. doi: 10.1016/j.biochi.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salie MJ, Oram DS, Kuipers DP, Scripture JP, Chenge J, MacDonald GJ, Louters LL. Nitroxyl (HNO) acutely activates the glucose uptake activity of GLUT1. Biochimie. 2012;94:864–869. doi: 10.1016/j.biochi.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scott J, Opejin A, Tidball A, Stehouwer N, Rekman J, Louters LL. Dual action of phenylarsine oxide on the glucose transport activity of GLUT1. Chem Biol Interact. 2009;182:199–203. doi: 10.1016/j.cbi.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 29.Gunnink SM, Kerk SA, Kuiper BD, Alabi OD, Kuipers DP, Praamsma RC, Wrobel KE, Louters LL. Alkaline pH activates the transport activity of GLUT1 in L929 fibroblast cells. Biochimie. 2013;99 doi: 10.1016/j.biochi.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gipson IK, Spurr-Michaud S, Argueso P, Tisdale A, Ng TF, Russo CL. Mucin gene expression in immortalized human corneal-limbal and conjunctival epithelial cell lines. Investig Ophthalmol Vis Sci. 2003;44:2496–2506. doi: 10.1167/iovs.02-0851. [DOI] [PubMed] [Google Scholar]
- 31.Van Dyke DA, Walters L, Frieswyk D, Kokmeyer D, Louters LL. Acute effects of troglitazone and nitric oxide on glucose uptake in L929 fibroblast cells. Life Sci. 2003;72:2321–2327. doi: 10.1016/s0024-3205(03)00119-x. [DOI] [PubMed] [Google Scholar]
- 32.Kuipers DP, Scripture JP, Gunnink SM, Salie MJ, Schotanus MP, Ubels JL, Louters LL. Differential regulation of GLUT1 activity in human corneal limbal epithelial cells and fibroblasts. Biochimie. 2013;95:258–263. doi: 10.1016/j.biochi.2012.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buller CL, Heilig CW, Brosius FC., 3rd GLUT1 enhances mTOR activity independently of TSC2 and AMPK. Am J Physiol Ren Physiol. 2011;301:F588–F596. doi: 10.1152/ajprenal.00472.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roelofs B, Tidball A, Lindborg AE, TenHarmsel A, Vander Kooy TO, Louters LL. Acute activation of glucose uptake by glucose deprivation in L929 fibroblast cells. Biochimie. 2006;88:1941–1946. doi: 10.1016/j.biochi.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 35.Cok A, Plaisier C, Salie MJ, Oram DS, Chenge J, Louters LL. Berberine acutely activates the glucose transport activity of GLUT1. Biochimie. 2011;93:1187–1192. doi: 10.1016/j.biochi.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Louters LL, Scripture JP, Kuipers DP, Gunnink SM, Kuiper BD, Alabi OD. Hydroxylamine acutely activates glucose uptake in L929 fibroblast cells. Biochimie. 2013;95:787–792. doi: 10.1016/j.biochi.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tang Y, Chen A. Curcumin prevents leptin raising glucose levels in hepatic stellate cells by blocking translocation of glucose transporter-4 and increasing glucokinase. Br J Pharmacol. 2010;161:1137–1149. doi: 10.1111/j.1476-5381.2010.00956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zottola RJ, Cloherty EK, Coderre PE, Hansen A, Hebert DN, Carruthers A. Glucose transporter function is controlled by transporter oligomeric structure. A single, intramolecular disulfide promotes GLUT1 tetramerization. Biochemistry. 1995;34:9734–9747. doi: 10.1021/bi00030a011. [DOI] [PubMed] [Google Scholar]
- 39.Carruthers A, DeZutter J, Ganguly A, Devaskar SU. Will the original glucose transporter isoform please stand up! Am J Physiol Endocrinol Metab. 2009;297:E836–E848. doi: 10.1152/ajpendo.00496.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zoccoli MA, Baldwin SA, Lienhard GE. The monosaccharide transport system of the human erythrocyte. Solubilization and characterization on the basis of cytochalasin B binding. J Biol Chem. 1978;253:6923–6930. [PubMed] [Google Scholar]
- 41.Sage JM, Cura AJ, Lloyd KP, Carruthers A. Caffeine inhibits glucose transport by binding at the GLUT1 nucleotide-binding site. Am J Physiol Cell physiol. 2015;308:C827–C834. doi: 10.1152/ajpcell.00001.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4:807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 43.Gutierres VO, Campos ML, Arcaro CA, Assis RP, Baldan-Cimatti HM, Peccinini RG, Paula-Gomes S, Kettelhut IC, Baviera AM, Brunetti IL. Curcumin pharmacokinetic and pharmacodynamic evidences in streptozotocin-diabetic rats support the antidiabetic activity to be via metabolite(s) Evid Based Complement Altern Med eCAM. 2015;2015:678218. doi: 10.1155/2015/678218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dvivedi J, Pandey S, Gupta R. Effect of curcumin on glucose absorption: an experimental study on albino rats. Indian J Physiol Pharmacol. 2011;55:207–212. [PubMed] [Google Scholar]
- 45.Furuta E, Okuda H, Kobayashi A, Watabe K. Metabolic genes in cancer: their roles in tumor progression and clinical implications. Biochim Biophys Acta. 2010;1805:141–152. doi: 10.1016/j.bbcan.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Szablewski L. Expression of glucose transporters in cancers. Biochim Biophys Acta. 2013;1835:164–169. doi: 10.1016/j.bbcan.2012.12.004. [DOI] [PubMed] [Google Scholar]