Abstract
AIMS: To compare the ability of four commonly used PCR techniques to demonstrate clonal IgH rearrangements in multiple myeloma. METHODS: Bone marrow samples (containing a minimum of 10% plasma cells) were obtained from 127 patients with confirmed multiple myeloma. Framework 3 (Fr3) PCR was performed in all cases and the Framework 1 (Fr1f) PCR, which utilises six VH family specific primers, in 98 cases. In addition, 44 cases were assessed by Fr3, Fr1f, Framework 2 (Fr2) and Framework 1 consensus (Fr1 con) PCR techniques. JH primer selection was also assessed such that each PCR strategy was performed twice in each of the 44 cases, using the JH consensus primer (JH con) alone and then repeated with an equimolar mixture of JH con, JH3 and JH6 (JH mix). RESULTS: Clonal rearrangements were demonstrated in 71 (56%) of 127 cases with the Fr3 PCR and in 52 (53%) of 98 with the Fr1f PCR. However, by using both techniques it was possible to demonstrate clonal IgH rearrangements in 92 (75%) of 122 cases. Forty four cases were assessed by all four PCR techniques; in these cases the Fr3 and Fr1f PCRs demonstrated clonal rearrangements in 26 (59%) cases with a combined yield of 34 (77%). The Fr2 and Fr1 con PCR techniques had inferior pick up rates, demonstrating clonal rearrangements in 21 (48%) of 44 cases and a combined yield of 28 (63%). The Fr2 PCR did, however, demonstrate a clonal rearrangement in one case negative by both Fr3 and Fr1f. Two additional rearrangements were demonstrated by using JH mix; one became positive by Fr3, Fr1f and Fr2 and the other positive by Fr1f, Fr1 con and Fr2. CONCLUSIONS: By utilising both the Fr3 and Fr1f PCR techniques it is possible to demonstrate definitive clonal rearrangements in the majority of patients with multiple myeloma. The Fr1 con and Fr2 PCR techniques have inferior pick up rates but may detect some additional rearrangements.
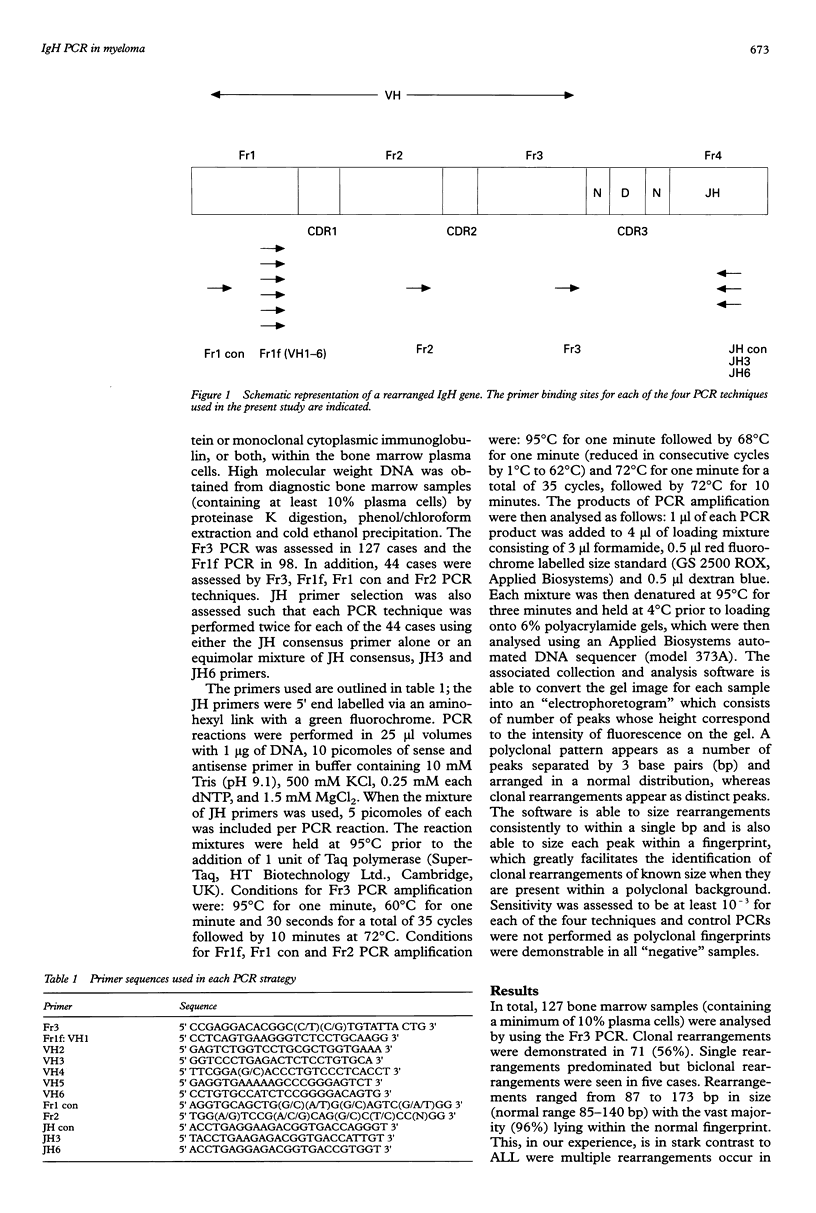
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aubin J., Davi F., Nguyen-Salomon F., Leboeuf D., Debert C., Taher M., Valensi F., Canioni D., Brousse N., Varet B. Description of a novel FR1 IgH PCR strategy and its comparison with three other strategies for the detection of clonality in B cell malignancies. Leukemia. 1995 Mar;9(3):471–479. [PubMed] [Google Scholar]
- Bahler D. W., Campbell M. J., Hart S., Miller R. A., Levy S., Levy R. Ig VH gene expression among human follicular lymphomas. Blood. 1991 Sep 15;78(6):1561–1568. [PubMed] [Google Scholar]
- Baker B. W., Deane M., Gilleece M. H., Johnston D., Scarffe J. H., Norton J. D. Distinctive features of immunoglobulin heavy chain variable region gene rearrangement in multiple myeloma. Leuk Lymphoma. 1994 Jul;14(3-4):291–301. doi: 10.3109/10428199409049681. [DOI] [PubMed] [Google Scholar]
- Bakkus M. H., Heirman C., Van Riet I., Van Camp B., Thielemans K. Evidence that multiple myeloma Ig heavy chain VDJ genes contain somatic mutations but show no intraclonal variation. Blood. 1992 Nov 1;80(9):2326–2335. [PubMed] [Google Scholar]
- Berenson J. R., Vescio R. A., Hong C. H., Cao J., Kim A., Lee C. C., Schiller G., Berenson R. J., Lichtenstein A. K. Multiple myeloma clones are derived from a cell late in B lymphoid development. Curr Top Microbiol Immunol. 1995;194:25–33. doi: 10.1007/978-3-642-79275-5_4. [DOI] [PubMed] [Google Scholar]
- Billadeau D., Quam L., Thomas W., Kay N., Greipp P., Kyle R., Oken M. M., Van Ness B. Detection and quantitation of malignant cells in the peripheral blood of multiple myeloma patients. Blood. 1992 Oct 1;80(7):1818–1824. [PubMed] [Google Scholar]
- Bird J. M., Bloxham D., Samson D., Marcus R. E., Russell N. H., Kelsey S. M., Newland A. C., Apperley J. F. Molecular detection of clonally rearranged cells in peripheral blood progenitor cell harvests from multiple myeloma patients. Br J Haematol. 1994 Sep;88(1):110–116. doi: 10.1111/j.1365-2141.1994.tb04985.x. [DOI] [PubMed] [Google Scholar]
- Bird J. M., Russell N. H., Samson D. Minimal residual disease after bone marrow transplantation for multiple myeloma: evidence for cure in long-term survivors. Bone Marrow Transplant. 1993 Dec;12(6):651–654. [PubMed] [Google Scholar]
- Brisco M. J., Tan L. W., Orsborn A. M., Morley A. A. Development of a highly sensitive assay, based on the polymerase chain reaction, for rare B-lymphocyte clones in a polyclonal population. Br J Haematol. 1990 Jun;75(2):163–167. doi: 10.1111/j.1365-2141.1990.tb02643.x. [DOI] [PubMed] [Google Scholar]
- Corradini P., Voena C., Astolfi M., Ladetto M., Tarella C., Boccadoro M., Pileri A. High-dose sequential chemoradiotherapy in multiple myeloma: residual tumor cells are detectable in bone marrow and peripheral blood cell harvests and after autografting. Blood. 1995 Mar 15;85(6):1596–1602. [PubMed] [Google Scholar]
- Corradini P., Voena C., Omedé P., Astolfi M., Boccadoro M., Dalla-Favera R., Pileri A. Detection of circulating tumor cells in multiple myeloma by a PCR-based method. Leukemia. 1993 Nov;7(11):1879–1882. [PubMed] [Google Scholar]
- Deane M., Baker B. W., Norton J. D. Immunoglobulin VH4 gene usage in B lymphoid leukaemias. Br J Haematol. 1993 Jun;84(2):242–249. doi: 10.1111/j.1365-2141.1993.tb03059.x. [DOI] [PubMed] [Google Scholar]
- Deane M., Norton J. D. Immunoglobulin gene 'fingerprinting': an approach to analysis of B lymphoid clonality in lymphoproliferative disorders. Br J Haematol. 1991 Mar;77(3):274–281. doi: 10.1111/j.1365-2141.1991.tb08570.x. [DOI] [PubMed] [Google Scholar]
- Deane M., Norton J. D. Immunoglobulin heavy chain variable region family usage is independent of tumor cell phenotype in human B lineage leukemias. Eur J Immunol. 1990 Oct;20(10):2209–2217. doi: 10.1002/eji.1830201009. [DOI] [PubMed] [Google Scholar]
- Deane M., Norton J. D. Preferential rearrangement of developmentally regulated immunoglobulin VH1 genes in human B-lineage leukaemias. Leukemia. 1991 Aug;5(8):646–650. [PubMed] [Google Scholar]
- Diss T. C., Peng H., Wotherspoon A. C., Isaacson P. G., Pan L. Detection of monoclonality in low-grade B-cell lymphomas using the polymerase chain reaction is dependent on primer selection and lymphoma type. J Pathol. 1993 Mar;169(3):291–295. doi: 10.1002/path.1711690303. [DOI] [PubMed] [Google Scholar]
- Gazitt Y., Reading C. C., Hoffman R., Wickrema A., Vesole D. H., Jagannath S., Condino J., Lee B., Barlogie B., Tricot G. Purified CD34+ Lin- Thy+ stem cells do not contain clonal myeloma cells. Blood. 1995 Jul 1;86(1):381–389. [PubMed] [Google Scholar]
- Holden J. T., Geller R. B., Farhi D. C., Holland H. K., Stempora L. L., Phillips C. N., Bray R. A. Characterization of Thy-1 (CDw90) expression in CD34+ acute leukemia. Blood. 1995 Jul 1;86(1):60–65. [PubMed] [Google Scholar]
- Kipps T. J., Carson D. A. Autoantibodies in chronic lymphocytic leukemia and related systemic autoimmune diseases. Blood. 1993 May 15;81(10):2475–2487. [PubMed] [Google Scholar]
- Maloum K., Magnac C., Pritsch O., Binet J. L., Merle-Béral H., Dighiero G. Skewed rearrangement of the VH4-21 gene during pre-B acute lymphoblastic leukemia. Leuk Lymphoma. 1995 May;17(5-6):435–441. doi: 10.3109/10428199509056854. [DOI] [PubMed] [Google Scholar]
- Mayer R., Logtenberg T., Strauchen J., Dimitriu-Bona A., Mayer L., Mechanic S., Chiorazzi N., Borche L., Dighiero G., Mannheimer-Lory A. CD5 and immunoglobulin V gene expression in B-cell lymphomas and chronic lymphocytic leukemia. Blood. 1990 Apr 1;75(7):1518–1524. [PubMed] [Google Scholar]
- Owen R. G., Haynes A. P., Evans P. A., Johnson R. J., Rawstron A. C., McQuaker G., Smith G. M., Galvin M. C., Barnard D. L., Russell N. H. Detection of clonal immunoglobulin gene rearrangements in the peripheral blood progenitor cells of patients with multiple myeloma: the potential role of purging with CD34 positive selection. Clin Mol Pathol. 1996 Apr;49(2):M112–M117. doi: 10.1136/mp.49.2.m112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph Q. M., Brisco M. J., Joshua D. E., Brown R., Gibson J., Morley A. A. Advancement of multiple myeloma from diagnosis through plateau phase to progression does not involve a new B-cell clone: evidence from the Ig heavy chain gene. Blood. 1993 Jul 1;82(1):202–206. [PubMed] [Google Scholar]
- Schiller G., Vescio R., Freytes C., Spitzer G., Sahebi F., Lee M., Wu C. H., Cao J., Lee J. C., Hong C. H. Transplantation of CD34+ peripheral blood progenitor cells after high-dose chemotherapy for patients with advanced multiple myeloma. Blood. 1995 Jul 1;86(1):390–397. [PubMed] [Google Scholar]
- Trainor K. J., Brisco M. J., Wan J. H., Neoh S., Grist S., Morley A. A. Gene rearrangement in B- and T-lymphoproliferative disease detected by the polymerase chain reaction. Blood. 1991 Jul 1;78(1):192–196. [PubMed] [Google Scholar]
- Wagner S. D., Martinelli V., Luzzatto L. Similar patterns of V kappa gene usage but different degrees of somatic mutation in hairy cell leukemia, prolymphocytic leukemia, Waldenstrom's macroglobulinemia, and myeloma. Blood. 1994 Jun 15;83(12):3647–3653. [PubMed] [Google Scholar]


