Abstract
Evidence indicates meditation facilitates affective regulation and reduces negative affect. It also influences resting-state functional connectivity between affective networks and the posterior cingulate (PCC)/precuneus, regions critically implicated in self-referential processing. However, no longitudinal study employing active control group has examined the effect of meditation training on affective processing, PCC/precuneus connectivity, and their association. Here, we report that eight-week meditation, but not relaxation, training ‘neutralized’ affective processing of positive and negative stimuli in healthy elderly participants. Additionally, meditation versus relaxation training increased the positive connectivity between the PCC/precuneus and the pons, the direction of which was largely directed from the pons to the PCC/precuneus, as revealed by dynamic causal modeling. Further, changes in connectivity between the PCC/precuneus and pons predicted changes in affective processing after meditation training. These findings indicate meditation promotes self-referential affective regulation based on increased regulatory influence of the pons on PCC/precuneus, which new affective-processing strategy is employed across both resting state and when evaluating affective stimuli. Such insights have clinical implications on interventions on elderly individuals with affective disorders.
Abbreviations: BMA, Bayesian model average; BMS, Bayesian model selection; DMN, default mode network; DRN, dorsal raphe nucleus; EPT, emotion processing task; HADS, Hospital Anxiety and Depression Scale; IAPS, International Affective Picture System; MDD, major depressive disorder; MRI, magnetic resonance imaging; rs-FC, resting-state functional connectivity; PCC, posterior cingulate cortex; PRN, pontine raphe nucleus; spDCM, spectral dynamic causal modeling; SVC, small volume correction; TONI-III, Test of Nonverbal Intelligence, third edition; YoE, years of education
Keywords: Pons, Functional connectivity, Resting-state MRI, Meditation, Affective processing
Highlights
-
•
Meditation training in the elderly “neutralized” affective processing
-
•
Meditation training in the elderly increased pons-to-PCC resting-state connectivity
-
•
Meditation promotes affective regulation via increased influence of pons on PCC.
As an increasingly popular practice, meditation is considered to facilitate affective regulation and positive mind state. Here we report meditation training versus active control ‘neutralized’ elderly participants' positive and negative affective processing, and led to more positive resting-state connectivity from the pontine nuclei, which regulates affective processing, to the posterior cingulate/precuneus which engages in self-referential processing. Further, the change in pons-to-posterior cingulate connectivity predicted change in affective processing in the meditation, but not active control, group. These findings indicate that meditation enhances affective regulatory capacities, with a neural basis of increased influence of the pontine nuclei on the posterior cingulate/precuneus.
1. Introduction
Accumulating evidence indicates that meditation facilitates affective regulation, enhances positive affect and reduces negative affect states (Jain et al., 2007, Robins et al., 2012, Chiesa et al., 2013). Such effects of meditation training are accompanied by neural functional connectivity changes (Brewer et al., 2011, Jang et al., 2011, Kilpatrick et al., 2011). Hence, further exploration on the associations between the affective and neural effects of meditation on brain connectivity patterns would enhance our theoretical understanding of the neural network mechanisms by which meditation training influences affective processing, as well as providing important clinical implications for improving the states of individuals with compromised affective regulation capacities, such as those with major depressive disorders (MDD) (Chiesa and Serretti, 2011, Simon and Engström, 2015).
Existing evidence indicates that both long- and short-term meditation practices change individuals' affective processing and regulatory patterns (Chiesa et al., 2013). Theoretically, meditation emphasizes non-judgmental, open observations of thoughts, feelings, and stimuli from a non-self-referential perspective, allowing for the development of more self-detached, experiential and objective analysis of sensory events rather than focusing on the subjective affective values (Ruby and Decety, 2004). Thereof, an even-minded mental state of ‘equanimity’ is ultimately developed towards all stimuli and experiences, regardless of their affective valence (Desbordes et al., 2015). Accordingly, meditation should lead to less differentiated affective processing of ‘positive’ and ‘negative’ stimuli, as well as reduced general affective reactivity and arousal (Farb et al., 2010, Goldin and Gross, 2010). Meditation also instructs people to maintain their attention on present experiences, which prevents them from engaging in recursive, ruminating processing of negative stimuli (Brewer et al., 2011). It has been shown that meditation reduces affective processing of negative and positive stimuli, as manifested in the level of affective interference (Ortner et al., 2007, Menezes et al., 2013), affective stimuli valence and arousal ratings (Menezes et al., 2013, Ho et al., 2015), limbic and striatal neural reactivities (Tang et al., 2009), and cortical functional connectivities during processing affective stimuli (Lutz et al., 2016), even when the participants are in a non-meditative state (for reviews, see Chiesa et al., 2013, Tang et al., 2015). However, relatively few studies have employed an appropriate active control group that enables an evaluation of the effects of meditation-specific components on affective processing. One such form of active control for meditation training is relaxation training, which can control for social interactions with a group or teacher, the amount of home exercise, motivation, and positive expectations (Tang et al., 2015). Menezes et al. (2013) found that relative to relaxation training, short-term meditation training led to reduced affective processing of negative pictorial stimuli from the International Affective Picture System (IAPS) for both valence and arousal. Nevertheless, such longitudinal research is lacking on the effect of meditation on the processing of positive affective stimuli, while cross-sectional studies have reported inconsistent findings (Ortner et al., 2007, Lee et al., 2012, Reva et al., 2014, Ho et al., 2015). Existing studies that involved comparing the effects of short-term meditation and active control training also indicate specific beneficial effects of meditation training on attentional and cognitive control capacities (Wenk-Sormaz, 2005, Tang et al., 2007, Zeidan et al., 2010), with a meta-analysis study concluding that meditation tends to exert medium-level effects on attention and cognitive control measures (Sedlmeier et al., 2012). Such improvements in attentional control following meditation practice can enhance disengagement from negative affective stimuli and lead to better affective regulation (Brewer et al., 2011).
The neural impact of meditation has been researched extensively in the context of the default mode network (DMN), a task-negative network that exhibits functionally coordinated activities when the individual is at rest (Raichle et al., 2001). Generally, the nature of meditation in facilitating observations of experiences from a self-detached viewpoint and in promoting focused attention on present-moment stimuli contrasts with the functioning of the DMN, which is considered to support stochastic mental drifting primarily from a self-centered perspective (Northoff et al., 2006, Brewer et al., 2011, Simon and Engström, 2015). A core ‘hub’ of the DMN is the posterior cingulate cortex (PCC) and the adjacent precuneus, both of which play critical roles in self-referential processing, self-consciousness, self-related mental representations and first-person perspective taking (Cavanna and Trimble, 2006, Dor-Ziderman et al., 2013, Tang et al., 2015). The connectivity patterns of those two regions with other cortico-limbic regions fundamentally determine how an individual perceives and evaluates the affective value of events and stimuli in relation to themselves (Josipovic, 2014). Consistent with this, individuals with MDD exhibit abnormal PCC resting-state functional connectivity (rs-FC) patterns, which correlated with negative self-referential rumination (Berman et al., 2010, Li et al., 2013). In contrast, meditation training is associated with more positive self-representation, higher self-esteem and higher self-acceptance (Emavardhana and Tori, 1997, Haimerl and Valentine, 2001), and it influences the functional connectivity of the PCC/precuneus with the medial and lateral prefrontal networks implicated for affective regulatory functions, during meditative (Brewer et al., 2011, Garrison et al., 2014), resting (Brewer et al., 2011, Jang et al., 2011, Taylor et al., 2013, Hasenkamp and Barsalou, 2012) and affective processing states (Lutz et al., 2016). These findings were generally interpreted as reflecting increased self-referential affective regulation and self-awareness following meditation training. However, all the existing studies on this topic have been cross-sectional, and longitudinal studies employing active control groups are much needed to provide more definitive evidence. Additionally, very few studies have attempted to link changes in PCC/precuneus rs-FC with changes in affective processing following meditation training. Specifically, it might be that meditation training alters the intrinsic functional connectivity patterns of the PCC/precuneus, which in turn triggers changes in affective processing. On the other hand, it might be that following meditation training, the association between changes in the PCC/precuneus rs-FC and in affective processing becomes stronger. This would be consistent with existing evidence indicating that after meditation training, the meditative neural patterns relevant to processing affective stimuli are maintained even at rest and become the default mode of brain activity (Manna et al., 2010, Brewer et al., 2011, Desbordes et al., 2012, Hasenkamp and Barsalou, 2012). Therefore, individuals with meditation experience might exhibit similar patterns of neural activity (i.e., the meditative mode) both at rest and when processing affective stimuli, which may not be true for non-meditators. No existing study has addressed these distinct, albeit non-mutually-exclusive, possibilities.
While existing meditation research has mostly focused on the functional connectivity between the PCC/precuneus and prefrontal regions, little evidence exists on whether the PCC/precuneus exhibits different functional connectivity patterns with subcortical affective networks following meditation training. In particular, the brain stem raphe nuclei constitute the major source of serotonergic projections that target the cortical, limbic and striatal regions implicated in affective processing (Deakin and Graeff, 1991, Hajós et al., 2003). Existing evidence indicates that serotonergic transmission in the brain may influence an individual's cognitive and neural processing of negative stimuli (Murphy et al., 2002, Cools et al., 2008, Roiser et al., 2008), and that reduction of serotonergic functions may be associated with heightened rates of neuroticism (Sen et al., 2004), consistent with a proposed role of serotonergic activity in regulating negative affective processing and in affective disorders (Fischer et al., 2015, Fakhoury, 2016). Similarly, the raphe nuclei have been found to regulate activity in downstream cortical and limbic affective networks, as well as processing of aversive stimuli and distress (Hajós et al., 2003, Amat et al., 2004, Selvaraj et al., 2015). While the dorsal raphe nucleus (DRN) might have received the most attention on its serotonergic-based regulations of downstream cortico-limbic-striatal networks and affective processing (Graeff et al., 1996, Selvaraj et al., 2015), recent evidence indicates that the pontine raphe nucleus (PRN) may also play critical roles in affective regulatory functions (Cannon et al., 2006, Cannon et al., 2007, Lee et al., 2015). Patients with bipolar disorders, which are fundamentally characterized by extreme euphoria and dysphoria states and affective dysregulations, exhibit reduced serotonergic functioning in the PRN (Cannon et al., 2006, Cannon et al., 2007), and both the anatomical and functional connectivity of the PRN with other affective networks predicted the affective states and emotional reactivity of healthy participants (Lee et al., 2015). Further, the PRN is critically involved in regulating negative stress (Vollmayr et al., 2000). Thus, the functional interplay between the PRN and PCC/precuneus, which likely underlies the processes by which individuals regulate self-referential negative affective processing, may be strengthened by meditation training. Such hypothesis is further based on known anatomical connections between the PCC/precuneus and pontine nuclei (Leichnetz, 2001, Cavanna and Trimble, 2006), existing findings indicating a brain stem serotonergic influence on the PCC rs-FC (Hahn et al., 2012), and evidence indicating that 8-week meditation training increased the gray matter concentration in a pontine region that correlated with increase in self-reported psychological well-being, as well as in the PCC (Hölzel et al., 2011, Singleton et al., 2014). Based on the theoretical framework that meditation training leads to an increased affective regulatory influence of the PRN on PCC/precuneus self-referential processing, we further expected that causal interactions between the PCC/precuneus and PRN would be mostly directed from the latter to the former structure, as revealed by spectral dynamic causal modeling (spDCM) (Razi et al., 2015).
In the present study, we investigated the effect of 8-week meditation versus relaxation training on affective processing and PCC/precuneus rs-FC patterns in elderly participants. We selected this age group because, despite evidence indicating beneficial cognitive and neural effects of meditation training among the elderly (Gard et al., 2014, Luders, 2014), no research has directly investigated the effect of short-term meditation training on affective processing networks in this age group. Given various existing studies have reported effects of meditation in reducing the valence (Ho et al., 2015, Lutz et al., 2016) and arousal (Menezes et al., 2013) processing of positive and negative stimuli, and based on the theorized impact of meditation in promoting non-judgmental observatory attitude (Tang et al., 2015), we hypothesized that following meditation versus relaxation training, participants would give less positive valence ratings to positive pictorial stimuli and more positive ratings to negative stimuli, as well as reduced arousal ratings for both positive and negative stimuli. On the neural network level, our primary hypothesis was that meditation relative to relaxation training would lead to increased rs-FC between the PCC/precuneus and brain stem regions containing the PRN, with the direction of the increased connectivity primarily from the latter to the former. Also, given the existing body of evidence implicating a role of the serotonergic transmission in the DRN in regulating the neural and behavioral patterns during processing negative stimuli (Graeff et al., 1996, Selvaraj et al., 2015), we additionally expected the rs-FC between the PCC/precuneus and the DRN to be affected by the meditation versus relaxation training. Furthermore, it has been proposed that meditation practice reduces self-referential processing of exteroceptive and interoceptive stimuli (Chiesa et al., 2013), which functions are respectively attributed to the somatosensory cortices and the posterior insula. Given the PCC/precuneus also has anatomical connections with other parts of the parietal cortices including the somatosensory cortices, we further hypothesized that meditation relative to relaxation training would lead to reduced PCC/precuneus rs-FC with the somatosensory cortex and/or posterior insula. Finally, the associations among meditation training, changes in affective ratings and changes in PCC/precuneus rs-FC would be accounted for by one or both of the following two models: i) rs-FC change mediates affective rating changes following meditation versus relaxation training, and ii) meditation training moderates the association between rs-FC and affective rating changes. As natural aging leads to particular functional declines in the dorsal prefrontal cortices (Shao and Lee, 2014), which might suggest that affective processing in the elderly is predominantly bottom-up and driven by subcortical networks (Chiesa et al., 2013), we did not explicitly hypothesize about changes in PCC/precuneus-prefrontal rs-FC following meditation training.
2. Material and Methods
2.1. Participants
Ethical approval was granted by the University of Hong Kong. Forty-five right-handed, healthy elderly adults with normal general intelligence and no prior meditation or relaxation training experience were recruited through community newsletters. Participants were randomly assigned to receiving either meditation training (n = 23; 16 females; mean age = 64.78 ± 2.71 years, range = 60–68 years) or relaxation training (n = 22; 14 females; mean age = 64.68 ± 2.19 years, range = 61–69 years). Demographically, the two groups were matched for age (p = 0.89), sex (p = 0.68), and years of education (YoE) (p = 0.17). Furthermore, the meditation and relaxation groups performed similarly on the Test of Nonverbal Intelligence, third edition (TONI-III) (Brown et al., 1997) (p = 0.91), and showed comparable levels of anxiety (p = 0.48) and depression (p = 0.66), as measured by the Hospital Anxiety and Depression Scale (HADS) (Leung et al., 1999). All participants were right-handed, as assessed with the Edinburgh Handedness Inventory (Oldfield, 1971); had normal or corrected-to-normal vision and hearing; reported no history of major physical illnesses, neurological or psychological conditions, such as substance abuse, psychotic disorders, or affective disorders; and were suitable to enter a magnetic resonance image (MRI) scanner.
For the rs-FC analysis (see below), 5 participants (2 from the meditation group, 3 from the relaxation group) were discarded due to excessive movements during scanning (> 1 voxel in any of the X, Y or Z directions) or poor data quality, leaving 40 participants (28 females) in the analysis. All participants gave written informed consent for participation in the study.
2.2. Meditation and Relaxation Training
Participants received either attention-based compassion meditation training or relaxation training in group settings for 8 weeks. Participants had no knowledge on which type of training they were going to receive prior to the training. The meditation training was conducted by an experienced meditator with 14 years of meditation practice and 4 years of teaching experience. Meditation participants were taught to 1) cultivate mindfulness through paying attention to the surrounding sounds and one's own breathing, feelings and sensations on the present moment, 2) apply non-judgmental and ‘acceptance’ attitudes on thoughts, feelings and sensations, 3) detach from a self-referential framework and observe one's own thoughts and feelings from an outsider's perspective, and 4) cultivate compassion and kindness towards self, family members, friends, strangers, and other living beings (Jain et al., 2007, Lutz et al., 2008). Relaxation training was conducted by a registered clinical psychologist with 4 years of teaching experience. Relaxation participants were taught diaphragmatic breathing, progressive muscle relaxation and imagery relaxation techniques aimed at enhancing body awareness and reducing body tension (Jain et al., 2007, Ortner et al., 2007). Each type of training involved 22 classes, with each class lasting 1.5 h. Each class started with a guided meditation or relaxation practice for ~ 30 min, didactic teaching for ~ 45 min and ended with another guided practice for ~ 15 min. Participants were asked to practice outside of class on a daily basis, and their practice durations were recorded. The total self-practice time was comparable between the meditation (average 710 min) and relaxation groups (average 711.23 min).
2.3. Procedure
All participants underwent a structural MRI and resting-state functional MRI (fMRI) scanning and performed the EPT both before (pre) and after training (post). In the pre-training session, participants were first screened for the exclusion criteria. Participants then completed the TONI-III IQ test and the HADS. The resting-state fMRI and structural MRI protocols were then conducted for the participants, followed by the behavioral Emotion Processing Task (EPT) performed outside of the scanner (see below). Participants then received 8 weeks of either meditation or relaxation training, as detailed above. Within 3 weeks following the completion of training, participants underwent post-training imaging and behavioral assessments, which were performed under the same protocols as for the pre-training assessment. After study completion, participants were debriefed, thanked and reimbursed 800 Hong Kong dollars.
2.4. The Emotion Processing Task (EPT) and Behavioral Analysis
The EPT was employed to evaluate potential changes in affective processing in relation to meditation or relaxation training. The EPT involved 60 affective pictorial stimuli obtained from the International Affective Picture System (IAPS) (Lang et al., 2008). The set included 20 positive (pos), 20 neutral (neu), and 20 negative (neg) pictures that were matched according to the proportion of human and non-human images. Using previously published data on IAPS ratings, we also confirmed that these categories differed significantly in valence (i.e., positive pictures received more positive ratings than neutral pictures, which in turn received more positive ratings than negative pictures, ps < 0.001). On the other hand, positive and negative pictures were matched by arousal level (p = 0.93), and both of these image types received higher arousal ratings than neutral stimuli (ps < 0.001).
During the EPT, participants were instructed to rate both the valence (very negative = 1 to very positive = 75) and arousal (very calm = 1 to very arousing = 75) levels of each positive, neutral and negative picture. Pictures were presented to participants on a Fujitsu computer monitor. Participants rated the same set of EPT pictures in the pre-training and post-training sessions. The order of picture presentations was randomized in different sessions. We adopted such design as the potential repetition effect on participants' ratings was expected to be minimal given the long time delay between the sessions (i.e. 8–11 weeks), and more importantly, we were primarily interested not in the main effect of session or session × valence, but in the 3-way interactive effect of training group × valence × session, which could not have been accounted for by simple repetition effect across sessions which occurred for all participants. The mean valence and arousal ratings (averaged across pictures) were analyzed using a 2 × 3 × 2 repeated-measures ANOVA with the within-subject factors of session (pre vs. post) and valence (pos vs. neu vs. neg) and the between-subjects factor of group (meditation vs. relaxation). The statistical threshold was set at p < 0.05; post hoc two-tailed t tests with Bonferroni correction were performed to characterize significant, higher-level ANOVA results.
2.5. Imaging Data Acquisition and rs-FC Analysis
Resting-state fMRI data were acquired with a 3T Philips scanner equipped with an 8-channel SENSE head coil. Using a T2*-weighted gradient-echo-planar imaging sequence, 180 functional volumes were acquired (slice number/TR/TE/flip angle = 32/2000 ms/30 ms/90°, matrix = 64 × 64, FOV = 230 × 230 × 128 mm3, voxel size = 3.9 × 3.9 × 4 mm3). Participants were instructed to stay awake with their eyes closed for the entire duration of the resting-state scanning, which lasted for a total of 6 min. A T1-weighted high-resolution anatomical scan was also acquired using the magnetization-prepared-rapid gradient-echo (MPRAGE) sequence (155 sagittal slices, TR/TE/flip angle = 6.9 ms/3.2 ms/8°, matrix = 240 × 240, FOV = 240 × 240 × 155 mm; voxel size = 1 × 1 × 1 mm3), for coregistration with the functional images.
The rs-FC fMRI data were preprocessed using Statistical Parametric Mapping (SPM8, Wellcome Department of Cognitive Neurology, London, UK). The first 10 volumes of the data were discarded to avoid tissue disequilibrium. Functional images were corrected for slice acquisition timing and realigned to the first image of the scan session to correct for head motion artifacts. The effects of 9 nuisance covariates, including the six head-motion parameters, mean signals of the whole brain, white-matter and cerebrospinal fluid signals, were removed (Yan and Zang, 2010, Jang et al., 2011). Given that some researchers argue that regressing out global brain signals can introduce spurious anticorrelated functional networks (Murphy et al., 2009), we also ran the rs-FC, spDCM and moderation analyses (see below and Results) without regressing out the global signals in order to ascertain the robustness of our main findings. The resulting images were then co-registered to their individual high-resolution T1 images, normalized to the 3 × 3 × 3 mm3 MNI template by unified segmentation, and spatially smoothed with a Gaussian kernel (full-width at half-maximum = 6 mm). The time courses were then detrended before being temporally band-pass filtered at 0.01–0.08 Hz (Yan and Zang, 2010). The FC correlation coefficiency maps generated by the rs-FC analyses were then z-transformed to obtain the zFC map, which approximates a normal distribution (Yan and Zang, 2010, Jang et al., 2011).
2.6. Whole-brain Seed-based rs-FC Analysis
For the whole-brain rs-FC analysis, a 6-mm sphere in the PCC/precuneus centered on (− 5, − 49, 40) was defined as the seed region for each participant, based on previous research (Yan and Zang, 2010, Fox et al., 2005). To replicate previous findings identifying the PCC/precuneus as a center in the DMN, and thus validate our current data, two-sided t tests were performed on the zFC-maps of the meditation and relaxation groups before training. These findings are presented in Supplementary Table S1.
The rs-FC pattern for the PCC/precuneus seed was analyzed using a 2 × 2 full factorial model with the within-subjects factor of session (pre vs. post) and the between-subjects factor of group (meditation vs. relaxation). To examine the main effect of group, we averaged each participant's rs-FC maps in a voxel-wise manner across the pre- and post-training sessions, which were then subjected to an independent-samples t-test at the group level. To examine the main effect of session, we subtracted each participant's pre-training rs-FC map from the post-training rs-FC map in a voxel-wise manner, and the resulted difference maps were then subjected to a one-sample t-test at the group level to evaluate whether participants' PCC/precuneus rs-FC patterns were, overall, different across the sessions. To examine the group × session interactive effect (Meditationpost-pre–Relaxationpost-pre and Relaxationpost-pre–Meditationpost-pre), we subjected participants' between-session difference maps to an independent-samples t-test to evaluate whether the change in PCC/precuneus rs-FC pattern across sessions depended on the type of training that participants received. All group-level analyses simultaneously controlled for age, sex and YoE to ensure that any differences in rs-FC between the meditation and relaxation groups were not due to between-group differences in the demographic variables.
As we hypothesized changes in PCC/precuneus rs-FC with the PRN and the DRN following meditation versus relaxation training, and in view of the importance and difficulty in localizing these deep nuclei in the brain stem, we have constructed a priori 6-mm spherical masks for both the PRN and the DRN, using anatomical coordinates from previous imaging studies. For the PRN, we used the peak coordinates from Cannon et al. (2006) which showed reduced serotonin transporter binding potentials in bipolar patients compared to healthy controls. The talairach coordinates that they provided (2, − 32, − 20) were transformed to MNI space to obtain the coordinates of (3, − 30, − 30), using Yale BioImage Suite Package based on the mapping of Lacadie et al. (2008). Notably, these coordinates are also very close to the ones obtained by Singleton et al. (2014) assessing the effect of 8-week meditation training on gray matter concentration change. Then, we overlapped the sphere on a standard MNI brain template and visually inspected the appropriateness of its location, and concluded that the entire sphere falls on the posterior portion of the pons slightly towards the rostral side, consistent with the localization of PRN using PET/MRI fusion imaging (Son et al., 2012). We then repeated the procedure for the DRN mask, for which we used the coordinates (− 2, − 24, − 9) provided by Table 1 of Acevedo et al. (2011), which was partly based on the ‘Atlas of the Human Brain’ (Mai et al., 2008). The resulted spherical mask was again visually inspected to ensure that it falls entirely in the anatomical region defined by Son et al. (2012) and Selvaraj et al. (2015). Please note that despite our efforts in locating the PRN, we acknowledge the likelihood that the mask that we constructed may not precisely correspond to the nucleus. Thus, in this paper we often reverted to more general terms such as ‘pons’ or ‘pontine region’ where plausible.
Table 1.
The mean and standard error of the mean (SE) of the participants' EPT valence and arousal ratings.
| Training group | Session | Valence | Valence |
Arousal |
||
|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | |||
| Meditation | Pre-training | Negative | 15.04 | 1.93 | 59.42 | 2.31 |
| Neutral | 41.38 | 0.91 | 32.45 | 2.18 | ||
| Positive | 61.58 | 1.77 | 54.64 | 2.85 | ||
| Post-training | Negative | 21.85 | 1.87 | 47.73 | 2.91 | |
| Neutral | 38.92 | 0.94 | 26.43 | 2.39 | ||
| Positive | 50.81 | 1.77 | 43.15 | 2.77 | ||
| Relaxation | Pre-training | Negative | 13.59 | 1.97 | 57.74 | 2.37 |
| Neutral | 39.02 | 0.93 | 28.07 | 2.23 | ||
| Positive | 59.45 | 1.81 | 48.33 | 2.92 | ||
| Post-training | Negative | 13.03 | 1.91 | 60.34 | 2.98 | |
| Neutral | 40.03 | 0.96 | 30.66 | 2.45 | ||
| Positive | 60.12 | 1.81 | 51.97 | 2.83 | ||
The ratings were made on a 75-point scale, averaged across all pictures of the same valence type (i.e., positive, neutral or negative), and presented separately for the meditation and relaxation groups before and after training. Lower values indicate less positive/more negative valence ratings or lower perceived arousal.
The PCC/precuneus-PRN and PCC/precuneus-DRN rs-FC patterns were characterized in two ways. The main analyses involved extracting the mean zFC values from the a priori PRN and the DRN regions-of-interest (ROI) masks, as described above, using SPM embedded functions. Such procedure provided us with the mean zFC values for each of the PRN and the DRN masks in each experimental condition (Pre-Meditation, Pre-Relaxation, Post-Meditation, Post-Relaxation) and for each participant. The resulting data were then subjected to a 2 (Meditation vs. Relaxation) × 2 (Pre vs. Post) mixed ANOVA analysis performed in SPSS v.20 (IBM Corp), with age, gender and YoE entered as covariates of no interest. To control for pre-training differences in the PCC/precuneus-PRN rs-FC patterns between the meditation and relaxation groups, we performed an extra ANOVA analysis examining the effect of group on the PCC/precuneus-PRN rs-FC change after training, with the pre-training rs-FC values entered as a covariate of no interest. The statistical threshold was set as p < 0.05, two-tailed. Second, in order to localize voxels within the PRN and DRN masks where rs-FC patterns were significantly influenced by the important group × session effect, we performed small-volume-correction (SVC) analyses based on those masks on the group-level results.
Besides the above ROI analyses, we also looked for other brain areas whose rs-FC with the PCC/precuneus was significantly influenced by the group × session effect at a whole brain level. To account for Type 1 errors, we applied a voxel-level uncorrected peak threshold of p < 0.005 and a family-wise-error (FWE) cluster-corrected threshold of p < 0.05, either within individual ROI masks (in case of SVC test) or at a whole brain level (in case of whole-brain analysis).
2.7. Spectral Dynamic Causal Modeling (spDCM) Analysis
Causal interactions between regions at rest were estimated for each subject before and after training by spectral DCM and using SPM, which is suitable for rs-FC studies (Friston et al., 2014, Razi et al., 2015). The following steps were performed: (1) selection of the PCC/precuneus seed region, as well as regions exhibiting rs-FC with the PCC/precuneus that showed a significant group × session effect as voxels of interests (VOIs); (2) extraction of the first eigenvariate of the time courses in the PCC/precuneus seed region and in other VOIs that were defined by drawing 6-mm spheres centered at the peak coordinates, which were then mean-corrected and adjusted for 9 nuisance variables including 6 head motion parameters, mean signals of the whole brain, white-matter and cerebrospinal fluid signals in each subject; (3) specification and estimation of all possible models; (4) performing a family-level Bayesian model selection (BMS) analysis (Penny et al., 2010) by grouping subsets of models based on some common feature shared by those models. We principally focused on exploring the directionality of the functional connectivity between the PCC/precuneus seed and the VOIs, i.e., bidirectional, PCC/precuneus to VOI, or VOI to PCC/precuneus; and (5) estimating the strengths of all connectivity parameters using Bayesian model averaging (BMA) analyses based on relative model family evidence generated by the BMS analysis. A 2 (pre vs. post) × 2 (meditation vs. relaxation) repeated-measures ANOVA was employed to assess the interactive effect of group and session on the connectivity parameters.
Please note that the construction of seed regions for the spDCM analyses were based on spheres centered at the locus of maxima of significant clusters to the group × session effect, as is typically done in resting-state DCM analyses (e.g. Admon and Pizzagalli, 2015, Crone et al., 2015, Razi et al., 2015). In order to more directly address our hypotheses that increased PCC/precuneus-pons rs-FC following meditation versus relaxation training would be directed from the pons to the PCC/precuneus, we also ran the spDCM analysis using the predefined PRN mask centering at (3, − 30, − 30) to assess whether the spDCM results were restricted to the significant voxels in the PRN mask.
2.8. Linking Behavioral and rs-FC Effects
We sought to examine the associations between the group × session interactive effects on the participants' EPT ratings and on PCC/precuneus rs-FC with the predefined PRN and DRN regions. First, we tested whether the group effect on the changes in EPT ratings following training was mediated by the group effect on the changes in PCC/precuneus rs-FC following training. For the EPT ratings, we focused on the positive and negative pictures, both of which are affectively salient and equally arousing, and the ratings they received are important indications of an individual's positive and negative affective processing patterns. To this end, we performed mediation analyses using a bootstrap approach that was implemented in SPSS (Hayes, 2013). Bootstrapping is a non-parametric approach to test hypotheses without making inherent assumptions on the distribution of the data. To predict changes in valence and arousal ratings of positive and negative pictures according to the variable of group with and without the mediating effect of changes in the PCC/precuneus-ROI rs-FC, we set up four separate models. In the first model, the change in the valence ratings of positive stimuli (ValPospost-pre) was the dependent variable, group was designated as the predictor and training-related change in PCC/precuneus-ROI rs-FC was set as the mediator. Additionally, the change in the arousal ratings in response to positive stimuli (AroPospost-pre) and pre-training PCC/precuneus-ROI rs-FC (rs-FCPre) were entered as covariates of no interest. Due to the moderate-to-high between-participant correlations between arousal and valence ratings (| r | s > 0.34), we controlled for changes in the arousal ratings when predicting changes in valence ratings (and vice versa, see below) to ensure that any observed influence of group or the PCC/precuneus-ROI rs-FC change on the latter was not due to its influence on the former. Moreover, the model controlled for age, sex and YoE. The second model resembled the first model, but AroPospost-pre was designated as the dependent variable and ValPospost-pre entered the model as a covariate of no interest. The third and fourth models replicated the first two models for predicting the training-related changes in the valence (model 3) and arousal (model 4) ratings of the negative pictures (ValNegpost-pre and AroNegpost-pre, respectively).
Next, we tested whether the association between training-related changes in participants' EPT ratings and PCC/precuneus-ROI rs-FC was moderated by group. Similar to the mediation analyses, the moderation analyses focused on the ratings of the positive and negative pictures and were performed using the bootstrap approach implemented in SPSS (Hayes, 2013). To predict changes in valence and arousal ratings of positive and negative pictures by changes in the PCC/precuneus-ROI rs-FC, we set up four separate models. In the first model, the change in the valence ratings of positive stimuli (ValPospost-pre) was the dependent variable and group was designated as the moderator. Additionally, the change of arousal ratings of positive stimuli (AroPospost-pre) and pre-training PCC/precuneus-ROI rs-FC (rs-FCPre), as well as age, sex and YoE, were entered as covariates of no interest. The second model resembled the first model, but AroPospost-pre was designated as the dependent variable and ValPospost-pre entered the model as a covariate. The third and fourth models replicated the first two models for predicting training-related changes in the valence (model 3) and arousal (model 4) ratings of the negative pictures (ValNegpost-pre and AroNegpost-pre, respectively).
Bootstrapping was carried out using a bias-corrected approach with 5000 samples. Statistical significance was set at p < 0.05 for all analyses.
3. Results
3.1. The Emotion Processing Task (EPT) Behavior
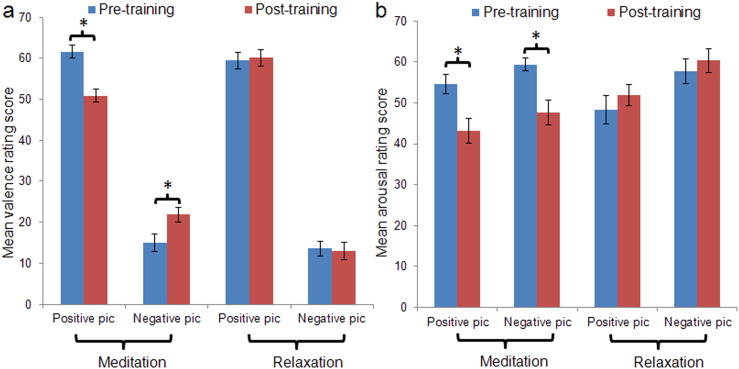
Participants' EPT valence and arousal ratings are presented in Table 1. For the valence ratings, a significant main effect of session (F (1, 43) = 5.05, p < 0.05) was observed, indicating overall less positive valence ratings after training than before training. There was also a main effect of valence (F (2, 86) = 355.37, p < 0.05), confirming that participants rated the positive pictures as significantly more positive than the neutral pictures (t (44) = 16.70, p < 0.05), which were in turn rated as significantly more positive than the negative pictures (t (44) = 17.38, p < 0.05). The meditation and relaxation groups overall showed comparable valence ratings (F (1, 43) = 0.69, p > 0.1). However, a significant interaction between training group and valence was observed (F (2, 86) = 3.79, p < 0.05), characterized by significantly more positive ratings of negative pictures (t (43) = 2.12, p < 0.05) but somewhat less positive ratings of positive pictures (t (43) = − 1.59, p = 0.12) in the meditation group compared to the relaxation group (Fig. 1a). Further, the group × valence effect was moderated by session (F (2, 86) = 16.63, p < 0.05). Prior to training, the interactive effect of training group and valence was non-significant (F (2, 86) = 0.035, p > 0.1), whereas this effect became significant after training (F (2, 86) = 12.919, p < 0.05). In other words, the meditation and relaxation groups were comparable at baseline in how their ratings were affected by the valence of pictures (Table 1, Fig. 1a and Supplementary Fig. S1a). However, following training, the valence ratings of the meditation group for the negative and positive pictures were less differentiated than those of the relaxation group (Table 1 and Fig. 1a). Post-hoc t-tests with Bonferroni correction confirmed that, after training, the meditation group gave more positive valence ratings to negative pictures (t (43) = 3.31, p < 0.05) but less positive ratings to positive pictures (t (43) = − 3.69, p < 0.05) compared to the relaxation group. The two groups did not differ in post-training valence ratings on neutral pictures (t (43) = − 0.82, p > 0.1) (Table 1 and Supplementary Fig. S1a). The meditation group showed significantly more positive ratings on negative pictures (t (22) = 3.02, p < 0.05) but significantly less positive ratings on positive pictures (t (22) = − 4.93, p < 0.05) after training compared to before training. This effect was not observed for the relaxation group (ps ≥ 0.2) (Table 1 and Fig. 1a).
Fig. 1.
EPT valence and arousal ratings. Participants' mean valence (a) and arousal (b) ratings are displayed for the 20 positive and 20 negative pictures presented during the emotion processing task (EPT) for the meditation and relaxation groups before and after training. All ratings were made on a 1–75 scale, with lower scores indicating less positive/more negative valence (for valence ratings) or less arousal (for arousal ratings). Error bars denote ± 1 standard error of the mean. * indicates statistically significant difference at p < 0.05.
Regarding the participants' arousal ratings on the EPT, a significant main effect of session (F (1, 43) = 5.85, p < 0.05) was observed, indicating an overall reduction in arousal ratings after training, and a main effect of valence (F (2, 86) = 196.16, p < 0.05), confirming that both positive (t (44) = 17.27, p < 0.05) and negative (t (44) = 14.24, p < 0.05) pictures were rated as more arousing than neutral pictures. The meditation and relaxation groups overall showed comparable arousal ratings (F (1, 43) = 0.59, p > 0.1). However, a significant interactive effect of training group and session was observed (F (1, 43) = 20.40, p < 0.05). While the meditation group generally gave lower arousal ratings following training compared with before training (t (22) = − 4.40, p < 0.05), such pattern was not observed for the relaxation group (t (21) = 1.74, p = 0.10) (Table 1, Fig. 1b and Supplementary Fig. S1b). Further, the group × session effect was moderated by valence (F (2, 86) = 3.06, p = 0.05). Specifically, the group × session effect was most significant for negative pictures (F (2, 86) = 22.49, p < 0.001), followed by positive pictures (F (2, 86) = 15.23, p < 0.001) and then neutral pictures (F (2, 86) = 9.56, p = 0.003) (Table 1, Fig. 1b and Supplementary Fig. S1b).
Further details about the behavioral analyses are included in Supplementary Materials (‘Additional Emotion Processing Task (EPT) behavioral analysis’).
3.2. PCC-based Resting-state Functional Connectivity
We first demonstrated that prior to training, both the meditation and the relaxation groups showed rs-FC patterns centered at the PCC/precuneus that matched closely with the previously described DMN (Yan and Zang, 2010). The resting-state activity of the PCC/precuneus seed showed positive correlations with the activity of areas including the bilateral inferior parietal and medial prefrontal cortices, and negative correlations with the activity of areas including the bilateral insula and dorsolateral prefrontal cortex (Supplementary Table S1).
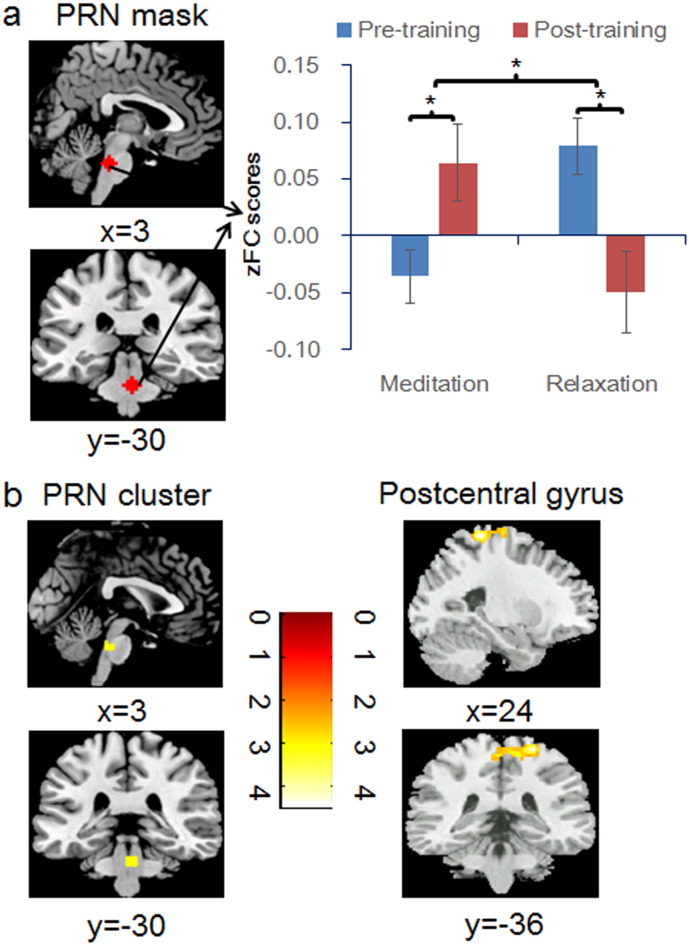
We then tested for the training group, session, and the critical group × session interactive effects on the PCC/precuneus rs-FC patterns. First, ROI analyses on extracted zFC values from the PRN and DRN revealed that the PRN rs-FC was not significantly influenced by either the main effect of group (F (1, 35) = 0.317, p = 0.577) or the main effect of session (F (1, 35) = 3.037, p = 0.09), but was significantly affected by the interaction of group and session (F (1, 35) = 13.933, p = 0.001) (Fig. 2a). Post-hoc tests showed that after training, the PCC/precuneus-PRN rs-FC became significantly more positive for the meditation group (t (20) = 2.53, p < 0.05) but less positive for the relaxation group (t (18) = − 3.00, p < 0.05) (Fig. 2a). An additional control ANOVA analysis confirmed that the effect of group on the changes in the PCC/precuneus-PRN rs-FC following training remained significant after controlling for pre-training differences between the groups in PCC/precuneus-PRN rs-FC (F (1, 37) = 5.092, p < 0.05). On the other hand, extracted zFC values from the predefined DRN mask showed no significant response to either the main effects of group or gender, or the group × session effect (ps > 0.09). Mask-based SVC test revealed a significant cluster in the PRN to the group × session effect (Meditationpost-pre–Relaxationpost-pre), which survived SVC FWE correction (Table 2, Fig. 2b). No significant clusters within the DRN mask were observed.
Fig. 2.
PCC/precuneus-based functional connectivity patterns. (a) The resting-state functional connectivity (rs-FC) between the PCC/precuneus seed and the predefined 6-mm spherical PRN mask centered at (3, − 30, − 30) was significantly influenced by the group × session effect (b) PRN-mask-based SVC test revealed a region of the pontine nucleus which survived SVC-level FWE correction (left), and whole-brain analysis revealed a region of the postcentral gyrus which survived whole-brain-level FWE correction (right), to the group × session effect. The PRN mask and significant clusters are overlaid on a standard anatomical template (ch2bet). MNI x and y coordinates are provided below the sagittal and coronal slices. Following training, the meditation group showed an upregulation of PCC/precuneus-PRN rs-FC in the positive direction. The opposite pattern was observed for the relaxation group after training. * indicates statistically significant difference at p < 0.05. Error bars represent ± 1 standard error of the mean. Colour bar denotes t-statistics. zFC scores: r-to-z transformed functional connectivity coefficiency values (Yan and Zang, 2010).
Table 2.
PCC/precuneus seed-based ROI and whole-brain functional connectivity analysis.
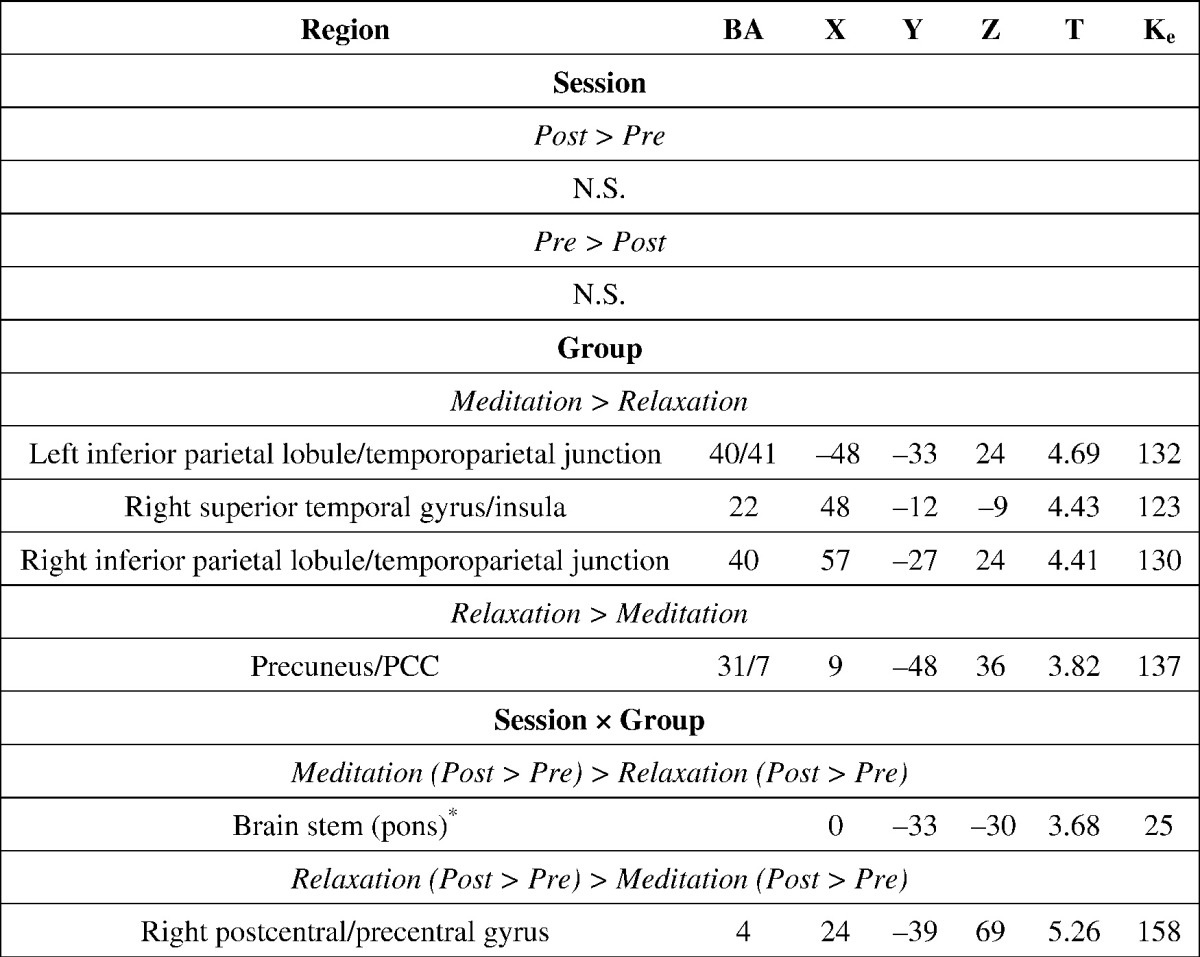
The Montreal Neurological Institute (MNI) coordinates and the associated t-statistics are displayed for the effects of session (pre- vs. post-training), group (meditation vs. relaxation), and session × group on the resting-state functional connectivity based on the posterior cingulate cortex seed region (6-mm sphere centered on − 5, − 49, 40), while controlling for the demographic factors of age, sex and years of education. Analyses were carried out both based on the predefined ROI mask in the PRN in the form of SVC test, and at a whole-brain level to explore other regions whose rs-FC may be significantly influenced by the main or interactive effect. The activation threshold was set at p < 0.005 at the peak level and p < 0.05 at the cluster level, family-wise-error (FWE) corrected. BA: Brodmann Area. Ke: cluster size. N.S.: no significant clusters. Pre/Post: Pre-training/Post-training * this pontine cluster survived both whole-brain FWE correction (locus of maxima = 6, − 33, − 30, t = 4.31, cluster size = 113) and SVC correction based on pre-defined PRN spherical mask centered at (3, − 30, − 30). The coordinates, peak T value and cluster size shown for the pontine cluster are for the significant cluster revealed by the SVC test.
At the whole-brain level, the main effect of session generated no significant clusters that survived FWE correction. When compared with the relaxation group, the meditation group showed more positive rs-FC between the PCC/precuneus seed and the right superior temporal gyrus extending to the insula and the bilateral inferior parietal lobule. The reverse contrast revealed a significant cluster at the PCC/precuneus (Table 2). The interactive effect of group and session revealed a cluster at the right postcentral gyrus, which showed rs-FC with the PCC/precuneus that became more positive after training than before training for the relaxation group compared to the meditation group (Table 2, Fig. 2b). Post-hoc tests indicated that the rs-FC with the PCC/precuneus for the right postcentral gyrus became significantly more negative following meditation training (t (20) = − 4.22, p < 0.05) but significantly more positive following relaxation training (t (18) = 4.56, p < 0.05).
We also ran the rs-FC analyses without regressing out global brain signals (see Materials and Methods–Imaging Data Acquisition and rs-FC analysis). The results of those analyses are included in Supplementary Materials (‘PCC-based resting-state functional connectivity analysis without global signal regression’). We successfully replicated the group × session effect on the pontine and postcentral gyrus rs-FC without regressing out the global signals.
3.3. Effective Connectivity
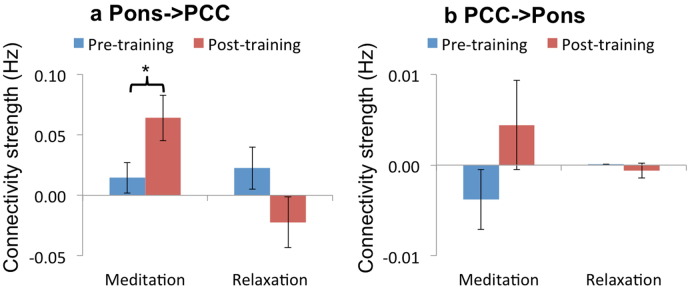
Spectral DCM was implemented to assess the causal relationships between the time course for the PCC/precuneus seed and those for the pons and postcentral gyrus, whose rs-FC with the PCC/precuneus showed significant group × session effects. Such analysis was performed for each individual before and after training. Although our data space contained the time course for the postcentral gyrus, we focused only on the effective connectivity between the PCC/precuneus and the pons, which according to our hypothesis would be directed from the latter to the former structure following meditation training. The pons seed region was centered at (0, − 33, − 30), which corresponded to the locus of maxima of the significant cluster to the group × session effect that survived SVC test with predefined PRN mask (see Materials and Methods).
To examine the group × session effect on the pons-to-PCC/precuneus connectivity, the connectivity strengths estimated from a BMA analysis were subjected to a 2 (pre vs. post) × 2 (meditation vs. relaxation) ANOVA. There was a significant group by session interactive effect (F (1, 38) = 7.02, p < 0.05) (Fig. 3a). Post-hoc analyses showed that the meditation group exhibited a significant increase in positive effective connectivity following training (t (20) = 2.25, p < 0.05), which effect was negative and non-significant for the relaxation group (t (18) = − 1.58, p = 0.13) (Fig. 3a). One-sample t tests indicated a significantly positive connectivity strength in the meditation group following training (t (20) = 3.39, p < 0.05) but not before training (t (20) = 1.15, p = 0.26). The relaxation group showed no significant pons-to-PCC/precuneus connectivity either pre-training (t (18) = 1.29, p = 0.21) or post-training (t (18) = − 1.06, p = 0.3).
Fig. 3.
BMA analysis on connectivity parameters (in Hz). ANOVA analyses on (a) the strength of connectivity from the pons (centered at 0, − 33, − 30) to the PCC/precuneus showed a significant group × session effect. The meditation group, but not the relaxation group, showed a significant increase in positive connectivity strength following training (b) The connectivity strength from the PCC/precuneus to the pons showed no group × session effect and no significant change following training in either the meditation or relaxation group. Error bars denote ± 1 standard error of the mean. * indicates a statistically significant difference at p < 0.05.
Similar control analyses were conducted for the PCC/precuneus-to-pons connectivity. No group × session interactive effect was found (F (1, 38) = 0.49, p = 0.49) (Fig. 3b). The connectivity strengths for the meditation and relaxation groups before and after training were all non-significant (ps > 0.5).
When the spDCM analyses were performed using averaged pontine signals extracted from the entire predefined PRN mask centered at (3, − 30, − 30), we obtained identical result patterns as above. Detailed results of these analyses are included in Supplementary Materials (‘PRN mask-based spDCM analyses’).
We also performed the spDCM analyses using signals from seed regions centering on the locus of maxima as revealed by the rs-FC analyses without global signal regression (see Supplementary Fig. S2 for the locations of these seed regions). The results of these analyses are consistent with the patterns described above (Supplementary Materials ‘spDCM analyses without global signal regression’).
3.4. Linking Behavioral and rs-FC Effects
In this part of the analysis, we focused on the valence and arousal ratings for the positive and negative pictures, which showed greater group × session effects than the neutral pictures (see above). The mediation and moderation analysis results for the neutral pictures are included in Supplementary Materials (‘Additional behavior-FC association analyses’). The behavior-FC association analyses were performed for the predefined PRN mask, which was our primary a priori ROI and was revealed by our a priori contrast of interest (i.e., Meditationpost-pre–Relaxationpost-pre). Such approach reduced the total number of tests conducted and the associated type I error. We also performed the association analyses in the predefined DRN mask for exploratory purpose.
We first examined whether changes in PCC/precuneus-PRN rs-FC following training mediated the effect of group on changes in the EPT affective ratings following training. We did not observe significant mediating effect of PCC/precuneus-PRN rs-FC (i.e., non-significant indirect effect, ps > 0.1) in any of the 4 mediation models predicting ValPospost-pre, AroPospost-pre, ValNegpost-pre and AroNegpost-pre, respectively.
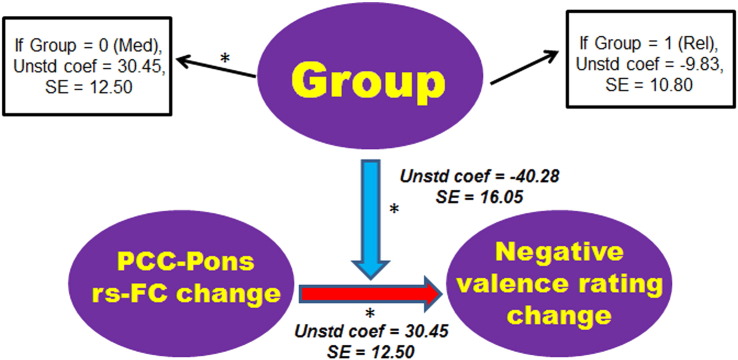
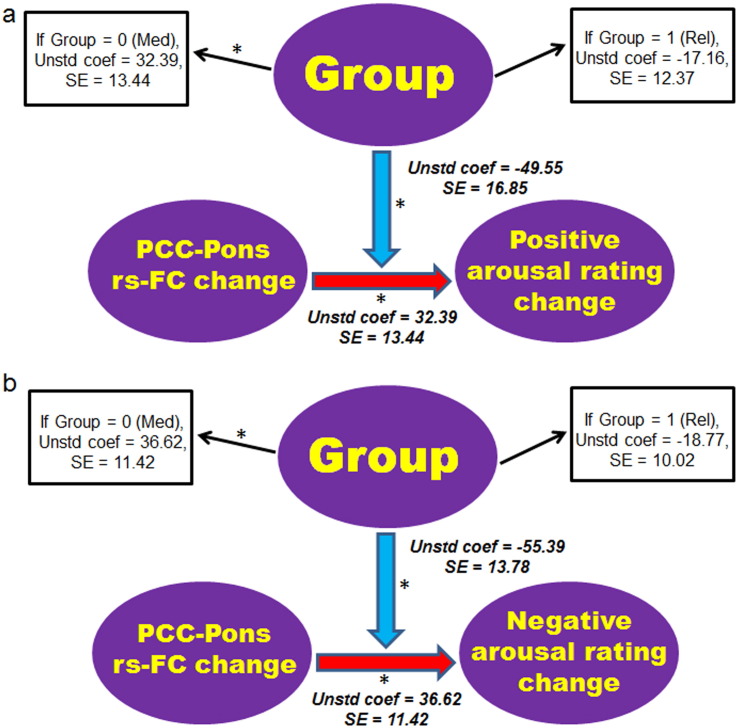
We then examined whether group moderated the association between changes in PCC/precuneus-PRN rs-FC and in EPT ratings. We found that group had a significant moderating influence over the association between changes in PCC/precuneus-PRN rs-FC and in valence ratings of negative pictures (t (31) = − 2.51, p < 0.05). Specifically, participants who showed larger positive increases in PCC/precuneus-PRN rs-FC following training also showed greater increases in positive valence ratings for the negative pictures after training in the meditation group (t (14) = 2.44, p < 0.05) but not in the relaxation group (t (12) = − 0.91, p > 0.1) (Fig. 4). No significant moderating influence of group was found for the association between changes in PCC/precuneus-pons rs-FC and in valence ratings of positive pictures (t (31) = 1.71, p = 0.1).
Fig. 4.
The moderation analysis for EPT valence ratings. The moderating effect of group (meditation vs. relaxation) is depicted on the association of changes in the rs-FC between the PCC/precuneus and the predefined PRN mask, and in participants' valence ratings of the negative pictures following training. The model controlled for the effects of the pre-training PCC/precuneus-PRN rs-FC, training-related changes in arousal ratings of the negative pictures, as well as age, sex and years of education. Both unstandardized regression coefficients and standard errors are shown for the main effect and the moderating effect. * indicates effects that are significant at p < 0.05. Red and blue arrows denote positive and negative effects, respectively. Please note that the direction of an arrow denotes the hypothetical rather than established direction of an effect. Med: Meditation group, PCC: posterior cingulate cortex, Rel: Relaxation group, Rs-FC: resting-state functional connectivity, SE: standard error, Unstd coef: unstandardized coefficients.
Group also had a significant moderating influence over the association between changes in PCC/precuneus-pons rs-FC and in arousal ratings of both negative (t (31) = − 4.02, p < 0.05) and positive (t (31) = − 2.94, p < 0.05) pictures. Specifically, participants who showed more positive increase in PCC/precuneus-pons rs-FC tended to show less reduction for arousal ratings of negative (t (14) = 3.21, p < 0.05) and positive (t (14) = 2.41, p < 0.05) pictures following training in the meditation group, but not in the relaxation group (ps > 0.05) (Fig. 5a and 5b).
Fig. 5.
The moderation analysis for EPT arousal ratings. The moderating effect of group (meditation vs. relaxation) is depicted on the association of changes in the rs-FC between the PCC/precuneus and the predefined PRN mask, and in participants' arousal ratings on the (a) positive- and (b) negative-valenced pictures following training. The models additionally controlled for the effects of the pre-training PCC/precuneus-PRN rs-FC, training-related changes in valence ratings of positive (a) and negative (b) pictures, as well as age, sex and years of education. Both unstandardized regression coefficients and the associated standard errors are shown for the main effect and the moderating effect. * indicates effects that are significant at p < 0.05. Red and blue arrows denote positive and negative effects, respectively. Please note that the direction of an arrow denotes the hypothetical rather than established direction of an effect. Med: Meditation group, PCC: posterior cingulate cortex, Rel: Relaxation group, Rs-FC: resting-state functional connectivity, SE: standard error, Unstd coef: unstandardized coefficients.
No significant results were observed for either mediation or moderation analyses on average rs-FC values extracted from the predefined DRN mask (ps > 0.1).
When the moderation analyses were rerun without regressing out the global signals, we were largely able to replicate the pattern of results as described above, although global signal regressing seemed to have had some effects on the absolute strength of the moderation analysis results (Supplementary Materials ‘Moderation analyses without global signal regression’).
4. Discussion
This study was the first to specifically investigate the effect of short-term meditation training on affective processing and the relevant neural networks in elderly individuals. We employed a longitudinal design and an active control group suitable for establishing the causal and specific effects of meditation training on affective processing and/or regulation. Our findings indicate that following 8-week meditation training, participants' affective ratings of positive and negative stimuli converged towards the middle and became more similar to the ratings of neutral stimuli compared with the ratings before training. Arousal ratings of the affective stimuli were also reduced following meditation training. However, none of those changes were evident in the relaxation group. Additionally, compared with relaxation training, meditation training led to more positive rs-FC between the PCC/precuneus and the pontine brain stem region but more negative rs-FC with the postcentral gyrus. Moreover, the positive PCC/precuneus-pons connectivity following meditation training was largely directed from the pons to the PCC/precuneus. Finally, the type of training participants received moderated the association between the changes in PCC/precuneus-pons rs-FC and in affective ratings, such that the rs-FC and rating changes were closely associated in the meditation group but not in the relaxation group. Such results have never been reported before, and they reveal novel and important information on the characteristics of meditation training in altering the ways in which individuals engage in affective regulatory processes.
One key component of meditation is the open, non-judgmental attitude towards internal and external experiences, which, when coupled with a self-detached evaluative approach, can ultimately lead to stimuli and events being perceived as neither ‘good’ nor ‘bad’, but rather as even entities devoid of subjective, dual valence meanings (Ruby and Decety, 2004, Josipovic, 2014, Desbordes et al., 2015). Moreover, meditation training emphasizes maintaining attention to the present stimuli, which reduces mind-wandering and prevents ruminations about negative thoughts and stimuli that are not directly present (Brewer et al., 2011, Chiesa et al., 2013). Accordingly, accumulating evidence confirms the effect of meditation training on reducing negative processing, negative affect and affective reactivity/arousal to negative stimuli (Jain et al., 2007, Tang et al., 2007, Taylor et al., 2011, Gard et al., 2012, Menezes et al., 2013, Lutz et al., 2016). However, existing evidence on the effect of meditation training on positive affective processing is mixed and cross-sectional only (Ortner et al., 2007, Taylor et al., 2011, Lee et al., 2012, Ho et al., 2015, Lutz et al., 2016). Our current findings provide convincing evidence that 8-week meditation training led to more and less positive ratings on negative and positive stimuli, respectively, as well as reduced arousal ratings for both positive and negative stimuli. Such results, obtained in an elderly sample, are consistent with the findings of recent cross-sectional studies including participants with more variable ages (Ho et al., 2015, Lutz et al., 2016), and strongly suggest that an important aspect of meditation training influence on affective processing is to approach an even-minded mental state towards all stimuli, regardless of their valence (Josipovic, 2014, Desbordes et al., 2015). This has the consequence of reduced affective reactivity and improved emotional stability (Lutz et al., 2008, Chambers et al., 2009, Goldin and Gross, 2010). While further replications in other age groups are needed, our current findings in elderly participants offer illuminating insights on the relationships among meditation training, converging affective processing of positive and negative stimuli, and improved affect state and/or regulation. Given existing evidence that elderly individuals exhibit increases in positive affect, decreases in negative affect, better emotional regulation and greater positive processing bias (Carstensen et al., 2000, Mather and Carstensen, 2003, Shao and Lee, 2014), the precise mechanism by which meditation training might both reduce positive affective processing, as evidenced in our current findings, and promote positive affect state and psychological well-being, as suggested by previous research (Jain et al., 2007, Ortner et al., 2007), warrants further exploration.
Emerging evidence indicates that resting-state (Brewer et al., 2011, Taylor et al., 2013, Hasenkamp and Barsalou, 2012), meditative state (Brewer et al., 2011, Garrison et al., 2014) and affective task-related (Lutz et al., 2016) functional connectivity patterns between the PCC/precuneus and the dorsolateral and medial prefrontal regions are altered following long-term meditation practice, which are thought to reflect greater levels of self-related affective regulation and self-awareness (Chiesa et al., 2013, Tang et al., 2015). However, no longitudinal study has examined whether short-term meditation training may be sufficient to alter the rs-FC of the PCC/precuneus. Moreover, the functional interplay between PCC/precuneus and subcortical affective neural circuitries has been largely unexplored. Our findings indicate that the rs-FC between the PCC/precuneus and the pontine region of the brain stem became more positive and was largely directed from the latter to the former following 8-week meditation training, whereas the rs-FC between the PCC/precuneus and the somatosensory cortices became more negative. Previous research indicates that the pontine region is critically involved in affective regulatory functions (Vollmayr et al., 2000, Cannon et al., 2006, Cannon et al., 2007), and its rs-FC with striatal and midbrain regions predicted individuals' positive and negative affect states (Lee et al., 2015). Thus, the increased positive connectivity from the pons to the PCC/precuneus following meditation training may indicate an enhanced self-referential affective regulatory system, possibly following a shift from a narrative, self-evaluative approach to a non-judgmental, self-aware approach (Hasenkamp and Barsalou, 2012). Fundamentally, such a shift may result from the central component of meditation in in promoting open observations of thoughts, feelings and events without judging their values in relation to oneself (Ives-Deliperi et al., 2011, Chiesa et al., 2013), which, when combined with the attentional focus on the present experience rather than recursive processing of distant events, changes the individual's overall affective processing and regulatory patterns. Given that the pons encompasses the PRN from which the serotonergic pathways originate and regulates affective functions of widespread downstream networks, increased positive connectivity from the pons to the PCC/precuneus may be central to the overarching effect of meditation training on the neural affective network, underlying the increase in positive self-representation, self-esteem and self-acceptance (Haimerl and Valentine, 2001). On the other hand, we obtained no evidence that the rs-FC between the PCC/precuneus and the DRN was affected by meditation versus relaxation training, which might be due to two possible reasons. First, the DRN and PRN have been found to show different brain projections (Cooper et al., 2003), with some evidence indicating that the DRN may have a primarily prefrontal projection in the cortex (McQuade and Sharp, 1995, Hajós et al., 2003). Second, animal evidence indicates that acute stress altered serotonergic functions in the PRN but not in the DRN (Vollmayr et al., 2000). As meditation training tends to focus on dealing with immediately present internal or external stimuli, improved affective regulatory function in response to acute stressors may have led to enhanced PRN, but not DRN, connectivities with the PCC/precuneus. The more negative rs-FC between the PCC/precuneus and the somatosensory cortices may represent reductions in self-referential and self-focused sensory attention and processing in the meditation group (Lutz et al., 2016), indicating a shift in focus from relating sensory events to oneself to simply observing the events from an outsider's perspective (Ruby and Decety, 2004).
The relaxation group showed the opposite patterns of PCC/precuneus connectivity changes to those of the meditation group; that is, more negative connectivity from the pons but more positive rs-FC with the somatosensory cortices following training. Evidence from previous studies suggests that relaxation training versus no intervention increases and decreases positive and negative affect states, respectively, although the strength of the effect may be smaller than that for meditation training (Ortner et al., 2007, Jain et al., 2007). While certain effects such as enhanced body awareness may be common to both meditation and relaxation training, the former embodies many key elements that are not present in the latter, such as non-judgmental observation, attention to the present, and a self-detached perspective (Jain et al., 2007). Consistent with this, previous studies directly comparing meditation with relaxation training showed that the former led to distinct influences on neural activity compared with the latter (Tang et al., 2009). Our findings provide further evidence that meditation and relaxation training are associated with distinct neural affective mechanisms. During relaxation training, body sensations, states and images may be linked more closely to the ‘self’ concept, such that self-referential affective processing is determined principally by one's voluntary efforts in regulating those bodily states. Such voluntary regulatory processes may involve the functional influence of prefrontal networks on exteroceptive or interoceptive networks (Kilpatrick et al., 2011) rather than low-level affective regions such as the pons. Future research employing more comprehensive rs-FC analysis methods, such as independent component analysis (ICA), might shed further light on this question.
The change in PCC/precuneus-pons rs-FC did not significantly mediate the change in affective ratings following meditation training versus relaxation training. Several reasons might explain the lack of a mediating effect. First, the PCC/precuneus-pons rs-FC change alone might not significantly account for the meditation training effect on the affective rating change. The functional connectivity between the PCC/precuneus and other regions, such as the somatosensory cortices, might also be involved. Second, rs-FC networks centering at the PCC/precuneus region may not capture the entire picture of neural network changes following meditation training. Changes in functional connectivity between prefrontal and subcortical networks could also contribute to the meditation training effect on affective processing (Kilpatrick et al., 2011). Third, the rs-FC pattern alone may not significantly explain the training group effect on the affective rating changes, at least in the total participant sample. However, the results of the moderation analyses showed that changes in PCC/precuneus-pons rs-FC significantly predicted changes in valence ratings on negative pictures, and arousal ratings on both positive and negative pictures, following meditation, but not relaxation, training. Specifically, participants who showed greater positive increases of PCC/precuneus-pons rs-FC following meditation training also exhibited greater increases in positive valence ratings of negative pictures. Such result is consistent with existing evidence implicating the functional interplay between the pontine region and cortical and subcortical networks in regulating affective processing and state (Cannon et al., 2006, Cannon et al., 2007, Lee et al., 2015), as well as research suggesting that the pontine region is particularly involved in controlling negative affect and distress (Vollmayr et al., 2000). Our results thus reveal a key role of the PCC/precuneus-pons functional interplay in underlying changes in negative affective regulation following meditation training. On the other hand, participants showing more positive increases in PCC/precuneus-pons rs-FC following meditation training exhibited less reduction in arousal ratings of the affective pictures. Given previous evidence implicating the pontine nuclei in supporting interoceptive and visceral perceptions (Critchley, 2005), a more positive increase in PCC/precuneus-pons rs-FC following meditation training might underlie an enhanced awareness of one's own physiological state (Fox et al., 2012, Chiesa et al., 2013), leading to higher (or less decreased) levels of self-reported arousal. Importantly, following meditation training, higher levels of perceived arousal as a result of greater self-awareness may not be accompanied by correspondingly high subjective valance processing, but could simply be objective observations and analysis of bodily states (Chiesa et al., 2013). Indeed, such increased awareness of one's own bodily states can further help with affective regulatory processes (Chiesa et al., 2013).
In contrast, we observed no evidence that changes in PCC/precuneus-pons rs-FC predicted changes in affective ratings among the relaxation group. Two reasons might explain why we specifically found the association in the meditation group. First, previous evidence that meditation training influences PCC/precuneus functional connectivity patterns consistently across resting, meditative and affective processing states indicates that meditation training may alter the neural default network mode for self-referential affective processing, with the new meditative mode employed regardless of the state that the individual is in (Manna et al., 2010, Brewer et al., 2011, Jang et al., 2011, Taylor et al., 2013, Hasenkamp and Barsalou, 2012, Garrison et al., 2014, Lutz et al., 2016). Based on our findings, this new state-free affective regulation system is at least partly underlain by more positive influences from the pons to the PCC/precuneus, which was not developed during relaxation training. This explains why associations between the PCC/precuneus-pons rs-FC and affective rating changes were only found in the meditation group. Second, following meditation training, the affective system might become more bottom-up and regulated via low-level subcortical networks, such as the pontine region (Chiesa et al., 2013), the transition of which might be particularly marked among elderly individuals with reduced dorsal prefrontal top-down regulatory functioning (Shao and Lee, 2014). Thus, it is possible that the PCC/precuneus-pons rs-FC change determined the change in affective ratings to a greater extent in the meditation than in the relaxation group.
Collectively, our findings further characterize the meditation training effect on affective processing, reveal important roles of the pons-to-PCC/precuneus connectivity in regulating negative affective processing and affective reactivity, and highlight the effect of meditation training in forming a new default mode for affective regulation patterns that are employed across resting and affective processing states. Clinically, our findings have important implications for improving the affective regulatory functions of elderly individuals. While meditation training has shown efficacy in treating depression (Teasdale et al., 2000) and anxiety (Goldin et al., 2009) in the general population, no well-controlled longitudinal study has examined the effectiveness of meditation in the elderly. Our findings support the use of meditation training for ameliorating affective symptoms among the aging population, as well as using rs-FC patterns as a biomarker for examining the therapeutic effects (Simon and Engström, 2015). Furthermore, our finding that a bottom-up system directed from the pons to the PCC/precuneus is critically involved in negative affective regulation following meditation training has implications in helping patients with affective disorders who have difficulties in exerting top-down affective regulatory control (Liotti et al., 2002). Our current study has the following limitations. First, despite that we matched the meditation and relaxation groups on various demographic and psychometric characteristics in an a priori manner, the two groups still showed some pre-training differences in PCC/precuneus rs-FC with the pons and the postcentral gyrus. Although we statistically adjusted for those differences in the data analyses, it remains possible that the two groups were different on some important aspects prior to training which contributed to the observed results. Future studies may employ more comprehensive cognitive and affective assessments to achieve better matching of the training groups. Second, the findings need to be replicated in other age groups. Third, instead of a seed-based approach, other methods for analyzing rs-FC patterns, such as ICA, can provide more comprehensive evidence on network connectivity changes following meditation training. Fourth, imaging modalities that have higher temporal resolution, such as electroencephalogram (EEG) and magnetoencephalography (MEG), can help delineate the earlier bottom-up sensory and affective processes from subsequent top-down regulatory processes (Reva et al., 2014, Ho et al., 2015). Finally, future studies incorporating each of the resting, meditative and affective processing states can provide more definite evidence on the effect of meditation training in forming a new state-free default affective system.
5. Conclusion
In the first longitudinal study comparing the effects of an 8-week meditation training versus relaxation training on affective processing and resting-state connectivity changes among elderly individuals, we found that meditation, but not relaxation, training ‘neutralized’ individuals' perceived valence and decreased the general perceived arousal of affective stimuli. On the neural level, the connectivity from the pons to the PCC/precuneus became more positive following meditation versus relaxation training. The PCC/precuneus-pons connectivity further played important roles in regulating negative affective processing and in facilitating increased physiological self-awareness after meditation training. Our findings have important clinical implications for using meditation training to treat affective symptoms among the elderly, and using rs-FC patterns to evaluate its efficacy.
Author Contributions
TMCLee designed the research; TMCLee supervised the data collection; RShao, KKeuper and XGeng searched the literature; RShao, KKeuper, and XGeng performed the research and analyzed the data; RShao, KKeuper, and XGeng interpreted the data; RShao, KKeuper, XGeng and TMCLee prepared the manuscript; RShao, KKeuper, XGeng and TMCLee approved the final version of the manuscript.
Competing Financial Interests
The authors declare no competing financial interests.
Acknowledgement
This study was supported by Research Grant Council General Research Fund (HKU-17613815) to Tatia M.C. Lee. The funding agency has no roles in study design, data collection, data analysis, finding interpretation, manuscript preparation or the decision of submission. None of the authors had been paid to prepare this manuscript by a pharmaceutical company or other agency.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2016.06.018.
Contributor Information
Xiujuan Geng, Email: gengx@hku.hk.
Tatia M.C. Lee, Email: tmclee@hku.hk.
Appendix A. Supplementary data
Supplementary material.
References
- Acevedo B.P., Aron A., Fisher H.E., Brown L.L. Neural correlates of long-term intense romantic love. Soc. Cogn. Affect. Neurosci. 2011;nsq092 doi: 10.1093/scan/nsq092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Admon R., Pizzagalli D.A. Corticostriatal pathways contribute to the natural time course of positive mood. Nat. Commun. 2015;6:10065. doi: 10.1038/ncomms10065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amat J. Microinjection of urocortin 2 into the dorsal raphe nucleus activates serotonergic neurons and increases extracellular serotonin in the basolateral amygdala. Neuroscience. 2004;129(3):509–519. doi: 10.1016/j.neuroscience.2004.07.052. [DOI] [PubMed] [Google Scholar]
- Berman M.G. Depression, rumination and the default network. Soc. Cogn. Affect. Neurosci. 2010;6(5):548–555. doi: 10.1093/scan/nsq080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer J.A. Meditation experience is associated with differences in default mode network activity and connectivity. Proc. Natl. Acad. Sci. USA. 2011;108(50):20254–20259. doi: 10.1073/pnas.1112029108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown L., Sherbenou R.J., Johnsen S.K. 1997. Manual of Test of Nonverbal Intelligence: Third Edition (Austin, TX: PRO-ED, 1997) [Google Scholar]
- Cannon D.M. Serotonin transporter binding in bipolar disorder assessed using [11 C] DASB and positron emission tomography. Biol. Psychiatry. 2006;60(3):207–217. doi: 10.1016/j.biopsych.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Cannon D.M. Elevated serotonin transporter binding in major depressive disorder assessed using positron emission tomography and [11 C] DASB; comparison with bipolar disorder. Biol. Psychiatry. 2007;62(8):870–877. doi: 10.1016/j.biopsych.2007.03.016. [DOI] [PubMed] [Google Scholar]
- Carstensen L.L., Pasupathi M., Mayr U., Nesselroade J.R. Emotional experience in everyday life across the adult life span. J. Pers. Soc. Psychol. 2000;79(4):644–655. [PubMed] [Google Scholar]
- Cavanna A.E., Trimble M.R. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129(3):564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Chambers R., Gullone E., Allen N.B. Mindful emotion regulation: an integrative review. Clin. Psychol. Rev. 2009;29(6):560–572. doi: 10.1016/j.cpr.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Chiesa A., Serretti A. Mindfulness based cognitive therapy for psychiatric disorders: a systematic review and meta-analysis. Psychiatry Res. 2011;187(3):441–453. doi: 10.1016/j.psychres.2010.08.011. [DOI] [PubMed] [Google Scholar]
- Chiesa A., Serretti A., Jakobsen J.C. Mindfulness: top–down or bottom–up emotion regulation strategy? Clin. Psychol. Rev. 2013;33(1):82–96. doi: 10.1016/j.cpr.2012.10.006. [DOI] [PubMed] [Google Scholar]
- Cools R., Roberts A.C., Robbins T.W. Serotoninergic regulation of emotional and behavioural control processes. Trends Cogn. Sci. 2008;12(1):31–40. doi: 10.1016/j.tics.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Cooper J. Oxford University Press; 2003. The Biochemical Basis of Neuropharmacology. [Google Scholar]
- Critchley H.D. Neural mechanisms of autonomic, affective, and cognitive integration. J. Comp. Neurol. 2005;493(1):154–166. doi: 10.1002/cne.20749. [DOI] [PubMed] [Google Scholar]
- Crone J.S. Impaired consciousness is linked to changes in effective connectivity of the posterior cingulate cortex within the default mode network. NeuroImage. 2015;110:101–109. doi: 10.1016/j.neuroimage.2015.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deakin J.W., Graeff F.G. 5-HT and mechanisms of defence. J. Psychopharmacol. 1991;5(4):305–315. doi: 10.1177/026988119100500414. [DOI] [PubMed] [Google Scholar]
- Desbordes G. Effects of mindful-attention and compassion meditation training on amygdala response to emotional stimuli in an ordinary, non-meditative state. Front. Hum. Neurosci. 2012;6 doi: 10.3389/fnhum.2012.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desbordes G. Moving beyond mindfulness: defining equanimity as an outcome measure in meditation and contemplative research. Mindfulness. 2015;6(2):356–372. doi: 10.1007/s12671-013-0269-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dor-Ziderman Y., Berkovich-Ohana A., Glicksohn J., Goldstein A. Mindfulness-induced selflessness: a MEG neurophenomenological study. Front. Hum. Neurosci. 2013;7:582. doi: 10.3389/fnhum.2013.00582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emavardhana T., Tori C.D. Changes in self-concept, ego defense mechanisms, and religiosity following seven-day Vipassana meditation retreats. J. Sci. Study Relig. 1997;36:194–206. [Google Scholar]
- Fakhoury M. Revisiting the serotonin hypothesis: implications for major depressive disorder. Mol. Neurobiol. 2016;53(5):2778–2786. doi: 10.1007/s12035-015-9152-z. [DOI] [PubMed] [Google Scholar]
- Farb N.A. Minding one's emotions: mindfulness training alters the neural expression of sadness. Emotion. 2010;10(1):25–33. doi: 10.1037/a0017151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A.G., Jocham G., Ullsperger M. Dual serotonergic signals: a key to understanding paradoxical effects? Trends Cogn. Sci. 2015;19(1):21–26. doi: 10.1016/j.tics.2014.11.004. [DOI] [PubMed] [Google Scholar]
- Fox M.D. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. USA. 2005;102(27):9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox K.C.R. Meditation experience predicts introspective accuracy. PLoS One. 2012;7:e45370. doi: 10.1371/journal.pone.0045370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K.J., Kahan J., Biswal B., Razi A. A DCM for resting state fMRI. NeuroImage. 2014;94:396–407. doi: 10.1016/j.neuroimage.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard T. Pain attenuation through mindfulness is associated with decreased cognitive control and increased sensory processing in the brain. Cereb. Cortex. 2012;22(11):2692–2702. doi: 10.1093/cercor/bhr352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard T., Hölzel B.K., Lazar S.W. The potential effects of meditation on age-related cognitive decline: a systematic review. Ann. NY Acad. Sci. 2014;1307(1):89–103. doi: 10.1111/nyas.12348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison K.A., Scheinost D., Constable R.T., Brewer J.A. BOLD signal and functional connectivity associated with loving kindness meditation. Brain Behav. 2014;4(3):337–347. doi: 10.1002/brb3.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin P.R., Gross J.J. Effects of mindfulness-based stress reduction (MBSR) on emotion regulation in social anxiety disorder. Emotion. 2010;10(1):83–91. doi: 10.1037/a0018441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin P., Ramel W., Gross J. Mindfulness meditation training and self-referential processing in social anxiety disorder: behavioral and neural effects. J. Cogn. Psychother. 2009;23(3):242. doi: 10.1891/0889-8391.23.3.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graeff F.G. Role of 5-HT in stress, anxiety and depression. Pharmacol. Biochem. Behav. 1996;54:129–141. doi: 10.1016/0091-3057(95)02135-3. [DOI] [PubMed] [Google Scholar]
- Hahn A. Differential modulation of the default mode network via serotonin-1A receptors. Proc. Natl. Acad. Sci. USA. 2012;109(7):2619–2624. doi: 10.1073/pnas.1117104109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haimerl C.J., Valentine E.R. The effect of contemplative practice on intrapersonal, interpersonal, and transpersonal dimensions of the self-concept. J. Transpers. Psychol. 2001;33:37–52. [Google Scholar]
- Hajós M., Gartside S.E., Varga V., Sharp T. In vivo inhibition of neuronal activity in the rat ventromedial prefrontal cortex by midbrain-raphe nuclei: role of 5-HT 1A receptors. Neuropharmacology. 2003;45(1):72–81. doi: 10.1016/s0028-3908(03)00139-4. [DOI] [PubMed] [Google Scholar]
- Hasenkamp W., Barsalou L.W. Effects of meditation experience on functional connectivity of distributed brain networks. Front. Hum. Neurosci. 2012;6 doi: 10.3389/fnhum.2012.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes A.F. Guilford Press; 2013. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-based Approach. [Google Scholar]
- Ho N.S., Sun D., Ting K.H., Chan C.C., Lee T. Mindfulness trait predicts neurophysiological reactivity associated with negativity bias: an ERP study. Evid. Based. Complement. Alternat. Med. 2015:212368. doi: 10.1155/2015/212368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölzel B.K. Mindfulness practice leads to increases in regional brain gray matter density. Psychiatry Res. Neuroimaging. 2011;191(1):36–43. doi: 10.1016/j.pscychresns.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ives-Deliperi V.L., Solms M., Meintjes E.M. The neural substrates of mindfulness: an fMRI investigation. Soc. Neurosci. 2011;6(3):231–242. doi: 10.1080/17470919.2010.513495. [DOI] [PubMed] [Google Scholar]
- Jain S. A randomized controlled trial of mindfulness meditation versus relaxation training: effects on distress, positive states of mind, rumination, and distraction. Ann. Behav. Med. 2007;33(1):11–21. doi: 10.1207/s15324796abm3301_2. [DOI] [PubMed] [Google Scholar]
- Jang J.H. Increased default mode network connectivity associated with meditation. Neurosci. Lett. 2011;487(3):358–362. doi: 10.1016/j.neulet.2010.10.056. [DOI] [PubMed] [Google Scholar]
- Josipovic Z. Neural correlates of nondual awareness in meditation. Ann. N. Y. Acad. Sci. 2014;1307:9–18. doi: 10.1111/nyas.12261. [DOI] [PubMed] [Google Scholar]
- Kilpatrick L.A. Impact of mindfulness-based stress reduction training on intrinsic brain connectivity. NeuroImage. 2011;56(1):290–298. doi: 10.1016/j.neuroimage.2011.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacadie C.M., Fulbright R.K., Rajeevan N., Constable R.T., Papademetris X. More accurate Talairach coordinates for neuroimaging using non-linear registration. NeuroImage. 2008;42(2):717–725. doi: 10.1016/j.neuroimage.2008.04.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang P.J., Bradley M.M., Cuthbert B.N. Technical Report A-8. University of Florida; Gainesvolle, FL: 2008. International affective picture system (IAPS): affective ratings of pictures and instruction manual. [Google Scholar]
- Lee T.M. Distinct neural activity associated with focused-attention meditation and loving-kindness meditation. PLoS One. 2012;7(8):e40054. doi: 10.1371/journal.pone.0040054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T.M. A pontine region is a neural correlate of the human affective processing network. EBioMedicine. 2015;2(11):1799–1805. doi: 10.1016/j.ebiom.2015.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leichnetz G.R. Connections of the medial posterior parietal cortex (area 7 m) in the monkey. Anat. Rec. 2001;263:215–236. doi: 10.1002/ar.1082. [DOI] [PubMed] [Google Scholar]
- Leung C.M., Wing Y.K., Kwong P.K., Shum A.L.K. Validation of the Chinese-Cantonese version of the Hospital Anxiety and Depression Scale and comparison with the Hamilton Rating Scale of depression. Acta Psychiatr. Scand. 1999;100(6):456–461. doi: 10.1111/j.1600-0447.1999.tb10897.x. [DOI] [PubMed] [Google Scholar]
- Li B. A treatment-resistant default mode subnetwork in major depression. Biol. Psychiatry. 2013;74(1):48–54. doi: 10.1016/j.biopsych.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Liotti M., Mayberg H.S., McGinnis S., Brannan S.L., Jerabek P. Unmasking disease-specific cerebral blood flow abnormalities: mood challenge in patients with remitted unipolar depression. Am. J. Psychiatry. 2002;159:1830–1840. doi: 10.1176/appi.ajp.159.11.1830. [DOI] [PubMed] [Google Scholar]
- Luders E. Exploring age-related brain degeneration in meditation practitioners. Ann. N. Y. Acad. Sci. 2014;1307(1):82–88. doi: 10.1111/nyas.12217. [DOI] [PubMed] [Google Scholar]
- Lutz A., Brefczynski-Lewis J., Johnstone T., Davidson R.J. Regulation of the neural circuitry of emotion by compassion meditation: effects of meditative expertise. PLoS One. 2008;3(3):e1897. doi: 10.1371/journal.pone.0001897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz J. Altered processing of self-related emotional stimuli in mindfulness meditators. NeuroImage. 2016;124:958–967. doi: 10.1016/j.neuroimage.2015.09.057. [DOI] [PubMed] [Google Scholar]
- Mai J.K., Paxinos G., Voss T. 3rd ed.) Elsevier Academic Press; San Diego: 2008. Atlas of the Human Brain. [Google Scholar]
- Manna A. Neural correlates of focused attention and cognitive monitoring in meditation. Brain Res. Bull. 2010;82(1):46–56. doi: 10.1016/j.brainresbull.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Mather M., Carstensen L.L. Aging and attentional biases for emotional faces. Psychol. Sci. 2003;14(5):409–415. doi: 10.1111/1467-9280.01455. [DOI] [PubMed] [Google Scholar]
- McQuade R., Sharp T. Release of cerebral 5-hydroxytryptamine evoked by electrical stimulation of the dorsal and median raphe nuclei: effect of a neurotoxic amphetamine. Neuroscience. 1995;68(4):1079–1088. doi: 10.1016/0306-4522(95)00214-4. [DOI] [PubMed] [Google Scholar]
- Menezes C.B. The improvement of emotion and attention regulation after a 6-week training of focused meditation: a randomized controlled trial. Evid. Based. Complement. Alternat. Med. 2013;984678 doi: 10.1155/2013/984678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy F., Smith K., Cowen P., Robbins T., Sahakian B. The effects of tryptophan depletion on cognitive and affective processing in healthy volunteers. Psychopharmacology. 2002;163(1):42–53. doi: 10.1007/s00213-002-1128-9. [DOI] [PubMed] [Google Scholar]
- Murphy K., Birn R.M., Handwerker D.A., Jones T.B., Bandettini P.A. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? NeuroImage. 2009;44(3):893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G. Self-referential processing in our brain—a meta-analysis of imaging studies on the self. NeuroImage. 2006;31(1):440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Oldfield R.C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Ortner C.N.M., Kilner S.J., Zelazo P.D. Mindfulness meditation and reduced emotional interference on a cognitive task. Motiv. Emot. 2007;31:271–283. [Google Scholar]
- Penny W.D. Comparing families of dynamic causal models. PLoS Comput. Biol. 2010;6:e1000709. doi: 10.1371/journal.pcbi.1000709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle M.E. A default mode of brain function. Proc. Natl. Acad. Sci. USA. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razi A., Kahan J., Rees G., Friston K.J. Construct validation of a DCM for resting state fMRI. NeuroImage. 2015;106:1–14. doi: 10.1016/j.neuroimage.2014.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reva N.V., Pavlov S.V., Loktev K.V., Korenyok V.V., Aftanas L.I. Influence of long-term Sahaja Yoga meditation practice on emotional processing in the brain: an ERP study. Neuroscience. 2014;281:195–201. doi: 10.1016/j.neuroscience.2014.09.053. [DOI] [PubMed] [Google Scholar]
- Robins C.J., Keng S.L., Ekblad A.G., Brantley J.G. Effects of mindfulness-based stress reduction on emotional experience and expression: a randomized controlled trial. J. Clin. Psychol. 2012;68(1):117–131. doi: 10.1002/jclp.20857. [DOI] [PubMed] [Google Scholar]
- Roiser J.P. The effect of acute tryptophan depletion on the neural correlates of emotional processing in healthy volunteers. Neuropsychopharmacology. 2008;33(8):1992–2006. doi: 10.1038/sj.npp.1301581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby P., Decety J. How would you feel versus how do you think she would feel? A neuroimaging study of perspective-taking with social emotions. J. Cogn. Neurosci. 2004;16(6):988–999. doi: 10.1162/0898929041502661. [DOI] [PubMed] [Google Scholar]
- Sedlmeier P. The psychological effects of meditation: a meta-analysis. Psychol. Bull. 2012;138(6):1139–1171. doi: 10.1037/a0028168. [DOI] [PubMed] [Google Scholar]
- Selvaraj S. Presynaptic serotoninergic regulation of emotional processing: a multimodal brain imaging study. Biol. Psychiatry. 2015;78(8):563–571. doi: 10.1016/j.biopsych.2014.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen S., Burmeister M., Ghosh D. Meta-analysis of the association between a serotonin transporter promoter polymorphism (5-HTTLPR) and anxiety-related personality traits. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2004;127(1):85–89. doi: 10.1002/ajmg.b.20158. [DOI] [PubMed] [Google Scholar]
- Shao R., Lee T.M.C. Aging and risk taking: toward an integration of cognitive, emotional, and neurobiological perspectives. Neurosci. Neuroecon. 2014;3:47–62. [Google Scholar]
- Simon R., Engström M. The default mode network as a biomarker for monitoring the therapeutic effects of meditation. Front. Psychol. 2015;6:776. doi: 10.3389/fpsyg.2015.00776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton O. Change in brainstem gray matter concentration following a mindfulness-based intervention is correlated with improvement in psychological well-being. Front. Hum. Neurosci. 2014;8:33. [Google Scholar]
- Son Y.D. Glucose metabolism of the midline nuclei raphe in the brainstem observed by PET–MRI fusion imaging. NeuroImage. 2012;59(2):1094–1097. doi: 10.1016/j.neuroimage.2011.09.036. [DOI] [PubMed] [Google Scholar]
- Tang Y.Y. Short-term meditation training improves attention and self-regulation. Proc. Natl. Acad. Sci. U. S. A. 2007;104:17152–17156. doi: 10.1073/pnas.0707678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y.Y. Central and autonomic nervous system interaction is altered by short-term meditation. Proc. Natl. Acad. Sci. U. S. A. 2009;106:8865–8870. doi: 10.1073/pnas.0904031106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y.Y., Hölzel B.K., Posner M.I. The neuroscience of mindfulness meditation. Nat. Rev. Neurosci. 2015;16(4):213–225. doi: 10.1038/nrn3916. [DOI] [PubMed] [Google Scholar]
- Taylor V.A. Impact of mindfulness on the neural responses to emotional pictures in experienced and beginner meditators. NeuroImage. 2011;57(4):1524–1533. doi: 10.1016/j.neuroimage.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Taylor V.A. Impact of meditation training on the default mode network during a restful state. Soc. Cogn. Affect. Neurosci. 2013;8(1):4–14. doi: 10.1093/scan/nsr087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teasdale J.D. Prevention of relapse/recurrence in major depression by mindfulness-based cognitive therapy. J. Consult. Clin. Psychol. 2000;68(4):615. doi: 10.1037//0022-006x.68.4.615. [DOI] [PubMed] [Google Scholar]
- Vollmayr B., Keck S., Henn F.A., Schloss P. Acute stress decreases serotonin transporter mRNA in the raphe pontis but not in other raphe nuclei of the rat. Neurosci. Lett. 2000;290(2):109–112. doi: 10.1016/s0304-3940(00)01346-x. [DOI] [PubMed] [Google Scholar]
- Wenk-Sormaz H. Meditation can reduce habitual responding. Altern. Ther. Health Med. 2005;11(2):42–58. [PubMed] [Google Scholar]
- Yan C., Zang Y. DPARSF: a MATLAB toolbox for “pipeline” data analysis of resting-state fMRI. Front. Syst. Neurosci. 2010;4(13) doi: 10.3389/fnsys.2010.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidan F., Johnson S.K., Diamond B.J., David Z., Goolkasian P. Mindfulness meditation improves cognition: evidence of brief mental training. Conscious. Cogn. 2010;19(2):597–605. doi: 10.1016/j.concog.2010.03.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.