Abstract
Background
Vaso-occlusive crisis (VOC), hallmark of sickle-cell disease (SCD), is the first cause of patients' Emergency-Room admissions and hospitalizations. Acute chest syndrome (ACS), a life-threatening complication, can occur during VOC, be fatal and prolong hospitalization. No predictive factor identifies VOC patients who will develop secondary ACS.
Methods
This prospective, monocenter, observational study on SS/S-β0thalassemia SCD adults aimed to identify parameters predicting ACS at Emergency-Department arrival. The primary endpoint was ACS onset within 15 days of admission. Secondary endpoints were hospitalization duration, morphine consumption, pain evaluation, blood transfusion(s) (BT(s)), requiring intensive care and mortality.
Findings
Among 250 VOCs included, 247 were analyzed. Forty-four (17.8%) ACSs occurred within 15 (median [IQR] 3 [2, 3]) days post-admission based on auscultation abnormalities; missing chest radiographs excluded three patients. Comparing ACS to VOC, respectively, median hospital stay was longer 9 [7–11] vs 4 [3–7] days (p < 0.0001), 7/41 (17%) vs 1/203 (0.5%) required intensive care (p < 0.0001), and 20/41 (48.7%) vs 6/203 (3%) required BTs (p < 0.0001). No patient died. The multivariate model retained reticulocyte and leukocyte counts, and spine and/or pelvis pain as being independently associated with ACS; the resulting ACS-predictive score's area under the ROC was 0.840 [95% CI 0.780–0.900], 98.8% negative-predictive value and 39.5% positive-predictive value for the real ACS incidence.
Interpretation
The ACS-predictive score is simple, easily applied and could change VOC management and therapeutic perspectives. Assessed ACS risk could lead to earlier discharges or close monitoring and rapid medical intensification to prevent ACS.
Keywords: Sickle cell disease, Vaso-occlusive crisis, Acute chest syndrome, Score, Prospective study
Highlights
-
•
Acute chest syndrome is a threatening complication.
-
•
Acute chest syndrome often occurs during a vaso occlusive crisis.
-
•
Our study provides a predictive score of acute chest syndrome.
1. Introduction
Vaso-occlusive crisis (VOC), hallmark of sickle-cell disease (SCD), is the primary cause of Emergency-Department admissions and patients' hospitalizations (Ballas and Lusardi, 2005). About 20% of all-age deaths in the United States occurred during VOCs, and more frequently for those 20–54 years old in an inpatient facility (Hamideh and Alvarez, 2013). Secondary acute chest syndrome (ACS), the main severe VOC complication (Platt et al., 1994, Perronne et al., 2002), represented 50% of ACSs in a prospective study, appearing a mean of 2.5 days post-admission for VOC (Vichinsky et al., 2000). ACS remains a major cause of SCD-associated adult mortality (Platt et al., 1994, Perronne et al., 2002, Vichinsky et al., 2000, Maitre et al., 2000) and a major risk factor for early death (Platt et al., 1994). ACS is also associated with shortened survival probability, even long after the episode (Castro et al., 1994), particularly in conjunction with acute pulmonary hypertension (Mekontso Dessap et al., 2008). Still, prospective studies on adult VOCs are rare. Ballas et al., who studied 117 VOCs in 36 SCD patients over 6 years (Charache et al., 1992), found higher leukocyte and reticulocyte counts, lactate dehydrogenase (LDH) levels and inflammatory markers at the initial VOC phase vs steady state. In another study, half of the 102 patients studied had a prodromal phase 1–2 days pre-hospitalization, after the following precipitating factors, in decreasing frequency: cold, exertion, tiredness, infection, stress or worry, dehydration, alcohol consumption and pregnancy (Murray and May, 1988).
However, no study has yet focused on differences at admission between VOC patients who will or will not develop ACS. Predictive clinical and biological markers could provide important information on VOC and ACS pathophysiologies needed to develop new therapeutic targets. Moreover, a score predicting ACS could generate innovative strategies to prevent ACS and/or simplify low-risk VOC management, thereby limiting morbidity and mortality, and shortening hospitalizations.
This predictive severity (PRESEV) study was undertaken to identify VOC-risk factors and construct a score predicting ACS at Emergency-Department arrival for VOC.
2. Patients and Methods
2.1. Participants
This prospective, monocenter, observational study included SS or S-β0thalassemia patients, ≥ 18 years old arriving at the Emergency Room with severe VOC requiring hospitalization in our university hospital's Adult Sickle-Cell Referral Center. Severe VOC was defined as pain or tenderness not controlled by grade-II analgesics (codeine or tramadol), affecting at least one part of the body, ie, limbs, ribs, sternum, head (skull), and spine and/or pelvis (S ± P), that required opioids and was not attributable to other causes. Exclusion criteria included: VOC with parenteral hydration lasting > 24 h (because 24 h of treatment could modify clinical and biological parameters), primary ACS, transfusion or blood-exchange transfusion (henceforth referred to as BT) during the preceding month or chronic BTs, previous delayed hemolysis-transfusion reaction making BT impossible, severe complication requiring BT at arrival, pregnancy, psychiatric disorder, proven sepsis and/or surgery < 15 days earlier. Patients with multiple hospitalizations separated by ≥ 1 month could be enrolled in the trial more than once if each episode satisfied inclusion criteria.
Standardized care included bed rest, fluid replacement with 5% glucose (2 L with 4 g of NaCl/L), oral alkaline water (500 mL/day), folinic acid (5 mg/day), intravenous paracetamol (1 g every 6 h), at least 2 L of O2/min to obtain 98% transcutaneous oxygen saturation (SpO2) and systematic preventive incentive spirometry. Hydroxyurea therapy was noted. Morphine was administered by patient-controlled analgesia, according a standardized procedure. For febrile (> 38 °C) patients, hemoculture and urinalysis (CBEU) were run; Legionella and streptococcal pneumonia urinary antigens were sought when an auscultatory abnormality or radiological infiltrate was present. Empirical antibiotic therapy (amoxicillin and/or macrolide for suspected allergy or intracellular bacteria) was prescribed. French guidelines defined BT indications (Perronne et al., 2002). To assure the reliability of specimen sampling, storage and analysis, patients arriving at the Emergency Department after midnight were included the next morning except weekends, and hospitalized in the Internal Medicine or Clinical Immunology Department specialized in SCD management (after January 2008). Transfusion indications according to French guidelines (Lionnet et al., 2009) were: anemia < 6/dL with symptoms, VOC lasting > 8 days without improvement, every acute severe complication, overt stroke, severe infection, multiorgan involvement, acute priapism lasting > 3 h and severe ACS. The latter was defined by one of the following: respiratory frequency > 30/min or < 10/min not related to opioid overdose, shallow breathing, breathlessness on speaking, impaired consciousness, important extension of auscultatory abnormality and/or radiological infiltrate, arterial blood–gas PO2 < 60 mm Hg.
2.2. Primary and Secondary Endpoints
VOC and ACS groups were formed. The primary endpoint was ACS onset within 15 days of admission, defined as the appearance of an auscultatory abnormality (crepitants and/or bronchial breathing) and/or chest pain and an infiltrate on chest film and/or thoracic computed-tomography (CT) scan but excluding atelectasia. Secondary endpoints were: hospitalization duration, BT, morphine consumption, visual analog scale (VAS: 0 mm, none; 100 mm, worst possible) for pain and our categorical pain score (CPS: range 0–3 points: 0, no pain; 1, mild pain, unaffected by mobilization; 2, moderate pain, increased by mobilization; 3, severe pain with disability) (Bartolucci et al., 2009) evaluations, BT, intensive care unit admission and mortality. Medical history and precipitating factors were systematically recorded on a standardized form.
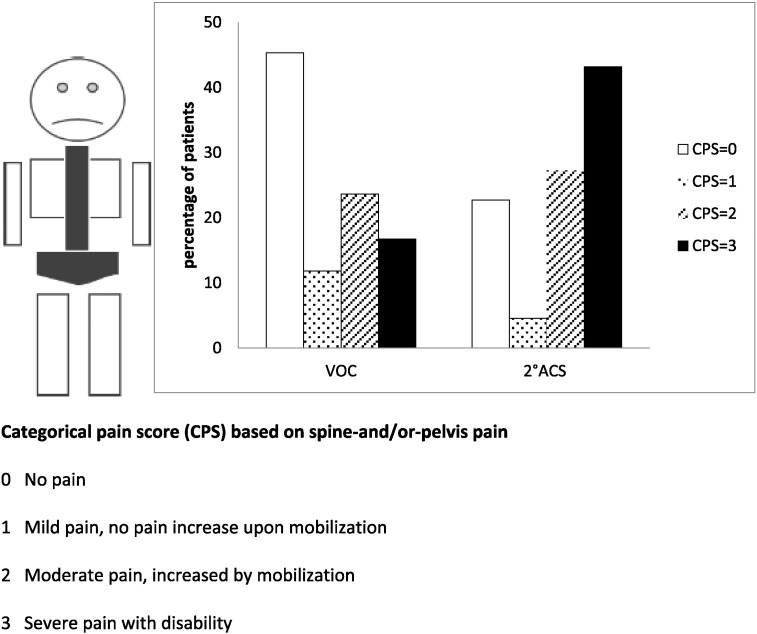
Each patient's clinical and biological parameters were determined at arrival. Every patient was examined by two physicians at least twice daily (for independent determinations of auscultatory abnormality), and nurses recorded temperature, pain, SpO2, respiratory and cardiac frequencies at least every 6 h. For the CPS, patients graded their pain in seven body sites (all four limbs, ribs and sternum, head, and S ± P; Fig. 1). Chest radiograph or thoracic CT scan was obtained for an auscultatory abnormality and/or chest pain. A radiologist and Referral Center physician blindly analyzed images. Daily morphine consumption was recorded on hospitalization days 1, 2 and 4. Patients could be discharged once pain was controlled by grade-II analgesics. All patients consulted at steady state, defined as ≥ 1 month post-hospitalization for VOC, infection, ACS, any other clinical event necessitating hospitalization and/or BT, and ≥ 3 months after the last BT, with biological parameter determinations.
Fig. 1.
Frequency of spine-and/or-pelvis categorical pain score determination at Emergency-Department arrival.
Our local Ethics Committee (CPP Île-de-France IX) approved this study, conducted in accordance with the Declaration of Helsinki, Good Clinical Practice guidelines, and local laws and regulations. Patients were enrolled after giving their written informed consent.
2.3. Statistical Analyses
To detect relevant major biological parameter differences with 80% power and a 5% alpha risk, 250 inclusions including 20% ACSs were required (e.g., differences of 1 g of hemoglobin/dL with standard deviation (SD) = 2 with unequal variance) and 40 × 109 reticulocytes/L (SD = 80, with unequal variance).
Continuous variables are expressed as means ± 1 SD or medians [interquartile range], depending on their normal or asymmetric distributions. Categorical variables are expressed as numbers (%). ACS-associated factors were first sought by univariate analyses, using Student's t-test or Mann–Whitney non-parametric test, depending on the distribution, or χ2 or Fisher's exact test, where appropriate. Analysis of variance for repeated measures compared morphine doses on the first 4 days of hospitalization. Continuous variables significantly associated with ACS were transformed into binary variables, based on whole-population medians and confirmed by receiver operating characteristics (ROC) curve-determined thresholds. ACS-associated factors (p ≤ 0.05) were entered into logistic-regression models with a backward-stepwise procedure, confirmed by a forward procedure adjusted to two day-1 hemoglobin-level classes. Variables achieving p ≤ 0.05 were kept in the model. Goodness-of-fit was assessed with the Hosmer–Lemeshow test and improvement by the final model of the explained variability by Nagelkerke's R2.
Each patient's ACS-predictive score was calculated using rounded linear transformation of the model coefficients and a binary value, 0/1, for the respective absence/presence of the event. The predictive score's performance was assessed with ROC curves and calculation of the area under them (AUROC) and its 95% confidence interval (CI). The threshold best discriminating between 2°ACS and VOC was retained, based on the score's highest sensitivity and negative-predictive value (NPV). Sensitivity analysis used a third reticulocyte-count category for patients with missing data. All statistical analyses were computed with SPSS v18 and/or Stata v11 software.
3. Results
3.1. Patients and Inclusion Characteristics
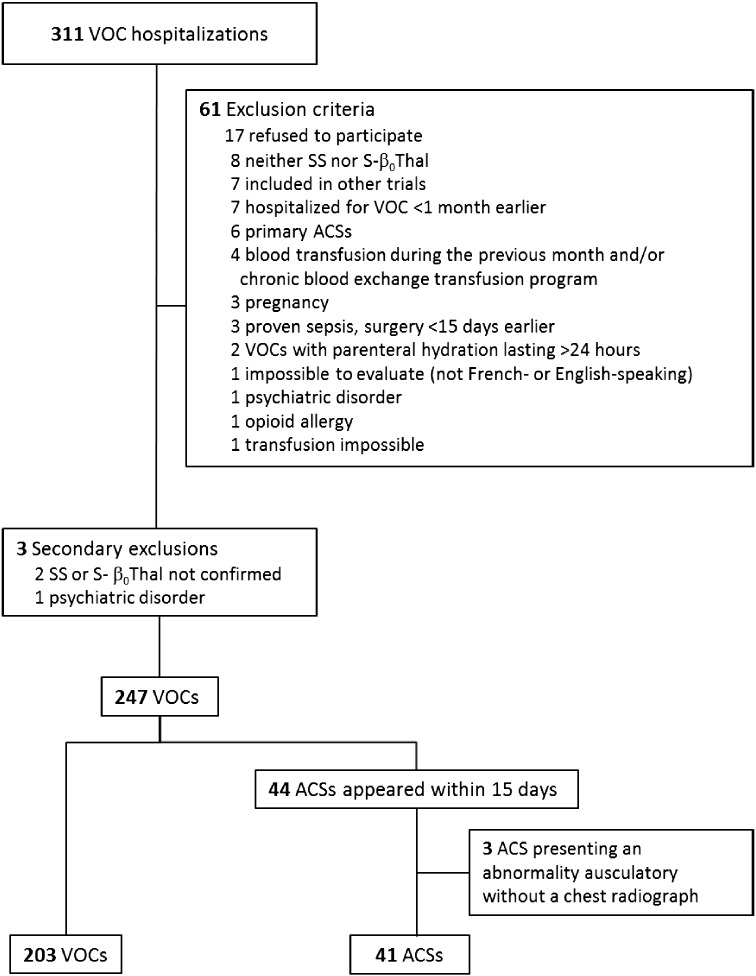
Recruitment lasted from July 2006 through September 2012 (Fig. 2), including 250 VOCs with 247 analyzed after excluding three (one psychiatric disorder and two non-SS or -S-β0thalassemia genotypes). One hundred and forty-five patients were included once and 43 more than once. Among patients who developed ACS and were included twice, all but one had a VOC during the other hospitalization. Forty-four/41 (17.8%) ACSs occurred within 15 (median 3 [2, 3]) days post-admission based on auscultation abnormalities and/or chest pain, but three patients were excluded from the analysis because of missing confirmatory chest radiographs. Only 2/41 chest radiograph-confirmed ACSs did not have an auscultatory abnormality. One VOC-group patient developed a late ACS 20 days post-hospitalization.
Fig. 2.
Study flow chart. VOC = vaso-occlusive crisis. ACS = acute chest syndrome.
Between-group clinical characteristics and SCD history at inclusion (Table 1) were comparable, except for the precipitating factor during the preceding 3 days. Patients were equally treated with hydroxyurea. VOC and ACS steady-state biological parameters for 222 episodes were comparable for the two groups, except red blood-cell (RBC) counts, respectively: 3.2 ± 0.7 × 1012/L and 2.9 ± 0.5 × 1012/L (p = 0.02).
Table 1.
Characteristics of the 244 vaso-occlusive crisis (VOC) episodes at inclusion.
| Characteristic | VOCs | ACSs | p |
|---|---|---|---|
| Number (%) | 203 (83.2) | 41 (16.8) | |
| Age, yr | 27.7 ± 7.3 | 26.9 ± 6.5 | 0.52 |
| Males, n (%) | 101 (49.8) | 22 (53.7) | 0.65 |
| Body mass index | 21.4 ± 3.3 | 20.9 ± 2.9 | 0.43 |
| Hydroxyurea treatment | 66 (32.5) | 15 (36.6) | 0.61 |
| SCD history | |||
| Transfusion during the last year | 55 (27.1) | 16 (36.4) | 0.27 |
| ICU admission during the last year | 43 (21.2) | 9 (20.5) | 1 |
| 0 VOC hospitalizations/yeara | 33 (16.3) | 10 (22.7) | 0.38 |
| 1–2 VOC hospitalizations/yeara | 85 (41.9) | 16 (36.4) | 0.61 |
| 3–5 VOC hospitalizations/yeara | 64 (31.5) | 16 (36.4) | 0.6 |
| > 5 VOC hospitalizations/yeara | 21 (10.3) | 2 (4.5) | 0.39 |
| Number of previous ACSs | 1 [0–3] | 1.5 [0–3] | 0.61 |
| Cerebral vasculopathy | 7 (3.4) | 1 (2.3) | 1 |
| Retinopathy | 28 (13.8) | 5 (11.4) | 0.81 |
| Kidney involvement | 15 (7.4) | 6 (13.6) | 0.23 |
| History of leg ulcer | 17 (8.4) | 1 (2.3) | 0.21 |
| Osteonecrosis | 48 (23.6) | 7 (15.9) | 0.32 |
| History of priapism | 32 (31.7) | 13 (54.2) | 0.057 |
| Precipitating factor in the past 3 days | 186 (91.6) | 37 (84.1) | 0.16 |
| Cold | 89 (43.8) | 8 (19.5) | 0.005 |
| Exertion | 62 (30.5) | 14 (31.8) | 0.71 |
| Stress | 46 (22.7) | 10 (24.4) | 0.84 |
| Infection (suspected) | 39 (19.2) | 10 (24.4) | 0.52 |
| Menstruation | 16 (15.7) | 3 (15.8) | 1 |
| Less oral hydration | 27 (13.3) | 4 (9.8) | 0.80 |
| Alcohol consumption | 12 (5.9) | 0 (0) | 0.23 |
| Vomiting or diarrhea | 11 (5.4) | 2 (4.9) | 1 |
| Steroids | 2 (1) | 2 (4.9) | 0.13 |
| Snoring | 12 (5.9) | 4 (9.8) | 0.32 |
| Limb compression | 1 (0.5) | 1 (2.4) | 0.31 |
| Airplane travel | 1 (0.5) | 0 (0) | 1 |
Bold value indicates significance at p < 0.05.
ACSs = secondary acute chest syndromes. SCD = sickle-cell disease. ICU = intensive care unit.
Results are expressed as n (%) or mean ± SD or median [IQR].
Mean number of VOC hospitalizations/patient/year for during the last 3 years.
A factor precipitating 186/203 (91.6%) VOCs and 34/41 (82.9%) ACSs was identified, with ≥ 2 for 92/203 (45.3%) and 18/41 (43.9%), respectively. The most frequent precipitating factors were cold (39·7%; significantly more frequent for VOCs; p = 0.004), exertion (31.1%), stress (23.4%), physician-suspected infection (20%), menstruation (14.9%) and decreased oral hydration (13.1%).
The mean VOC-onset-to-arrival (Table 2) interval was ~ 1 day; pain appeared at night for ~ 25% of the patients with no between-group differences. SpO2 was < 96% in 9.8% of VOC and 19.5% of ACS patients (p = 0.04). Despite similar respiratory frequency values, tachypnea (> 20 breaths/min) was more frequent in ACS (39% vs 29.1% in VOC; p = 0.001). The mean VAS-assessed global pain intensity and total CPS were comparable, but ACS patients had significantly more intense S ± P (Table 3, Fig. 1) and milder left arm pain. Pain intensity in the five other body sites, including chest, was similar for both groups. According to univariate analyses, ACS patients' leukocyte, neutrophil and reticulocyte counts, and LDH, direct bilirubin, aspartate aminotransferase, alkaline phosphatase and C-reactive protein levels were significantly higher, but their RBC counts and hemoglobin levels were lower than those of VOC patients.
Table 2.
Clinical and biological parameters at Emergency-Room arrival.
| Parameter | VOCs | ACSs | p | Missing |
|---|---|---|---|---|
| Number (%) | 203 (83.2) | 41 (16.8) | ||
| VOC-onset-to-hospitalization interval (h) | 22 [14–36] | 21 [14–310] | 0.55 | 11 |
| VOC night appearance | 45 (22.2) | 11 (26.8) | 0.52 | 0 |
| Temperature (°C) | 36.9 [36.5–37.2] | 36.9 [36.7–37.3] | 0.092 | 20 |
| Transcutaneous O2 saturation (%) | 99 [97–100] | 98.5 [95–100] | 0.17 | 11 |
| Breaths/min | 18 [16–20] | 20 [16–25] | 0.041 | 70 |
| Systolic blood pressure (mm Hg) | 122 [110–135] | 123 [111–135] | 0.47 | 18 |
| Diastolic blood pressure (mm Hg) | 70 [62–80] | 69 [64–78] | 0.76 | 18 |
| Arrival pain VAS (mm) | 8 [7–9] | 8 [7–10] | 0.25 | 6 |
| CPS sums for the 7 body parts | 5 [4–8] | 7 [5–12] | 0.56 | 0 |
| Spine and/or pelvis | 1 [0–2] | 2 [1–3] | < 0.001 | 3 |
| Ribs and sternum | 0 [0–1 | 0 [0–2] | 0.155 | 3 |
| Skull | 0 [0–0] | 0 [0–0] | 0.705 | 3 |
| Right upper limb | 0 [0–2] | 0 [0–1] | 0.70 | 3 |
| Left upper limb | 0 [0–2] | 0 [0–0] | 0.023 | 3 |
| Right lower limb | 1 [0–2] | 1 [0–2] | 0.94 | 3 |
| Left lower limb | 0 [0–2] | 1 [0–2] | 0.686 | 3 |
| Morphine consumption on day 1 | 42 [21–81] | 56 [30–77.5] | 0.3 | 19 |
| Red blood cells (× 1012/L) | 3.0 [2.6–3.5] | 2.7 [2.4–3.4] | 0.017 | 1 |
| Hemoglobin (g/dL) | 9.1 [8.3–9.9] | 8.2 [7.5–10.1] | 0.023 | 0 |
| White blood cells (× 109/L) | 11.3 [8.8–15.8] | 16.3 [13.3–20.1] | < 0.001 | 0 |
| Neutrophils (× 109/L) | 6.8 [5.2–10.4] | 8.6 [6.9–14.7] | 0.011 | 36 |
| Platelets (× 109/L) | 345 [280–412] | 371 [282–395] | 0.075 | 2 |
| Reticulocytes (× 109/L) | 206 [158–253] | 262 [232–343] | < 0.001 | 16 |
| Lactate dehydrogenase (IU/L) | 397 [322–526] | 501 [409–601] | 0.001 | 3 |
| Total bilirubin (μmol/L) | 36 [25–54] | 48 [33–76] | 0.005 | 3 |
| Direct bilirubin (μmol/L) | 10 [7–13] | 12 [9–17] | 0.002 | 3 |
| Indirect bilirubin (μmol/L) | 25 [17–38] | 34 [22–50 | 0.007 | 4 |
| Aspartate aminotransferase (IU/L) | 39 [29–54] | 53 [33–69] | 0.02 | 5 |
| Alanine aminotransferase (IU/L) | 23 [16–36] | 31 [16–50] | 0.17 | 3 |
| γ-Glutamyltransferase (IU/L) | 43 [24–85] | 47 [32–104] | 0.11 | 7 |
| Alkaline phosphatase (IU/L) | 77 [60–104] | 104 [80–129] | < 0.001 | 5 |
| Creatinine (μmol/L) | 59 [47–69] | 59 [47–68] | 0.81 | 2 |
| Blood urea nitrogen (mmol/L) | 2.5 [1.8–3.1] | 2.6 [2.1–3.3] | 0.20 | 2 |
| Creatine phosphokinase (IU/L) | 58 [43–91] | 67 [44–92] | 0.46 | 29 |
| C-reactive protein (mg/L) | 15 [5–42] | 35 [13–86] | 0.001 | 20 |
Bold values indicate significance at p < 0.05.
VOC, vaso-occlusive crisis; ACSs: secondary acute chest syndromes; Missing, missing data; CPS, categorical pain score.
Results are expressed as n (%), mean ± SD or median [IQR].
Table 3.
ACS-predictive model derived from the multivariate analysis.
| Day-1 variable | β-Coefficient | aOR [95% CI] | p | Pointsa |
|---|---|---|---|---|
| Reticulocytes (109/L) | ||||
| ≤ 216 | 0 | 1 | 0 | |
| > 216 | 2.153 | 8.613 [3.01–24.69] | < 0.001 | 6 |
| Spine and/or pelvis CPS | 0 | |||
| 0 or 1 | 0 | 1 | ||
| 2 | 1.401 | 4.060 [1.46–11.26] | 0.007 | 4 |
| 3 | 1.852 | 6.371 [2.37–17.15] | < 0.001 | 6 |
| Leukocytes (109/L) | ||||
| ≤ 11 | 0 | 1 | 0 | |
| > 11 | 1.160 | 3.190 [1.17–8.72] | 0.024 | 3 |
| Hemoglobin (g/dL)b | ||||
| > 9 | 0 | 1 | 0 | |
| ≤ 9 | 0.246 | 1.279 [0.55–2.96 | 0.567 | 1 |
| Predictive model performance on the study population | |||||
|---|---|---|---|---|---|
| Predictive scorec | ACSs | VOCs | Total | Risk | |
| ≥ 11 | 21 | 26 | 47 | PPV = 44.7% | High |
| 6–10 | 15 | 75 | 90 | Intermediate | |
| ≤ 5 | 1 | 88 | 89 | NPV = 98.9% | Low |
| Total | 37 | 189 | 226 | ||
aOR = adjusted odds ratio. CPS = categorical pain score. ACSs = secondary acute chest syndromes. VOCs = vaso-occlusive crises. PPV = predictive-positive value. NPV = negative-predictive value.
Points were calculated by multiplying the model's β-coefficients for each independent parameter × 3 and rounded to the nearest integer (AUC = 0.836 [CI 0.773–0.898]).
Forced into the model to adjust to the Hosmer–Lemeshow statistic = 0.967. Nagelkerke R2 = 0.338.
The ACS-predictive score is the sum of the points accorded each of the four day-1 variables.
3.2. Scores
Multivariate analyses retained day-1 > 216 × 109 reticulocytes, > 11 × 109 leukocytes and S ± P CPS = 2 or 3 as being independently associated with ACS. The model was adjusted to hemoglobin ≤ or > 9 g/dL (Table 3). The final score is the sum of the points accorded those four parameters; it was unchanged when adjusted for hydroxyurea. A score predicting ACS, calculated by adding the transformed β-coefficients for the 226 (189 VOC and 37 ACS) patients with no missing data for the parameters, ranged between 0 and 16.
The AUROC was 0.836 [0.773–0.898] (Supplementary fig. S1). The ROC curve determined two cutoffs, yielding three ACS-risk groups, each representing a third of the patients: score ≤ 5 (n = 89): low, with NPV = 98.9% (88/89); score > 11 (n = 47): high, with positive-predictive value (PPV) = 44.7% (21/47); and score 6–10 (n = 90): intermediate NPV = 83.3% and PPV = 16.7% (75/90) (Table 3). Applying the ACS-predictive score to the study population would have missed only 1/40 analyzable ACSs.
3.3. Outcomes
Median hospital stay was longer for ACS than VOC patients, respectively: 9 [7–11] vs 4 [3–7] days (p < 0.0001). Seven/41 (17%) ACSs vs 1/203 (0.5%) VOC (p < 0.0001) patients required intensive care. No patient died. ACS patients required more BTs than VOC patients, respectively: 20/41 (48.7%) vs 6/203 (3%) (p < 0.0001). For all but one (pre-cholecystectomy) BT, ACS severity was the BT indication, according to French guidelines, given almost simultaneously with ACS onset at a median of 3 [2, 3] days. VOC patients were transfused at a median 8 [5–11] days because of prolonged pain (3/6), late ACS on day 20 (1/6), severe cytomegalovirus infection (1/6) or severe anemia and low reticulocyte count (1/6).
Four/41 (9·7%) ACS vs 28/203 (13.8%) VOC (p = 0.4) patients were readmitted within 2 weeks of discharge. ACS- or VOC-patient morphine consumptions on days 1 (Table 1), as thereafter on days 2 and 4 (for 36 ACSs and 113 VOCs; p = 0.5), were comparable. At inclusion, only 7.3% of ACS and 3.9% of VOC patients were febrile (p = 0.64), with the former's mean temperature being slightly higher (p = 0.05). Their hemocultures and CBEU were negative; colchicine attenuated one patient's wrist arthritis and another had valganciclovir-treated cytomegalovirus infection with cytolytic hepatitis. Throughout hospitalization, 12 (29%) of the 41 ACS patients were febrile, all were Legionella- and streptococcal pneumonia urinary antigen-negative; amoxicillin was prescribed for all but one and 6/12 also received a macrolide.
4. Discussion
VOC is the main cause of SCD-patient Emergency-Department arrival and ACS is one of its most severe complications, especially in adults, with high morbidity and mortality (Platt et al., 1994, Perronne et al., 2002, Maitre et al., 2000, Castro et al., 1994, Mekontso Dessap et al., 2008). ACS occurred in ~ 50% of hospitalized VOC patients a mean of 2.5 days post-admission (Vichinsky et al., 2000), similar to the 2.8 days herein (median: 3 days). A recent American epidemiological study (Hamideh and Alvarez, 2013) found higher mortality rates among rural SCD patients than their urban counterparts, highlighting the need to improve rural medical care. No biological or clinical marker predictive of developing ACS was previously available.
Our results enabled construction of a very simple ACS-predictive score based on four day-1 parameters (i.e., reticulocyte and leukocyte counts, hemoglobin and S ± P CPS). It has excellent NPV, enabling identification, as of Emergency-Department admission for VOC, of patients at very low or high risk (one-third each) of developing ACS. When applied routinely, this score enables emergency-care physicians to orient the former towards short hospital stays or outpatient monitoring, as in many African countries, and to transfer the latter to referral centers where ACS-preventive therapy-intensification protocols can be applied. Indeed, therapeutic trials primarily aimed at lowering the ACS rate during VOC, based on the score to select patients at risk, would be more appropriate than the previous goal of shortening pain duration (Bartolucci et al., 2009, Orringer et al., 2001) or hospital stays (Bernini et al., 1998), or improving VOC resolution, as defined by composite scores (Gladwin et al., 2011). We emphasize that the score was determined during severe VOCs, defined as pain not controlled by grade-II analgesics. However, it is unlikely that the score would be different if less severe VOCs were included.
Because no consensual ACS definition exists, we used a definition requiring chest radiograph. However, auscultation abnormality alone should suffice for ACS diagnosis, based on French guidelines and our clinical practice. Crepitants and/or bronchial breathing occur earlier than radiographic abnormality, and chest pain is not always present despite severe ACS. The major advantage of a definition based on the appearance of those signs (but not other anomalies) is its broad applicability, even where radiography is expensive and/or not available, as in many areas in Africa. Moreover, the onset of those signs should be sufficient to diagnose ACS.
Our ACS-predictive model's clinical parameter is S ± P pain. Spine and pelvis trabecular bone characteristics probably explain our findings. VAS does not locate pain, unlike our CPS that we used previously (Bartolucci et al., 2009). Pain categorized at four levels and determined for seven body parts was easily determined and discriminant. S ± P “Severe pain with disability” was the most frequent ACS criterion, while “No pain” was the most frequent for uncomplicated VOC. Surprisingly, chest pain at arrival did not differ but ~ 80% of ACS patients suffered chest pain at its onset. Decreased respiratory frequency did not differ either, whereas arrival SpO2 was slightly lower for future ACS patients. Intriguingly, cold was a less frequent precipitating factor for ACS patients, perhaps because limb-bone vascular perfusion is more probably influenced by low temperature-induced vascular changes. Infections did not differ between groups but have been associated with ACSs in children and adults (Vichinsky et al., 2000). However, at arrival, our patients were rarely febrile and systematic hemocultures, CBEU, and searches for Legionella and streptococcal pneumonia urinary antigens, when abnormal lung sounds were heard during hospitalization, were negative. Chlamydia, Mycoplasma and Haemophilus were not sought because such tests were not easily and routinely done during the investigation period, and thus outside the study's scope.
Notably, the arrival reticulocyte count contributed strongly to the predictive score, being significantly higher on day 1 than at steady state for 2°ACSs (272 ± 88 vs 237 ± 95, respectively p = 0.006, n = 33 paired values), while the VOC group's values were stable (p = 0.98). Moreover, increased reticulocyte adhesion to activated endothelium and subendothelial matrix compounds (Hebbel, 1997, Swerlick et al., 1993) was mainly attributed to more membrane-adhesion molecules (El Nemer et al., 1998, Cartron and Elion, 2008), some of which disappear on mature RBCs, e.g., α4β1 or CD36 (Swerlick et al., 1993, Browne and Hebbel, 1996, Joneckis et al., 1993), and to functional signaling pathways more active in reticulocytes than mature RBCs (Bartolucci et al., 2010, Limbird et al., 1980). Leukocytes are the other major score component. Their increase during VOCs has been proven; (Ballas et al., 1988) however, they were less able discriminate between ACS and VOC than reticulocytes. Leukocytes are known to be an independent factor predictive of SCD-associated death (Platt et al., 1994). Reported results demonstrated enhanced leukocyte adhesion to SS RBCs (Chaar et al., 2010) and vascular endothelium (Turhan et al., 2002), and their subsequent leukocyte participation in vaso-occlusion (Belcher et al., 2000, Hidalgo et al., 2009).
Pertinently, hydroxyurea did not modify the score, perhaps because patients taking it have lower reticulocyte and leukocyte counts. Hence, a reticulocyte or leukocyte rise under it would probably discriminate better between groups than without such increases. Furthermore, the comparable percentages of hydroxyurea-treated patients in our two groups indicate that, when treated patients develop severe VOCs requiring hospitalization, their risk of worsening is comparable to that of untreated patients, which should not be confused with hydroxyurea's protective effect against ACS and VOC (Charache et al., 1995). Because the hydroxyurea dose was not considered in this study, we could not exclude a difference according to its dose. Morphine consumption on hospital days 1, 2 and 4 did not differ between groups, thereby excluding its possible effect on ACS appearance, in contrast to previous hypotheses (Kopecky et al., 2004, Buchanan et al., 2005).
Our study was homogenous because of its monocenter design with the same physicians, biologists and guidelines for all patients. Our results enabled a score to be built that could bring new perspectives to SCD management, but remains to be validated in other centers and countries, and tested on pediatric populations, before concluding as to its usefulness. In conclusion, a score predicting ACS as of Emergency-Department arrival for VOC, based on reticulocyte and leukocyte counts, hemoglobin and S ± P CPS, is simple enough to be easily applied and could change VOC therapeutic perspectives.
Authors Contributions
PB designed and performed research, analyzed data, and wrote the manuscript; BG and FG designed the research, analyzed data and gave advice; FRT performed the statistical analysis; AH, MK, YL, BR, AS, MM and ASL provided patients' clinical data and discussed the results, gave advice, and commented on the manuscript; SL, SM and OWB performed biological analysis; and JB performed the monitoring.
Disclosure of Conflicts of Interest
The authors have declared that no conflicts of interest exist.
Acknowledgments
We thank Janet Jacobson for editorial assistance, Hélène Jouault and Mirna Saloum for their participation.
No funding was needed for this study.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2016.06.038.
Appendix A. Supplementary data
Supplementary material.
References
- Ballas S.K., Lusardi M. Hospital readmission for adult acute sickle cell painful episodes: frequency, etiology, and prognostic significance. Am. J. Hematol. 2005;79(1):17–25. doi: 10.1002/ajh.20336. [DOI] [PubMed] [Google Scholar]
- Ballas S.K., Larner J., Smith E.D., Surrey S., Schwartz E., Rappaport E.F. Rheologic predictors of the severity of the painful sickle cell crisis. Blood. 1988;72(4):1216–1223. [PubMed] [Google Scholar]
- Bartolucci P., El Murr T., Roudot-Thoraval F. A randomized, controlled clinical trial of ketoprofen for sickle-cell disease vaso-occlusive crises in adults. Blood. 2009;114(18):3742–3747. doi: 10.1182/blood-2009-06-227330. [DOI] [PubMed] [Google Scholar]
- Bartolucci P., Chaar V., Picot J. Decreased sickle red blood cell adhesion to laminin by hydroxyurea is associated with inhibition of Lu/BCAM protein phosphorylation. Blood. 2010;116(12):2152–2159. doi: 10.1182/blood-2009-12-257444. [DOI] [PubMed] [Google Scholar]
- Belcher J.D., Marker P.H., Weber J.P., Hebbel R.P., Vercellotti G.M. Activated monocytes in sickle cell disease: potential role in the activation of vascular endothelium and vaso-occlusion. Blood. 2000;96(7):2451–2459. [PubMed] [Google Scholar]
- Bernini J.C., Rogers Z.R., Sandler E.S., Reisch J.S., Quinn C.T., Buchanan G.R. Beneficial effect of intravenous dexamethasone in children with mild to moderately severe acute chest syndrome complicating sickle cell disease. Blood. 1998;92(9):3082–3089. [PubMed] [Google Scholar]
- Browne P.V., Hebbel R.P. CD36-positive stress reticulocytosis in sickle cell anemia. J. Lab. Clin. Med. 1996;127(4):340–347. doi: 10.1016/s0022-2143(96)90181-x. [DOI] [PubMed] [Google Scholar]
- Buchanan I.D., Woodward M., Reed G.W. Opioid selection during sickle cell pain crisis and its impact on the development of acute chest syndrome. Pediatr. Blood Cancer. 2005;45(5):716–724. doi: 10.1002/pbc.20403. [DOI] [PubMed] [Google Scholar]
- Cartron J.P., Elion J. Erythroid adhesion molecules in sickle cell disease: effect of hydroxyurea. Transfus. Clin. Biol. J. Soc. Fr. Transfus. Sanguine. 2008;15(1–2):39–50. doi: 10.1016/j.tracli.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Castro O., Brambilla D.J., Thorington B. The acute chest syndrome in sickle cell disease: incidence and risk factors. The Cooperative Study of Sickle Cell Disease. Blood. 1994;84(2):643–649. [PubMed] [Google Scholar]
- Chaar V., Picot J., Renaud O. Aggregation of mononuclear and red blood cells through an α4β1-Lu/basal cell adhesion molecule interaction in sickle cell disease. Haematologica. 2010;95(11):1841–1848. doi: 10.3324/haematol.2010.026294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charache S., Dover G.J., Moore R.D. Hydroxyurea: effects on hemoglobin F production in patients with sickle cell anemia. Blood. 1992;79(10):2555–2565. [PubMed] [Google Scholar]
- Charache S., Terrin M.L., Moore R.D. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia. N. Engl. J. Med. 1995;332(20):1317–1322. doi: 10.1056/NEJM199505183322001. [DOI] [PubMed] [Google Scholar]
- El Nemer W., Gane P., Colin Y. The Lutheran blood group glycoproteins, the erythroid receptors for laminin, are adhesion molecules. J. Biol. Chem. 1998;273(27):16686–16693. doi: 10.1074/jbc.273.27.16686. [DOI] [PubMed] [Google Scholar]
- Gladwin M.T., Kato G.J., Weiner D. Nitric oxide for inhalation in the acute treatment of sickle cell pain crisis: a randomized controlled trial. JAMA. 2011;305(9):893–902. doi: 10.1001/jama.2011.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamideh D., Alvarez O. Sickle cell disease related mortality in the United States (1999–2009) Pediatr. Blood Cancer. 2013;60(9):1482–1486. doi: 10.1002/pbc.24557. [DOI] [PubMed] [Google Scholar]
- Hebbel R.P. Adhesive interactions of sickle erythrocytes with endothelium. J. Clin. Invest. 1997;100(11 Suppl.):S83–S86. [PubMed] [Google Scholar]
- Hidalgo A., Chang J., Jang J.E., Peired A.J., Chiang E.Y., Frenette P.S. Heterotypic interactions enabled by polarized neutrophil microdomains mediate thromboinflammatory injury. Nat. Med. 2009;15(4):384–391. doi: 10.1038/nm.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joneckis C.C., Ackley R.L., Orringer E.P., Wayner E.A., Parise L.V. Integrin alpha 4 beta 1 and glycoprotein IV (CD36) are expressed on circulating reticulocytes in sickle cell anemia. Blood. 1993;82(12):3548–3555. [PubMed] [Google Scholar]
- Kopecky E.A., Jacobson S., Joshi P., Koren G. Systemic exposure to morphine and the risk of acute chest syndrome in sickle cell disease. Clin. Pharmacol. Ther. 2004;75(3):140–146. doi: 10.1016/j.clpt.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Limbird L.E., Gill D.M., Stadel J.M., Hickey A.R., Lefkowitz R.J. Loss of beta-adrenergic receptor-guanine nucleotide regulatory protein interactions accompanies decline in catecholamine responsiveness of adenylate cyclase in maturing rat erythrocytes. J. Biol. Chem. 1980;255(5):1854–1861. [PubMed] [Google Scholar]
- Lionnet F., Arlet J.B., Bartolucci P., Habibi A., Ribeil J.A., Stankovic K. Guidelines for management of adult sickle cell disease. La Rev. Med. Interne. 2009;30(Suppl. 3):S162–S223. doi: 10.1016/j.revmed.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Maitre B., Habibi A., Roudot-Thoraval F. Acute chest syndrome in adults with sickle cell disease. Chest. 2000;117(5):1386–1392. doi: 10.1378/chest.117.5.1386. [DOI] [PubMed] [Google Scholar]
- Mekontso Dessap A., Leon R., Habibi A. Pulmonary hypertension and cor pulmonale during severe acute chest syndrome in sickle cell disease. Am. J. Respir. Crit. Care Med. 2008;177(6):646–653. doi: 10.1164/rccm.200710-1606OC. [DOI] [PubMed] [Google Scholar]
- Murray N., May A. Painful crises in sickle cell disease—patients' perspectives. BMJ. 1988;297(6646):452–454. doi: 10.1136/bmj.297.6646.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orringer E.P., Casella J.F., Ataga K.I. Purified poloxamer 188 for treatment of acute vaso-occlusive crisis of sickle cell disease: a randomized controlled trial. JAMA. 2001;286(17):2099–2106. doi: 10.1001/jama.286.17.2099. [DOI] [PubMed] [Google Scholar]
- Perronne V., Roberts-Harewood M., Bachir D. Patterns of mortality in sickle cell disease in adults in France and England. Hematol. J. Off. J. Eur. Haematol. Assoc./EHA. 2002;3(1):56–60. doi: 10.1038/sj.thj.6200147. [DOI] [PubMed] [Google Scholar]
- Platt O.S., Brambilla D.J., Rosse W.F. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N. Engl. J. Med. 1994;330(23):1639–1644. doi: 10.1056/NEJM199406093302303. [DOI] [PubMed] [Google Scholar]
- Swerlick R.A., Eckman J.R., Kumar A., Jeitler M., Wick T.M. Alpha 4 beta 1-integrin expression on sickle reticulocytes: vascular cell adhesion molecule-1-dependent binding to endothelium. Blood. 1993;82(6):1891–1899. [PubMed] [Google Scholar]
- Turhan A., Weiss L.A., Mohandas N., Coller B.S., Frenette P.S. Primary role for adherent leukocytes in sickle cell vascular occlusion: a new paradigm. Proc. Natl. Acad. Sci. U. S. A. 2002;99(5):3047–3051. doi: 10.1073/pnas.052522799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vichinsky E.P., Neumayr L.D., Earles A.N. Causes and outcomes of the acute chest syndrome in sickle cell disease. National Acute Chest Syndrome Study Group. N. Engl. J. Med. 2000;342(25):1855–1865. doi: 10.1056/NEJM200006223422502. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.