Abstract
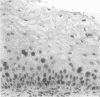
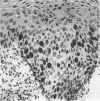
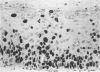
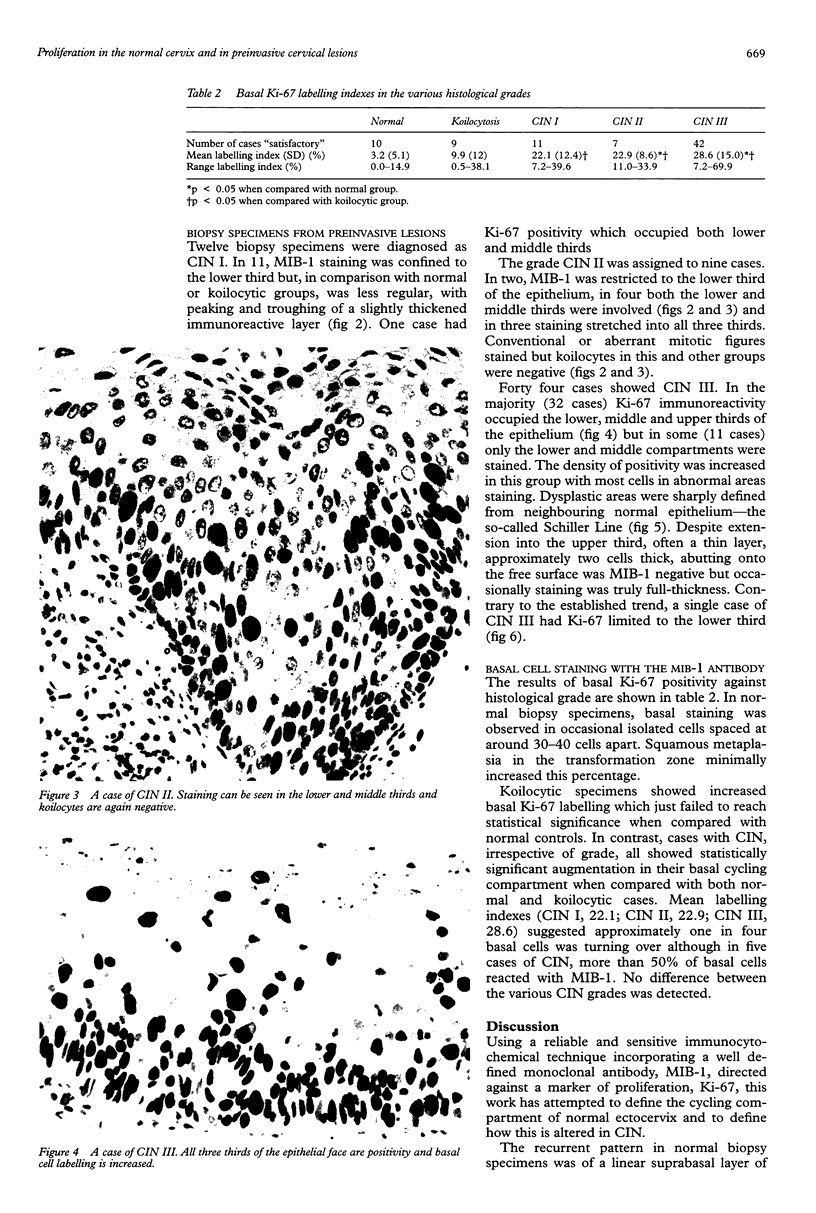
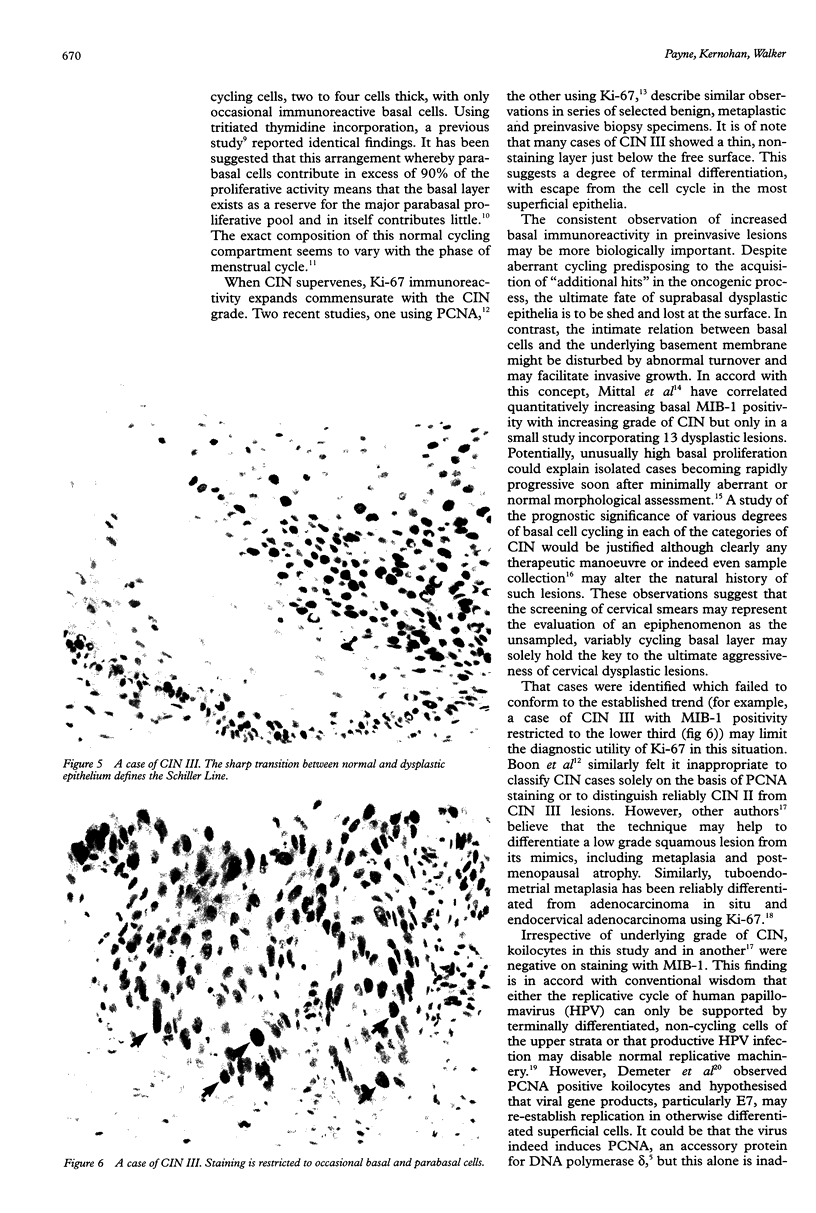
AIMS: To characterise further the proliferative compartment of the normal cervix and to document its alteration, if any, in the various grades of cervical intraepithelial neoplasia (CIN), particularly changes to the basal epithelial layer; to hypothesise as to the diagnostic and biological significance of any observed differences. METHOD: Proliferative compartments from 86 cervical biopsy specimens (10 normal, 11 with koilocytic change only, 12 CIN I, nine CIN II, and 44 CIN III) were determined using microwave antigen retrieval and a standard three-step Streptavidin biotin peroxidase immunocytochemical technique incorporating the MIB-1 monoclonal antibody (directed against the Ki-67 antigen). Immunoreactivity was assessed as occupying either the lower one third, lower two thirds or all three thirds of the squamous epithelium. Basal cell positivity was also quantitated. RESULTS: Specimens without CIN showed a thin suprabasal proliferative compartment two to four cells thick. True basal positivity was infrequent. With increasing grade of CIN, the growth compartment stretched evermore superficially so that in lesions of CIN III almost the full thickness of epithelium was cycling. In all grades of CIN, basal cell proliferation was significantly increased. CONCLUSIONS: In normal cervix, the parabasal layers represent the main proliferative pool with the basal layer providing a reserve. When CIN supervenes, this proliferative compartment expands commensurate with the grade of dysplasia and as basal turnover is increased specifically the intimate relation between epithelium and basement membrane might be disturbed, facilitating invasion. The diagnostic utility of these changes in growth compartments is limited.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Averette H. E., Weinstein G. D., Frost P. Autoradiographic analysis of cell proliferation kinetics in human genital tissues. I. Normal cervix and vagina. Am J Obstet Gynecol. 1970 Sep 1;108(1):8–17. doi: 10.1016/0002-9378(70)90195-x. [DOI] [PubMed] [Google Scholar]
- Berkeley A. S., LiVolsi V. A., Schwartz P. E. Advanced squamous cell carcinoma of the cervix with recent normal Papanicolaou tests. Lancet. 1980 Aug 16;2(8190):375–376. doi: 10.1016/s0140-6736(80)90378-5. [DOI] [PubMed] [Google Scholar]
- Boon M. E., Luzzatto R., Beck S., Bosch M. M., Hermans J., Rietveld W. J. Proliferation profile of benign and premalignant cervical epithelium as established by PCNA staining pattern. Pathol Res Pract. 1994 Apr;190(4):372–377. doi: 10.1016/S0344-0338(11)80409-7. [DOI] [PubMed] [Google Scholar]
- Brown D. C., Gatter K. C. Monoclonal antibody Ki-67: its use in histopathology. Histopathology. 1990 Dec;17(6):489–503. doi: 10.1111/j.1365-2559.1990.tb00788.x. [DOI] [PubMed] [Google Scholar]
- Buxton E. J., Luesley D. M., Shafi M. I., Rollason M. Colposcopically directed punch biopsy: a potentially misleading investigation. Br J Obstet Gynaecol. 1991 Dec;98(12):1273–1276. doi: 10.1111/j.1471-0528.1991.tb15401.x. [DOI] [PubMed] [Google Scholar]
- Cattoretti G., Pileri S., Parravicini C., Becker M. H., Poggi S., Bifulco C., Key G., D'Amato L., Sabattini E., Feudale E. Antigen unmasking on formalin-fixed, paraffin-embedded tissue sections. J Pathol. 1993 Oct;171(2):83–98. doi: 10.1002/path.1711710205. [DOI] [PubMed] [Google Scholar]
- Demeter L. M., Stoler M. H., Broker T. R., Chow L. T. Induction of proliferating cell nuclear antigen in differentiated keratinocytes of human papillomavirus-infected lesions. Hum Pathol. 1994 Apr;25(4):343–348. doi: 10.1016/0046-8177(94)90141-4. [DOI] [PubMed] [Google Scholar]
- Gerdes J., Lemke H., Baisch H., Wacker H. H., Schwab U., Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984 Oct;133(4):1710–1715. [PubMed] [Google Scholar]
- Hall P. A., Levison D. A., Woods A. L., Yu C. C., Kellock D. B., Watkins J. A., Barnes D. M., Gillett C. E., Camplejohn R., Dover R. Proliferating cell nuclear antigen (PCNA) immunolocalization in paraffin sections: an index of cell proliferation with evidence of deregulated expression in some neoplasms. J Pathol. 1990 Dec;162(4):285–294. doi: 10.1002/path.1711620403. [DOI] [PubMed] [Google Scholar]
- Kamel O. W., Franklin W. A., Ringus J. C., Meyer J. S. Thymidine labeling index and Ki-67 growth fraction in lesions of the breast. Am J Pathol. 1989 Jan;134(1):107–113. [PMC free article] [PubMed] [Google Scholar]
- Konishi I., Fujii S., Nonogaki H., Nanbu Y., Iwai T., Mori T. Immunohistochemical analysis of estrogen receptors, progesterone receptors, Ki-67 antigen, and human papillomavirus DNA in normal and neoplastic epithelium of the uterine cervix. Cancer. 1991 Sep 15;68(6):1340–1350. doi: 10.1002/1097-0142(19910915)68:6<1340::aid-cncr2820680626>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- McCluggage W. G., Maxwell P., McBride H. A., Hamilton P. W., Bharucha H. Monoclonal antibodies Ki-67 and MIB1 in the distinction of tuboendometrial metaplasia from endocervical adenocarcinoma and adenocarcinoma in situ in formalin-fixed material. Int J Gynecol Pathol. 1995 Jul;14(3):209–216. doi: 10.1097/00004347-199507000-00003. [DOI] [PubMed] [Google Scholar]
- Mittal K. R., Demopoulos R. I., Goswami S. Proliferating cell nuclear antigen (cyclin) expression in normal and abnormal cervical squamous epithelia. Am J Surg Pathol. 1993 Feb;17(2):117–122. doi: 10.1097/00000478-199302000-00003. [DOI] [PubMed] [Google Scholar]
- Schellhas H. F., Heath G. Cell renewal in the human cervix uteri; a radioautographic study DNA, RNA, and protein synthesis. Am J Obstet Gynecol. 1969 Jul 1;104(5):617–632. doi: 10.1016/0002-9378(69)90595-x. [DOI] [PubMed] [Google Scholar]
- Shi S. R., Key M. E., Kalra K. L. Antigen retrieval in formalin-fixed, paraffin-embedded tissues: an enhancement method for immunohistochemical staining based on microwave oven heating of tissue sections. J Histochem Cytochem. 1991 Jun;39(6):741–748. doi: 10.1177/39.6.1709656. [DOI] [PubMed] [Google Scholar]
- Yu C. C., Filipe M. I. Update on proliferation-associated antibodies applicable to formalin-fixed paraffin-embedded tissue and their clinical applications. Histochem J. 1993 Dec;25(12):843–853. [PubMed] [Google Scholar]
- al-Saleh W., Delvenne P., Greimers R., Fridman V., Doyen J., Boniver J. Assessment of Ki-67 antigen immunostaining in squamous intraepithelial lesions of the uterine cervix. Correlation with the histologic grade and human papillomavirus type. Am J Clin Pathol. 1995 Aug;104(2):154–160. doi: 10.1093/ajcp/104.2.154. [DOI] [PubMed] [Google Scholar]