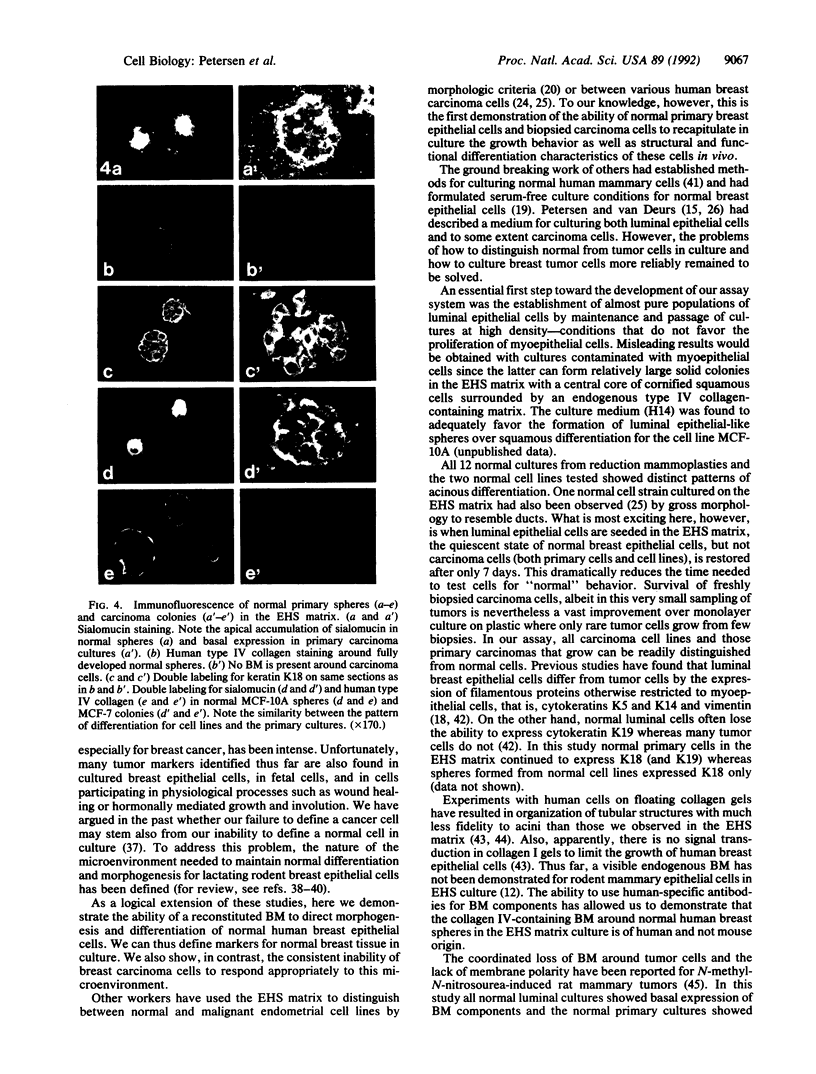
Abstract
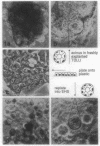
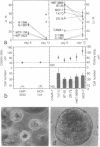
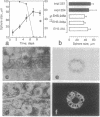
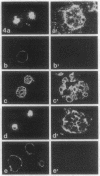
Normal human breast epithelial cells show a high degree of phenotypic plasticity in monolayer culture and express many traits that otherwise characterize tumor cells in vivo. Paradoxically, primary human breast carcinoma cells are difficult to establish in culture: most outgrowths arise from the normal tissue surrounding the tumor. These characteristics have posed major obstacles to the establishment of simple reliable criteria for mammary epithelial transformation in culture. In the present study, we show that a reconstituted basement membrane (BM) can be used to culture all normal human breast epithelial cells and a subset of human breast carcinoma cells. The two cell types can be readily distinguished by virtue of the ability of normal cells to reexpress a structurally and functionally differentiated phenotype within BM. Twelve specimens of normal breast tissue and 2 normal breast epithelial cell lines (total 14 samples) embedded in BM as single cells were able to form multicellular spherical colonies with a final size close to that of true acini in situ. Sections of mature spheres revealed a central lumen surrounded by polarized luminal epithelial cells expressing keratins 18 and 19 and sialomucin at the apical membrane. Significantly, two-thirds of normal spheres deposited a visible endogenous type IV collagen-containing BM even though they were in contact with exogenously provided BM. Growth was arrested completely within the same time period. In contrast, none of 6 carcinoma cell lines or 2 cultures of carcinoma from fresh samples (total 8 samples) responded to BM by growth regulation, lumen formation, correct polarity, or deposition of endogenous BM. These findings may provide the basis of a rapid assay for discriminating normal human breast epithelial cells from their malignant counterparts. Furthermore, we propose that the ability to sense BM appropriately and to form three-dimensional organotypic structures may be the function of a class of "suppressor" genes that are lost as cells become malignant.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Band V., Zajchowski D., Kulesa V., Sager R. Human papilloma virus DNAs immortalize normal human mammary epithelial cells and reduce their growth factor requirements. Proc Natl Acad Sci U S A. 1990 Jan;87(1):463–467. doi: 10.1073/pnas.87.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcellos-Hoff M. H., Aggeler J., Ram T. G., Bissell M. J. Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane. Development. 1989 Feb;105(2):223–235. doi: 10.1242/dev.105.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell M. J. The differentiated state of normal and malignant cells or how to define a "normal" cell in culture. Int Rev Cytol. 1981;70:27–100. doi: 10.1016/s0074-7696(08)61130-4. [DOI] [PubMed] [Google Scholar]
- Blum J. L., Zeigler M. E., Wicha M. S. Regulation of mammary differentiation by the extracellular matrix. Environ Health Perspect. 1989 Mar;80:71–83. doi: 10.1289/ehp.898071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum J. L., Zeigler M. E., Wicha M. S. Regulation of rat mammary gene expression by extracellular matrix components. Exp Cell Res. 1987 Dec;173(2):322–340. doi: 10.1016/0014-4827(87)90274-6. [DOI] [PubMed] [Google Scholar]
- Boyd J. A., Rinehart C. A., Jr, Walton L. A., Siegal G. P., Kaufman D. G. Ultrastructural characterization of two new human endometrial carcinoma cell lines and normal human endometrial epithelial cells cultured on extracellular matrix. In Vitro Cell Dev Biol. 1990 Jul;26(7):701–708. doi: 10.1007/BF02624426. [DOI] [PubMed] [Google Scholar]
- Briand P., Lykkesfeldt A. E. Long-term cultivation of a human breast cancer cell line, MCF-7, in a chemically defined medium. Effect of estradiol. Anticancer Res. 1986 Jan-Feb;6(1):85–90. [PubMed] [Google Scholar]
- Briand P., Petersen O. W., Van Deurs B. A new diploid nontumorigenic human breast epithelial cell line isolated and propagated in chemically defined medium. In Vitro Cell Dev Biol. 1987 Mar;23(3):181–188. doi: 10.1007/BF02623578. [DOI] [PubMed] [Google Scholar]
- Caron de Fromentel C., Nardeux P. C., Soussi T., Lavialle C., Estrade S., Carloni G., Chandrasekaran K., Cassingena R. Epithelial HBL-100 cell line derived from milk of an apparently healthy woman harbours SV40 genetic information. Exp Cell Res. 1985 Sep;160(1):83–94. doi: 10.1016/0014-4827(85)90238-1. [DOI] [PubMed] [Google Scholar]
- Coopman P. J., Bracke M. E., Lissitzky J. C., De Bruyne G. K., Van Roy F. M., Foidart J. M., Mareel M. M. Influence of basement membrane molecules on directional migration of human breast cell lines in vitro. J Cell Sci. 1991 Mar;98(Pt 3):395–401. doi: 10.1242/jcs.98.3.395. [DOI] [PubMed] [Google Scholar]
- Dairkee S. H., Blayney C., Smith H. S., Hackett A. J. Monoclonal antibody that defines human myoepithelium. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7409–7413. doi: 10.1073/pnas.82.21.7409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darcy K. M., Black J. D., Hahm H. A., Ip M. M. Mammary organoids from immature virgin rats undergo ductal and alveolar morphogenesis when grown within a reconstituted basement membrane. Exp Cell Res. 1991 Sep;196(1):49–65. doi: 10.1016/0014-4827(91)90455-4. [DOI] [PubMed] [Google Scholar]
- Dulbecco R., Armstrong B., Allen R. Reversion toward an earlier stage of differentiation and loss of polarity during progression of N-methyl-N-nitrosourea-induced rat mammary tumours. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9292–9296. doi: 10.1073/pnas.85.23.9292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel L. W., Young N. A. Human breast carcinoma cells in continuous culture: a review. Cancer Res. 1978 Nov;38(11 Pt 2):4327–4339. [PubMed] [Google Scholar]
- Fogh J., Wright W. C., Loveless J. D. Absence of HeLa cell contamination in 169 cell lines derived from human tumors. J Natl Cancer Inst. 1977 Feb;58(2):209–214. doi: 10.1093/jnci/58.2.209. [DOI] [PubMed] [Google Scholar]
- Foster C. S., Smith C. A., Dinsdale E. A., Monaghan P., Neville A. M. Human mammary gland morphogenesis in vitro: the growth and differentiation of normal breast epithelium in collagen gel cultures defined by electron microscopy, monoclonal antibodies, and autoradiography. Dev Biol. 1983 Mar;96(1):197–216. doi: 10.1016/0012-1606(83)90323-8. [DOI] [PubMed] [Google Scholar]
- Fridman R., Giaccone G., Kanemoto T., Martin G. R., Gazdar A. F., Mulshine J. L. Reconstituted basement membrane (matrigel) and laminin can enhance the tumorigenicity and the drug resistance of small cell lung cancer cell lines. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6698–6702. doi: 10.1073/pnas.87.17.6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridman R., Kibbey M. C., Royce L. S., Zain M., Sweeney M., Jicha D. L., Yannelli J. R., Martin G. R., Kleinman H. K. Enhanced tumor growth of both primary and established human and murine tumor cells in athymic mice after coinjection with Matrigel. J Natl Cancer Inst. 1991 Jun 5;83(11):769–774. doi: 10.1093/jnci/83.11.769. [DOI] [PubMed] [Google Scholar]
- Gaffney E. V. A cell line (HBL-100) established from human breast milk. Cell Tissue Res. 1982;227(3):563–568. doi: 10.1007/BF00204786. [DOI] [PubMed] [Google Scholar]
- Hammond S. L., Ham R. G., Stampfer M. R. Serum-free growth of human mammary epithelial cells: rapid clonal growth in defined medium and extended serial passage with pituitary extract. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5435–5439. doi: 10.1073/pnas.81.17.5435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilkens J., Buijs F., Ligtenberg M. Complexity of MAM-6, an epithelial sialomucin associated with carcinomas. Cancer Res. 1989 Feb 15;49(4):786–793. [PubMed] [Google Scholar]
- Kramer R. H., Bensch K. G., Wong J. Invasion of reconstituted basement membrane matrix by metastatic human tumor cells. Cancer Res. 1986 Apr;46(4 Pt 2):1980–1989. [PubMed] [Google Scholar]
- Lee E. Y., Lee W. H., Kaetzel C. S., Parry G., Bissell M. J. Interaction of mouse mammary epithelial cells with collagen substrata: regulation of casein gene expression and secretion. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1419–1423. doi: 10.1073/pnas.82.5.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M. L., Aggeler J., Farson D. A., Hatier C., Hassell J., Bissell M. J. Influence of a reconstituted basement membrane and its components on casein gene expression and secretion in mouse mammary epithelial cells. Proc Natl Acad Sci U S A. 1987 Jan;84(1):136–140. doi: 10.1073/pnas.84.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen O. W., van Deurs B. Characterization of epithelial membrane antigen expression in human mammary epithelium by ultrastructural immunoperoxidase cytochemistry. J Histochem Cytochem. 1986 Jun;34(6):801–809. doi: 10.1177/34.6.3009605. [DOI] [PubMed] [Google Scholar]
- Petersen O. W., van Deurs B. Growth factor control of myoepithelial-cell differentiation in cultures of human mammary gland. Differentiation. 1988 Dec;39(3):197–215. doi: 10.1111/j.1432-0436.1988.tb00094.x. [DOI] [PubMed] [Google Scholar]
- Petersen O. W., van Deurs B. Preservation of defined phenotypic traits in short-term cultured human breast carcinoma derived epithelial cells. Cancer Res. 1987 Feb 1;47(3):856–866. [PubMed] [Google Scholar]
- Rudland P. S. Stem cells and the development of mammary cancers in experimental rats and in humans. Cancer Metastasis Rev. 1987;6(1):55–83. doi: 10.1007/BF00047609. [DOI] [PubMed] [Google Scholar]
- Rønnov-Jessen L., Celis J. E., Van Deurs B., Petersen O. W. A fibroblast-associated antigen: characterization in fibroblasts and immunoreactivity in smooth muscle differentiated stromal cells. J Histochem Cytochem. 1992 Apr;40(4):475–486. doi: 10.1177/40.4.1552184. [DOI] [PubMed] [Google Scholar]
- Rønnov-Jessen L., Van Deurs B., Nielsen M., Petersen O. W. Identification, paracrine generation, and possible function of human breast carcinoma myofibroblasts in culture. In Vitro Cell Dev Biol. 1992 Apr;28A(4):273–283. doi: 10.1007/BF02634244. [DOI] [PubMed] [Google Scholar]
- Satyaswaroop P. G., Tabibzadeh S. S. Extracellular matrix and the patterns of differentiation of human endometrial carcinomas in vitro and in vivo. Cancer Res. 1991 Oct 15;51(20):5661–5666. [PubMed] [Google Scholar]
- Soule H. D., Maloney T. M., Wolman S. R., Peterson W. D., Jr, Brenz R., McGrath C. M., Russo J., Pauley R. J., Jones R. F., Brooks S. C. Isolation and characterization of a spontaneously immortalized human breast epithelial cell line, MCF-10. Cancer Res. 1990 Sep 15;50(18):6075–6086. [PubMed] [Google Scholar]
- Stampfer M. R., Bartley J. C. Induction of transformation and continuous cell lines from normal human mammary epithelial cells after exposure to benzo[a]pyrene. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2394–2398. doi: 10.1073/pnas.82.8.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stampfer M., Hallowes R. C., Hackett A. J. Growth of normal human mammary cells in culture. In Vitro. 1980 May;16(5):415–425. doi: 10.1007/BF02618365. [DOI] [PubMed] [Google Scholar]
- Streuli C. H., Bailey N., Bissell M. J. Control of mammary epithelial differentiation: basement membrane induces tissue-specific gene expression in the absence of cell-cell interaction and morphological polarity. J Cell Biol. 1991 Dec;115(5):1383–1395. doi: 10.1083/jcb.115.5.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Papadimitriou J., Stampfer M., Bartek J., Lewis A., Boshell M., Lane E. B., Leigh I. M. Keratin expression in human mammary epithelial cells cultured from normal and malignant tissue: relation to in vivo phenotypes and influence of medium. J Cell Sci. 1989 Nov;94(Pt 3):403–413. doi: 10.1242/jcs.94.3.403. [DOI] [PubMed] [Google Scholar]
- Thompson E. W., Paik S., Brünner N., Sommers C. L., Zugmaier G., Clarke R., Shima T. B., Torri J., Donahue S., Lippman M. E. Association of increased basement membrane invasiveness with absence of estrogen receptor and expression of vimentin in human breast cancer cell lines. J Cell Physiol. 1992 Mar;150(3):534–544. doi: 10.1002/jcp.1041500314. [DOI] [PubMed] [Google Scholar]
- Trask D. K., Band V., Zajchowski D. A., Yaswen P., Suh T., Sager R. Keratins as markers that distinguish normal and tumor-derived mammary epithelial cells. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2319–2323. doi: 10.1073/pnas.87.6.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellings S. R., Jensen H. M., Marcum R. G. An atlas of subgross pathology of the human breast with special reference to possible precancerous lesions. J Natl Cancer Inst. 1975 Aug;55(2):231–273. [PubMed] [Google Scholar]
- White T. E., di Sant'Agnese P. A., Miller R. K. Human endometrial cells grown on an extracellular matrix form simple columnar epithelia and glands. In Vitro Cell Dev Biol. 1990 Jun;26(6):636–642. doi: 10.1007/BF02624214. [DOI] [PubMed] [Google Scholar]
- Wicha M. S., Lowrie G., Kohn E., Bagavandoss P., Mahn T. Extracellular matrix promotes mammary epithelial growth and differentiation in vitro. Proc Natl Acad Sci U S A. 1982 May;79(10):3213–3217. doi: 10.1073/pnas.79.10.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Balakrishnan A., Hamamoto S., Elias J. J., Rosenau W., Beattie C. W., Das Gupta T. K., Wellings S. R., Nandi S. Human breast epithelial cells in serum-free collagen gel primary culture: growth, morphological, and immunocytochemical analysis. J Cell Physiol. 1987 Nov;133(2):228-34, 254-5. doi: 10.1002/jcp.1041330205. [DOI] [PubMed] [Google Scholar]
- Yaswen P., Smoll A., Peehl D. M., Trask D. K., Sager R., Stampfer M. R. Down-regulation of a calmodulin-related gene during transformation of human mammary epithelial cells. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7360–7364. doi: 10.1073/pnas.87.19.7360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajchowski D. A., Band V., Trask D. K., Kling D., Connolly J. L., Sager R. Suppression of tumor-forming ability and related traits in MCF-7 human breast cancer cells by fusion with immortal mammary epithelial cells. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2314–2318. doi: 10.1073/pnas.87.6.2314. [DOI] [PMC free article] [PubMed] [Google Scholar]