Abstract
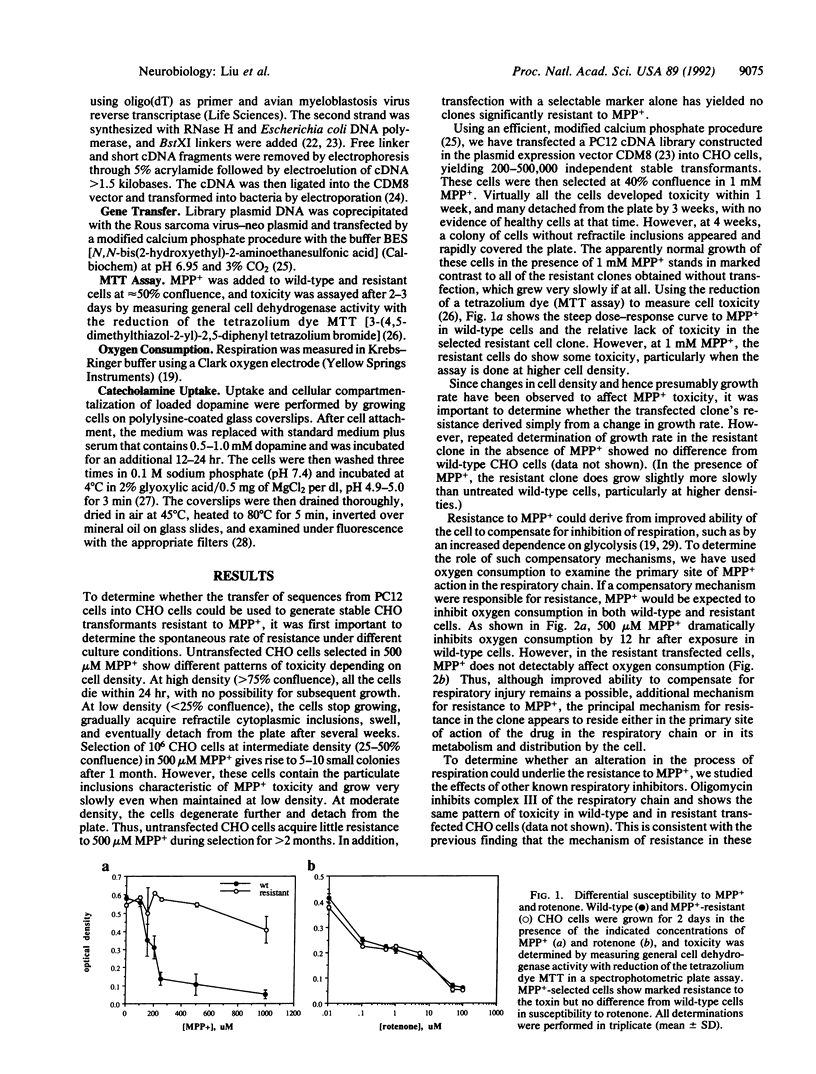
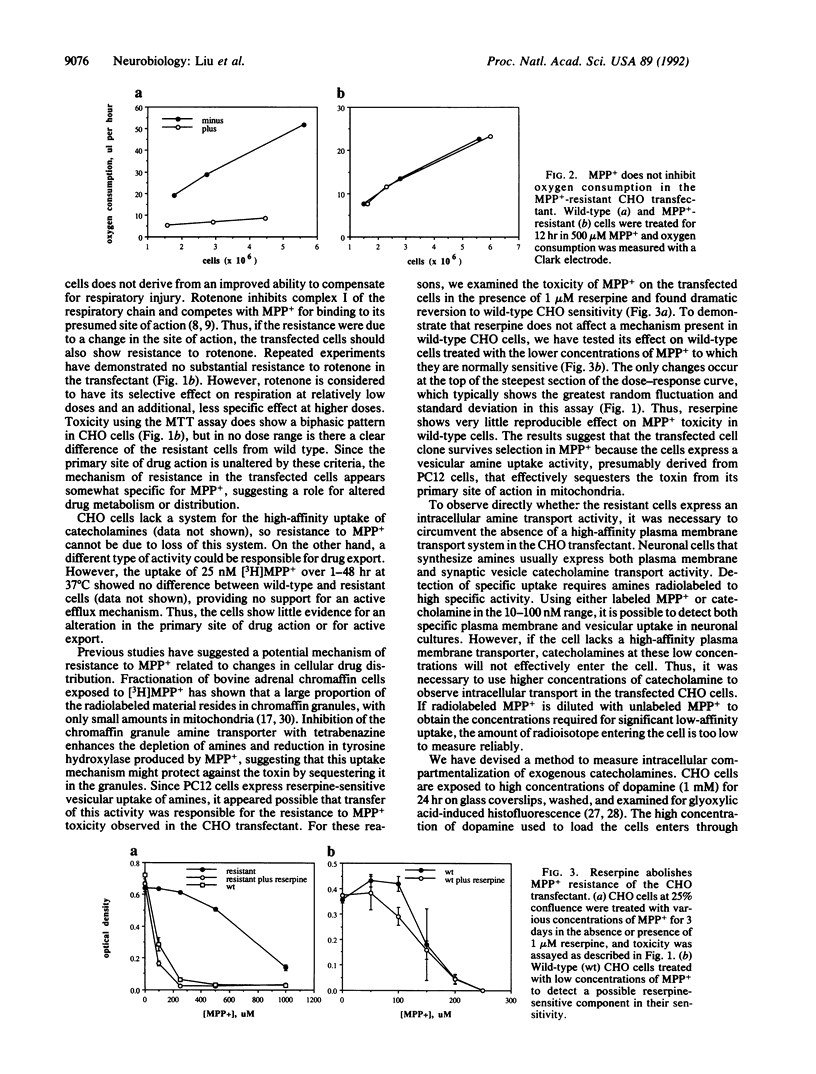
The toxin N-methyl-1,2,3,6-tetrahydropyridine produces a model of neural degeneration very similar to idiopathic Parkinson disease. To understand the cellular mechanisms that modulate susceptibility to its active metabolite N-methyl-4-phenylpyridinium (MPP+), we have transfected a cDNA expression library from the relatively MPP(+)-resistant rat pheochromocytoma PC12 cells into MPP(+)-sensitive Chinese hamster ovary (CHO) fibroblasts. Selection of the stable transformants in high concentrations of MPP+ has yielded a clone extremely resistant to the toxin. Reserpine reverses the resistance to MPP+, suggesting that a transport activity protects against this form of toxicity, perhaps by sequestering the toxin within an intracellular compartment. In support of this hypothesis, dopamine loaded into the CHO transformant shows a localized distribution that is distinct from the pattern observed in wild-type cells and is also reversed by reserpine.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aruffo A., Seed B. Molecular cloning of a CD28 cDNA by a high-efficiency COS cell expression system. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8573–8577. doi: 10.1073/pnas.84.23.8573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cohen G. Monoamine oxidase and oxidative stress at dopaminergic synapses. J Neural Transm Suppl. 1990;32:229–238. doi: 10.1007/978-3-7091-9113-2_33. [DOI] [PubMed] [Google Scholar]
- Daniels A. J., Reinhard J. F., Jr Energy-driven uptake of the neurotoxin 1-methyl-4-phenylpyridinium into chromaffin granules via the catecholamine transporter. J Biol Chem. 1988 Apr 15;263(11):5034–5036. [PubMed] [Google Scholar]
- De la Torre J. C. An improved approach to histofluorescence using the SPG method for tissue monoamines. J Neurosci Methods. 1980 Oct;3(1):1–5. doi: 10.1016/0165-0270(80)90029-1. [DOI] [PubMed] [Google Scholar]
- Denton T., Howard B. D. A dopaminergic cell line variant resistant to the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. J Neurochem. 1987 Aug;49(2):622–630. doi: 10.1111/j.1471-4159.1987.tb02909.x. [DOI] [PubMed] [Google Scholar]
- Dower W. J., Miller J. F., Ragsdale C. W. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 1988 Jul 11;16(13):6127–6145. doi: 10.1093/nar/16.13.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene L. A., Rein G. Release, storage and uptake of catecholamines by a clonal cell line of nerve growth factor (NGF) responsive pheo-chromocytoma cells. Brain Res. 1977 Jul 1;129(2):247–263. doi: 10.1016/0006-8993(77)90005-1. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hansen M. B., Nielsen S. E., Berg K. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J Immunol Methods. 1989 May 12;119(2):203–210. doi: 10.1016/0022-1759(89)90397-9. [DOI] [PubMed] [Google Scholar]
- Heikkila R. E., Manzino L., Cabbat F. S., Duvoisin R. C. Protection against the dopaminergic neurotoxicity of 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine by monoamine oxidase inhibitors. Nature. 1984 Oct 4;311(5985):467–469. doi: 10.1038/311467a0. [DOI] [PubMed] [Google Scholar]
- Hyman C., Hofer M., Barde Y. A., Juhasz M., Yancopoulos G. D., Squinto S. P., Lindsay R. M. BDNF is a neurotrophic factor for dopaminergic neurons of the substantia nigra. Nature. 1991 Mar 21;350(6315):230–232. doi: 10.1038/350230a0. [DOI] [PubMed] [Google Scholar]
- Javitch J. A., D'Amato R. J., Strittmatter S. M., Snyder S. H. Parkinsonism-inducing neurotoxin, N-methyl-4-phenyl-1,2,3,6 -tetrahydropyridine: uptake of the metabolite N-methyl-4-phenylpyridine by dopamine neurons explains selective toxicity. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2173–2177. doi: 10.1073/pnas.82.7.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilty J. E., Lorang D., Amara S. G. Cloning and expression of a cocaine-sensitive rat dopamine transporter. Science. 1991 Oct 25;254(5031):578–579. doi: 10.1126/science.1948035. [DOI] [PubMed] [Google Scholar]
- Kish P. E., Fischer-Bovenkerk C., Ueda T. Active transport of gamma-aminobutyric acid and glycine into synaptic vesicles. Proc Natl Acad Sci U S A. 1989 May;86(10):3877–3881. doi: 10.1073/pnas.86.10.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knigge K. M., Hoffman G., Scott D. E., Sladek J. R., Jr Identification of catecholamine and luteinizing hormone-releasing hormone (LHRH)-containing neurons in primary cultures of dispersed cells of the basal hypothalamus. Brain Res. 1977 Jan 28;120(3):393–405. doi: 10.1016/0006-8993(77)90394-8. [DOI] [PubMed] [Google Scholar]
- Krueger M. J., Singer T. P., Casida J. E., Ramsay R. R. Evidence that the blockade of mitochondrial respiration by the neurotoxin 1-methyl-4-phenylpyridinium (MPP+) involves binding at the same site as the respiratory inhibitor, rotenone. Biochem Biophys Res Commun. 1990 May 31;169(1):123–128. doi: 10.1016/0006-291x(90)91442-u. [DOI] [PubMed] [Google Scholar]
- Langston J. W., Ballard P., Tetrud J. W., Irwin I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983 Feb 25;219(4587):979–980. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- Langston J. W., Irwin I., Langston E. B., Forno L. S. Pargyline prevents MPTP-induced parkinsonism in primates. Science. 1984 Sep 28;225(4669):1480–1482. doi: 10.1126/science.6332378. [DOI] [PubMed] [Google Scholar]
- Markey S. P., Johannessen J. N., Chiueh C. C., Burns R. S., Herkenham M. A. Intraneuronal generation of a pyridinium metabolite may cause drug-induced parkinsonism. Nature. 1984 Oct 4;311(5985):464–467. doi: 10.1038/311464a0. [DOI] [PubMed] [Google Scholar]
- Mizuno Y., Ohta S., Tanaka M., Takamiya S., Suzuki K., Sato T., Oya H., Ozawa T., Kagawa Y. Deficiencies in complex I subunits of the respiratory chain in Parkinson's disease. Biochem Biophys Res Commun. 1989 Sep 29;163(3):1450–1455. doi: 10.1016/0006-291x(89)91141-8. [DOI] [PubMed] [Google Scholar]
- Pacholczyk T., Blakely R. D., Amara S. G. Expression cloning of a cocaine- and antidepressant-sensitive human noradrenaline transporter. Nature. 1991 Mar 28;350(6316):350–354. doi: 10.1038/350350a0. [DOI] [PubMed] [Google Scholar]
- Parker W. D., Jr, Boyson S. J., Parks J. K. Abnormalities of the electron transport chain in idiopathic Parkinson's disease. Ann Neurol. 1989 Dec;26(6):719–723. doi: 10.1002/ana.410260606. [DOI] [PubMed] [Google Scholar]
- Ramsay R. R., Krueger M. J., Youngster S. K., Gluck M. R., Casida J. E., Singer T. P. Interaction of 1-methyl-4-phenylpyridinium ion (MPP+) and its analogs with the rotenone/piericidin binding site of NADH dehydrogenase. J Neurochem. 1991 Apr;56(4):1184–1190. doi: 10.1111/j.1471-4159.1991.tb11409.x. [DOI] [PubMed] [Google Scholar]
- Ramsay R. R., Singer T. P. Energy-dependent uptake of N-methyl-4-phenylpyridinium, the neurotoxic metabolite of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, by mitochondria. J Biol Chem. 1986 Jun 15;261(17):7585–7587. [PubMed] [Google Scholar]
- Reinhard J. F., Jr, Carmichael S. W., Daniels A. J. Mechanisms of toxicity and cellular resistance to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and 1-methyl-4-phenylpyridinium in adrenomedullary chromaffin cell cultures. J Neurochem. 1990 Jul;55(1):311–320. doi: 10.1111/j.1471-4159.1990.tb08853.x. [DOI] [PubMed] [Google Scholar]
- Reinhard J. F., Jr, Diliberto E. J., Jr, Viveros O. H., Daniels A. J. Subcellular compartmentalization of 1-methyl-4-phenylpyridinium with catecholamines in adrenal medullary chromaffin vesicles may explain the lack of toxicity to adrenal chromaffin cells. Proc Natl Acad Sci U S A. 1987 Nov;84(22):8160–8164. doi: 10.1073/pnas.84.22.8160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudnick G. ATP-driven H+ pumping into intracellular organelles. Annu Rev Physiol. 1986;48:403–413. doi: 10.1146/annurev.ph.48.030186.002155. [DOI] [PubMed] [Google Scholar]
- Scherman D., Henry J. P. Reserpine binding to bovine chromaffin granule membranes. Characterization and comparison with dihydrotetrabenazine binding. Mol Pharmacol. 1984 Jan;25(1):113–122. [PubMed] [Google Scholar]
- Shimada S., Kitayama S., Lin C. L., Patel A., Nanthakumar E., Gregor P., Kuhar M., Uhl G. Cloning and expression of a cocaine-sensitive dopamine transporter complementary DNA. Science. 1991 Oct 25;254(5031):576–578. doi: 10.1126/science.1948034. [DOI] [PubMed] [Google Scholar]
- Shoffner J. M., Watts R. L., Juncos J. L., Torroni A., Wallace D. C. Mitochondrial oxidative phosphorylation defects in Parkinson's disease. Ann Neurol. 1991 Sep;30(3):332–339. doi: 10.1002/ana.410300304. [DOI] [PubMed] [Google Scholar]
- Stern-Bach Y., Greenberg-Ofrath N., Flechner I., Schuldiner S. Identification and purification of a functional amine transporter from bovine chromaffin granules. J Biol Chem. 1990 Mar 5;265(7):3961–3966. [PubMed] [Google Scholar]
- Turski L., Bressler K., Rettig K. J., Löschmann P. A., Wachtel H. Protection of substantia nigra from MPP+ neurotoxicity by N-methyl-D-aspartate antagonists. Nature. 1991 Jan 31;349(6308):414–418. doi: 10.1038/349414a0. [DOI] [PubMed] [Google Scholar]




