Pancreatic cysts were found to be more prevalent in patients with autosomal dominant polycystic kidney disease (ADPKD) compared with matched control subjects without ADPKD in this cross-sectional study and were associated with the presence of a mutation in the PKD2 gene.
Abstract
Purpose
To define the magnetic resonance (MR) imaging prevalence of pancreatic cysts in a cohort of patients with autosomal dominant polycystic kidney disease (ADPKD) compared with a control group without ADPKD that was matched for age, sex, and renal function.
Materials and Methods
In this HIPAA-compliant, institutional review board–approved study, all patients with ADPKD provided informed consent; for control subjects, informed consent was waived. Patients with ADPKD (n = 110) with mutations identified in PKD1 or PKD2 and control subjects without ADPKD or known pancreatic disease (n = 110) who were matched for age, sex, estimated glomerular filtration rate, and date of MR imaging examination were evaluated for pancreatic cysts by using axial and coronal single-shot fast spin-echo T2-weighted images obtained at 1.5 T. Total kidney volume and liver volume were measured. Univariate and multivariable logistic regression analyses were conducted to evaluate potential associations between collected variables and presence of pancreatic cysts among patients with ADPKD. The number, size, location, and imaging characteristics of the cysts were recorded.
Results
Patients with ADPKD were significantly more likely than control subjects to have at least one pancreatic cyst (40 of 110 patients [36%] vs 25 of 110 control subjects [23%]; P = .027). In a univariate analysis, pancreatic cysts were more prevalent in patients with ADPKD with mutations in PKD2 than in PKD1 (21 of 34 patients [62%] vs 19 of 76 patients [25%]; P = .0002). In a multivariable logistic regression model, PKD2 mutation locus was significantly associated with the presence of pancreatic cysts (P = .0004) and with liver volume (P = .038). Patients with ADPKD and a pancreatic cyst were 5.9 times more likely to have a PKD2 mutation than a PKD1 mutation after adjusting for age, race, sex, estimated glomerular filtration rate, liver volume, and total kidney volume.
Conclusion
Pancreatic cysts were more prevalent in patients with ADPKD with PKD2 mutation than in control subjects or patients with PKD1 mutation.
© RSNA, 2016
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) affects up to 12 million patients worldwide and accounts for 10% of patients with end-stage renal disease (1,2). It is caused by mutations in the PKD1 and PKD2 genes and is characterized by abnormal proliferation of renal tubular epithelium, leading to massive kidney enlargement and progressive chronic kidney disease (1,2). Extrarenal manifestations involve the cardiovascular (3–5), gastrointestinal (3,6–8), and genitourinary (2,3,9) systems.
Animal models of ADPKD develop pancreatic cysts in the presence of either a PKD1 or PKD2 mutation (10–13). Pancreatic cyst prevalence in ADPKD is 5%–9% by using abdominal ultrasonography (US) (7,8). In one US study of patients with ADPKD, investigators found an association of pancreatic cysts with germline PKD1 mutations (7). However, US of the pancreas has substantial technical limitations, owing to its retroperitoneal location and interference by bowel gas (14). Magnetic resonance (MR) imaging has a higher sensitivity for detecting pancreatic cysts, with a prevalence of 14%–45% in the general population (14–18).
The purpose of this study was to define the MR imaging prevalence of pancreatic cysts in a cohort of patients with ADPKD compared with a control group without ADPKD that was matched for age, sex, and renal function.
Materials and Methods
Patients
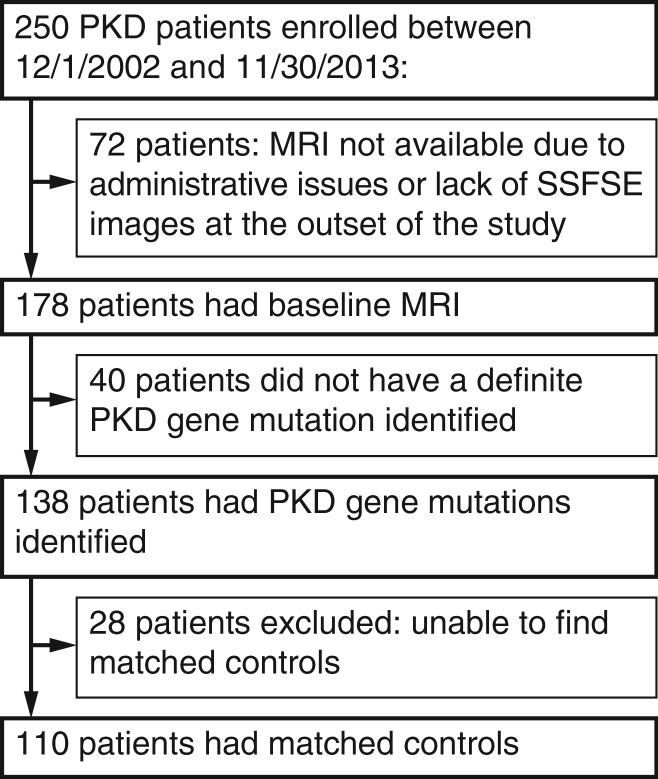
All patients with ADPKD participated in the Rogosin Institute Polycystic Kidney Disease Repository (no. NCT00792155), an ongoing, single-center, longitudinal study initiated in December 2002, designed to evaluate genotype and phenotype data (19). The study was approved by the institutional review board committees at Weill Cornell Medical College and The Rockefeller University (New York, NY), and written informed consent was obtained. Data are collected biennially on these patients with ADPKD. The study is financially supported in part by grant no. UL1RR024143 from the National Center for Advancing Translational Sciences, National Institutes of Health. Patients with ADPKD who underwent MR imaging between December 2002 and November 2013 were eligible for inclusion in this retrospective analysis of those prospectively acquired MR images. After excluding patients without a detectable mutation in PKD1 or PKD2 and patients for whom no matching control subject could be found, 110 patients with ADPKD were included in the current study (Fig 1). Pathogenic variants of PKD1 or PKD2 were determined by means of mutation analysis, as reported previously (19). Age, sex, race, height, estimated glomerular filtration rate (eGFR) attained by using the serum creatinine level obtained within 3 months of the MR imaging examination, and PKD genotype data were obtained from the Rogosin Institute ADPKD repository database.
Figure 1:
Flowchart shows the accrual flow, demonstrating subject enrollment in the study. PKD = polycystic kidney disease, SSFSE = single-shot fast spin-echo.
The control group comprised patients who underwent abdominal MR imaging and were randomly selected from the picture archiving and communication software database at Weill Cornell Medical College. Retrospective collection of data from control subjects was compliant with the Health Insurance Portability and Accountability Act and was institutional review board approved; informed consent was not required. Individuals with ADPKD or those who were known to have or were suspected of having pancreatic disease were excluded from the control group. By using clinical and laboratory data obtained retrospectively from their medical records, control subjects were matched with the ADPKD group for age, sex, eGFR, and date of MR imaging performed during their initial visit in the ADPKD repository study. Only the data needed for matching were obtained from the control subjects. For patients with ADPKD who underwent multiple follow-up MR imaging examinations, only the images from their first MR examination were analyzed for this study. To avoid bias related to changes in MR imaging techniques during the 11-year period of recruitment for the ADPKD repository study, control subjects and patients with ADPKD were also matched so that their MR imaging examinations were performed within 1 year of each other. eGFR was calculated with the four-variable Modification of Diet in Renal Disease study formula by using the serum creatinine level obtained within 3 months of the MR imaging examination. The medical records of patients with ADPKD and pancreatic cysts were reviewed for any reports of procedures and pathologic findings.
PKD Gene Sequence Analysis
Genomic DNA was extracted from peripheral blood lymphocytes by using a Gentra Puregene blood kit (Qiagen, Valencia, Calif). PKD1 (reference sequence NM_000296.2) and PKD2 (reference sequence NM_000297.2) were analyzed by using Surveyor Nuclease Wave (Transgenomic, Omaha, Neb) denaturing high-performance liquid chromatography screening, followed by confirmatory sequencing (19) or directly by Sanger sequencing (20) by using an ABI 3130 genetic analyzer (Applied Biosystems, Foster City, Calif). Alternatively, long-range polymerase chain reaction products covering the entire exonic regions and most of the 5ʹ and 3ʹ untranslated regions of PKD1 and PKD2 were sequenced by using next-generation sequencing on an Illumina MiSeq system (San Diego, Calif), followed by confirmatory Sanger sequencing of the pathogenic mutations (21). To avoid amplification of PKD1 pseudogenes, PKD1 was amplified by using primers anchored either in the rare mismatched region with the human homologs or in the single-copy region of PKD1. Sanger and next-generation sequencing data were analyzed by using Mutation Surveyor software version 4.0 (Soft Genetics, State College, Pa) (20) and the laboratory-developed pipeline (21), respectively.
Imaging Protocol
All patients underwent imaging at 1.5 T (Signa Excite 12.0–15.0; General Electric, Waukesha, Wis) by using a body phased-array coil. Axial and/or coronal SSFSE images were used to evaluate pancreatic cysts. Coronal and axial SSFSE images were obtained over single or multiple breath holds by using the parameters presented in Table 1.
Table 1.
Imaging Parameters
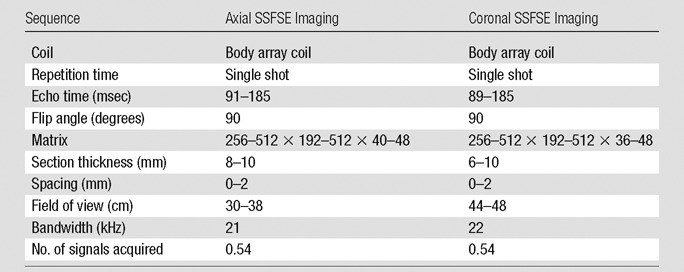
Note.—For imaging, a Signa Excite 12.0–15.0 (General Electric) MR imaging unit was used.
Image Analysis
Pancreatic cysts were identified on baseline axial and/or coronal SSFSE images in all patients with ADPKD and control subjects. MR images were reviewed independently by three radiologists (M.R.P., J.K., S.C.), one attending physician with 20 years of body MR imaging experience and two 2nd-year MR imaging fellows. Consensus among all readers, with repeat review of images, was used to resolve differences. This was necessary in 12% of the population (27 of 220 individuals). All viewers were blinded to the clinical, laboratory, and PKD mutation data; blinding of ADPKD status was not possible because this diagnosis was apparent on MR images. Pancreatic cyst maximum dimension was measured from inner wall to inner wall (Fig 2); a minimum threshold diameter of 2 mm was used. The number, size, location, and imaging characteristics of pancreatic cysts were recorded. The volumes of both kidneys and the liver of each patient were measured by tracing the outer boundary on a three-dimensional workstation (Advantage Windows workstation 4.1 software; General Electric).
Figure 2:
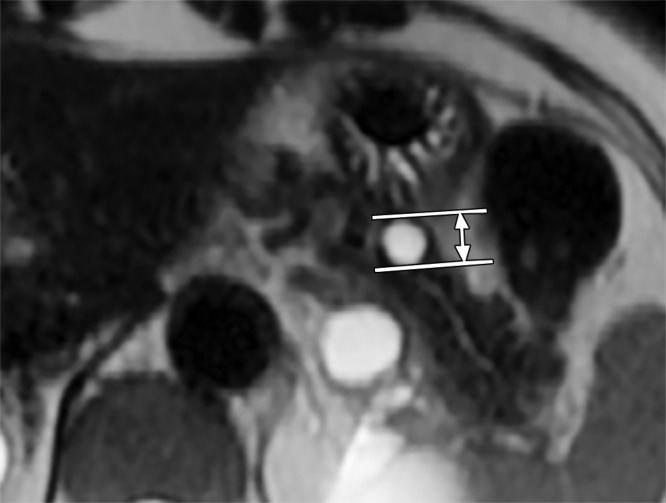
Axial SSFSE MR image shows that cyst size was measured by using an electronic caliper from the inner wall to the inner wall. In this case, it was 11 mm.
When follow-up studies of patients with ADPKD were available, changes in the imaging characteristics of pancreatic cysts were evaluated.
Statistical Analysis
Continuous variables are presented as means with standard deviations or as medians with interquartile ranges. Log transformations of total kidney volume (TKV) and liver volume were used to establish normal distributions. Differences in demographic variables between patients and control subjects were assessed with the Student t test, Wilcoxon test, or χ2 test, as appropriate. A χ2 test was used to evaluate any difference in the prevalence of cysts in patients with a PKD1 or PKD2 gene mutation and the presence or absence of cysts in patients with ADPKD. The Wilcoxon rank sum test was used to evaluate any associations between pancreas cyst properties and characteristics of PKD gene mutations. The Fisher exact test was used to evaluate any associations between the presence of cysts in patients with ADPKD and location of mutations in PKD1 and PKD2.
Univariate and multivariable logistic regression analyses helped determine whether variables were associated with presence of pancreatic cysts in patients with ADPKD. Covariates included in the models, selected on the basis of pathophysiological considerations, were age, sex, race, eGFR, log transformation of height-adjusted TKV and liver volume, and PKD gene mutation locus. The interaction between age and eGFR was also evaluated. All P values were two sided and were evaluated at the .05 α level. Interobserver reliability was evaluated by using the κ statistic for a binary “yes or no” presence of cysts, and an intraclass correlation coefficient for a continuous number of cysts was identified. All analyses were performed with SAS version 9.3 software (SAS Institute, Cary, NC).
Results
This study included 110 patients with ADPKD and 110 control subjects who were matched for age, sex, and eGFR (Table 2). Clinical indications for the MR imaging studies of the control subjects were evaluation for hepatobiliary lesions (n = 63), gastrointestinal lesions (n = 16), renal lesions (n = 8), pelvic lesions (n = 5), or other lesions (n = 6). Although ADPKD and control groups were of predominantly white race, the prevalence of white subjects was significantly greater in the ADPKD group (97 of 110 vs 82 of 110, respectively; P = .009). The ADPKD group had a threefold greater height-adjusted TKV (640 mL vs 197 mL, respectively; P < .0001) and significantly greater height-adjusted liver volume (1102 mL vs 896 mL, respectively; P < .0001) than did control subjects. In the ADPKD group, 36.4% of patients (40 of 110) had at least one pancreatic cyst, compared with 22.7% of control subjects (25 of 110; P = .027; Table 2). The distribution and mean size of pancreatic cysts did not differ in the ADPKD and control groups (Table E1 [online]). There was high reader agreement and reliability among the radiologists who evaluated the MR images (κ = 0.84 [P < .0001]; intraclass correlation coefficient = 0.97; 95% confidence interval: 0.96, 0.98).
Table 2.
Comparison of Clinical and Radiologic Features, Including Pancreatic Cyst Prevalence in Patients with ADPKD and Control Subjects
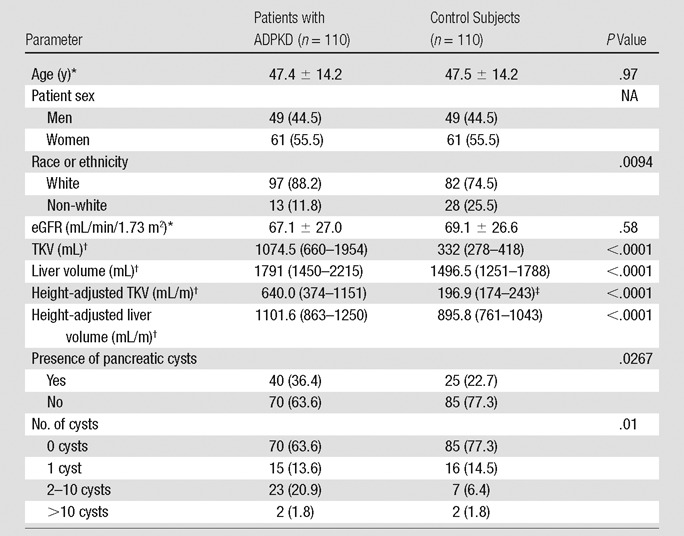
Note.—Numbers in parentheses are percentages, unless indicated otherwise. NA = not applicable.
*Data are means ± standard deviations.
†Data are medians, and numbers in parentheses are interquartile ranges.
‡Height data were missing in seven control subjects.
In the ADPKD group, 69% (76 of 110 patients) had a PKD1 mutation, and 31% (34 of 110 patients) had a PKD2 mutation (Table 3). Patients with a PKD2 mutation were older than those with a PKD1 mutation (53.0 years vs 44.9 years, respectively; P = .005). Height-adjusted TKV and liver volumes were not significantly different in the PKD1 and PKD2 groups. Although mutations in PKD1 were more prevalent overall, pancreatic cysts were more than twice as prevalent in the PKD2 group than in the PKD1 group (62% [21 of 34 patients] vs 25% [19 of 76 patients], respectively; P = .0002). The number of pancreatic cysts was also greater when a mutation in PKD2 was present; more than one cyst was found in 47% of the PKD2 group (16 of 34 patients), compared with only 12% of the PKD1 group (nine of 76 patients, P = .0004). The size and distribution of pancreatic cysts did not differ significantly in the PKD1 and PKD2 groups (Table E2 [online]).
Table 3.
Prevalence of Pancreatic Cysts according to PKD Gene Mutation
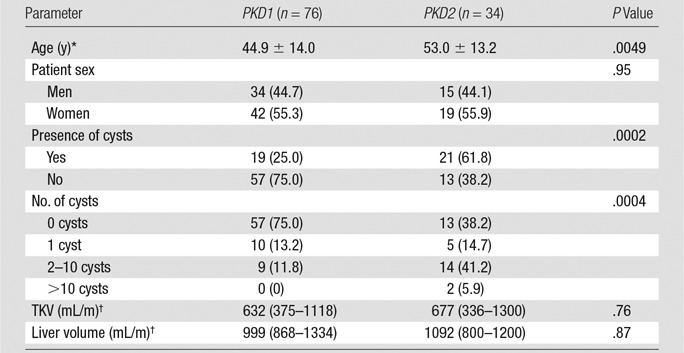
Note.—Numbers in parentheses are percentages, unless indicated otherwise.
*Data are means ± standard deviations.
†Data are medians, and numbers in parentheses are interquartile ranges.
In the univariate regression analysis, age (odds ratio [OR], 1.03; P = .04) and presence of a PKD2 mutation (OR, 4.85; P < .0001; Table 4) were associated with pancreatic cyst prevalence. In the multivariable model, an association with the presence of a PKD2 mutation remained (OR, 5.91; P = .0004), but the association with age did not persist after adjustment for confounders. The multivariable model was also used to identify an association of pancreatic cysts with log height-adjusted liver volume (OR, 3.64; P = .038); log height-adjusted TKV was of borderline significance (OR, 0.51; P = .08). There was no significant interaction between age and eGFR; thus, the analysis was performed without an age*eGFR interaction variable. There was no association of race or sex with the presence of pancreatic cysts.
Table 4.
Association of Pancreatic Cysts with Mutations in PKD2
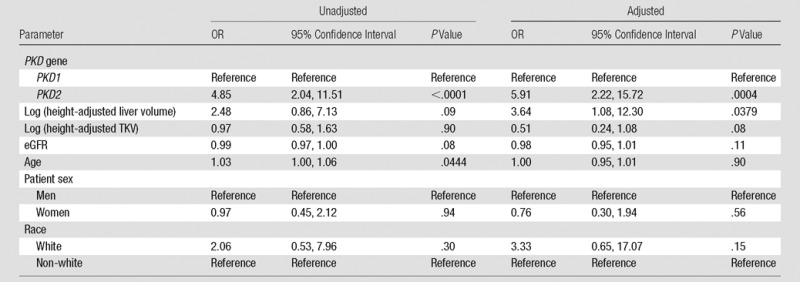
Note.—“Reference” refers to the reference group selected for analysis.
Truncating mutations were identified in 57.9% of the PKD1 group (11 of 19 patients) and 76.2% of the PKD2 group (16 of 21 patients). However, there was no association of the mutation type (truncating vs nontruncating) with the size or number of pancreatic cysts (Table E3 [online]). Moreover, there was no association of the presence of a pancreatic cyst with the mutation location in either PKD1 or PKD2 (Table E3 [online]).
Among the 40 patients with ADPKD and pancreatic cysts, six of 40 (15%) had cyst diameters that were 1 cm and larger, and five had a PKD2 mutation. Of those six patients, four had complex cysts with multilobulated or multiseptated appearance (Figs 3, 4) or internal debris (Fig 5); three had cysts with connection to the main pancreatic duct or uncinate duct, indicating possible side-branch intraductal papillary mucinous neoplasms or dilated side branches (Fig 4). One of these cysts measured 2.6 cm, with characteristics of serous cystadenoma (Fig 3). None of these six patients had symptoms or signs of pancreatic pathologic findings, and none underwent surgical resection. One patient underwent endoscopic US-guided fine-needle aspiration biopsy (Fig 4a). The pathologic findings indicated a possible low-grade, mucinous, cystic lesion.
Figure 3:
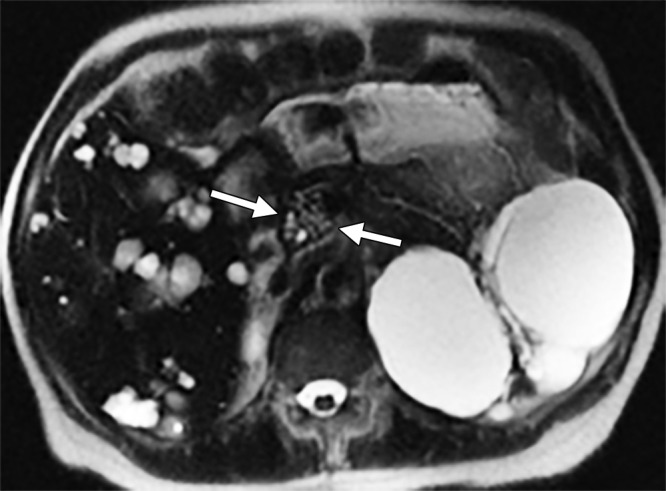
Axial MR image obtained in a 71-year-old woman with PKD2 mutation. There is a cluster of tiny cysts in the pancreatic head, measuring 26 mm in maximum dimension.
Figure 4a:
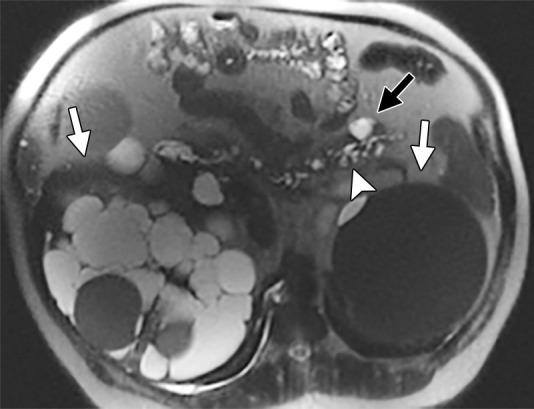
Axial MR images obtained in a 58-year old man with PKD2 mutation show multiple pancreatic cysts, with the largest in the tail of the pancreas, measuring 25 mm (black arrow). Most of the cysts demonstrate connection to the main pancreatic duct (arrowhead). Note numerous cysts in the right kidney and a large hemorrhagic cyst in the left kidney (white arrows). Endoscopic US–guided fine-needle aspiration biopsy was performed, and the result was benign. The size and the number of cysts increased in the 3 years after the biopsy. (a) Initially, there were up to 20 cysts, and the largest cyst size was 25 mm. (b) After interval biopsy and 3 years, there were at least 31 cysts, and the largest cyst size was 35 mm.
Figure 5a:
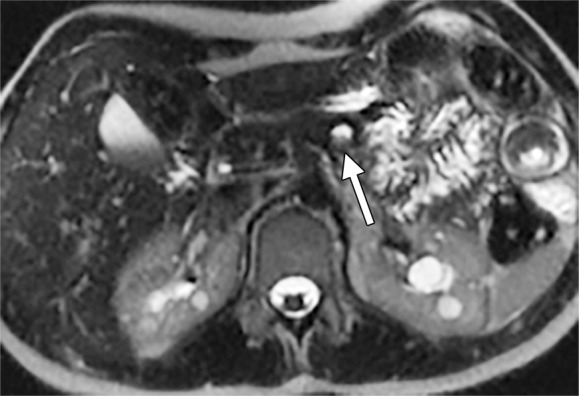
Axial MR images obtained in a 19-year old woman with PKD1 mutation. (a) A 10-mm pancreatic cyst with internal debris is seen (arrow). (b) After 3 years, the debris was cleared (arrow), with no interval size growth of the cyst.
Figure 4b:
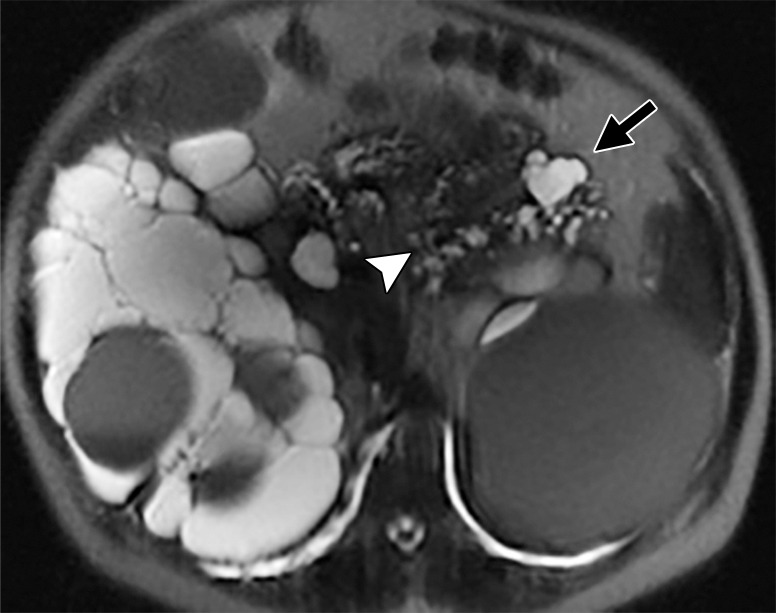
Axial MR images obtained in a 58-year old man with PKD2 mutation show multiple pancreatic cysts, with the largest in the tail of the pancreas, measuring 25 mm (black arrow). Most of the cysts demonstrate connection to the main pancreatic duct (arrowhead). Note numerous cysts in the right kidney and a large hemorrhagic cyst in the left kidney (white arrows). Endoscopic US–guided fine-needle aspiration biopsy was performed, and the result was benign. The size and the number of cysts increased in the 3 years after the biopsy. (a) Initially, there were up to 20 cysts, and the largest cyst size was 25 mm. (b) After interval biopsy and 3 years, there were at least 31 cysts, and the largest cyst size was 35 mm.
Figure 5b:
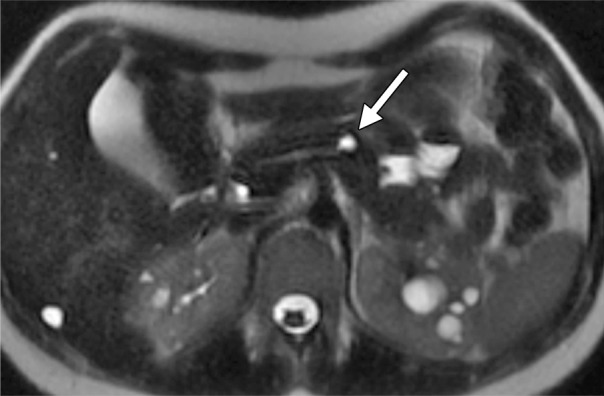
Axial MR images obtained in a 19-year old woman with PKD1 mutation. (a) A 10-mm pancreatic cyst with internal debris is seen (arrow). (b) After 3 years, the debris was cleared (arrow), with no interval size growth of the cyst.
Follow-up MR images were available for 23 patients with ADPKD and pancreatic cysts, with follow-up intervals ranging from 279 days to 7.5 years. Patients with longer follow-up intervals were more likely to show a change (P = .02; Table E4 [online]). Nine of 23 patients (39%) showed changes in pancreatic cysts (Fig 4): Five of 23 (22%) had interval increases in the number of cysts, and one of 23 (4%) had an interval decrease in the number of cysts. All nine patients with a change in pancreatic cyst size showed interval growth. Internal cyst debris cleared spontaneously in one patient during follow-up (Fig 5).
Discussion
In this cross-sectional study, we found the prevalence of pancreatic cysts to be significantly higher in an ADPKD cohort compared with a matched control population without ADPKD. Patients with ADPKD and a PKD2 gene mutation were five times more likely to have pancreatic cysts than patients in the PKD1 group; this finding persisted after adjusting for potential confounders.
PKD1 has a more aggressive disease course, with an earlier age of symptom onset, end-stage renal disease, and death (1,2). Thus, the potential to discriminate PKD1 from PKD2 on MR images has important prognostic implications. MR imaging identification of pancreatic cysts in ADPKD significantly increases the likelihood that a PKD2 mutation is present.
The mechanism(s) whereby PKD gene mutations cause pancreatic cysts has not been established. Homozygous mutations of PKD1 or PKD2 genes in animal models are embryonically lethal, causing structural cardiac defects, edema, focal hemorrhage, skeletal deformities, and development of cysts in the kidneys, liver, and pancreas (10–12,14). One generally accepted view is that ADPKD is recessive at the cellular level and that clonal proliferation of a renal tubular epithelial cell follows an inactivating somatic mutation (1,10,11,13,22,23). Rodent models of ADPKD develop pancreatic cysts when PKD1 or PKD2 is either globally or conditionally inactivated (10–12), consistent with this “two-hit” mechanism. Polycystin-2, the product of PKD2, also appears to contribute to pancreatic development at or after the point at which common pancreatic progenitor cells are committed to become ductal epithelium (10). Our current finding of an association of PKD2 mutations with pancreatic cysts supports the conclusion that polycystin-2, the gene product of PKD2, is required for the maintenance of the normal pancreatic duct structure in adults. However, causality cannot be determined from the observational data reported in this cross-sectional study.
Overall, we found PKD2 gene mutations in 30% of the patients with ADPKD in this study, which is approximately twice that reported previously (2,24–26). However, the PKD2 mutation prevalence in our cohort is similar to that in population-based studies in which PKD2 mutations were found in 24%–36% of patients (2,24–26). Higher PKD1 mutation prevalence in previous studies may have been overrepresented because of ascertainment bias, as clinical manifestations are more severe in patients with PKD1 mutations, whereas less severely affected patients with PKD2 mutations likely did not receive diagnoses (25). Torra et al (7) reported linkage to PKD1 in six patients with ADPKD and pancreatic cysts. However, their conclusions may have been confounded by the small number of patients evaluated.
The position of a PKD1 mutation (24) and the presence of a truncating mutation (25) contributed to the severity of the ADPKD phenotype in previous studies. In our study, however, neither of these variables was associated with pancreatic cyst prevalence; this may reflect the relatively small sample size.
Pancreatic cysts are more likely to be discovered incidentally at MR imaging than at US in the general population (14%–20% vs 0.21%, respectively) (14,16,17) and even more frequently at MR cholangiopancreatography (45%) (18), reflecting the higher spatial resolution and excellent image contrast of T2-weighted MR imaging. This is consistent with our finding of pancreatic cysts in more than 20% of the control population. Postmortem examinations of patients with ADPKD have yielded a prevalence of approximately 9% (27,28). However, autopsy studies can be confounded by selection bias, which limits generalization of the findings.
Few investigators have systematically evaluated pancreatic cysts in ADPKD. In the HALT Progression of Polycystic Kidney Disease, or HALT-PKD, study, a multicenter clinical trial of antihypertensive drug treatment in ADPKD in which MR imaging was limited to the coronal plane, pancreatic cysts were identified at baseline in only 1.8% of 560 patients with ADPKD, with 62% having only one cyst (29). By contrast, in our study, 36% of all patients with ADPKD (40 of 110) had at least one pancreatic cyst; 63% of those patients (25 of 40) had more than one cyst. Our findings suggest that pancreatic cysts are among the most prevalent extrarenal manifestations of ADPKD. The discrepancy between our findings and those of HALT-PKD may reflect the limited imaging plane available in HALT; pancreatic cysts are difficult to detect on coronal images (30). Moreover, the HALT-PKD population was limited to young subjects with early-stage kidney disease; the mean age was approximately 10 years younger than that in the present study. Although kidney cysts develop at a later age in ADPKD with a PKD2 mutation (31), it is unclear whether pancreatic cyst formation is associated with age. We identified an association of age with pancreatic cysts in a univariate model, but this association did not persist after adjustment for potential confounders.
The natural history and risk of complications of pancreatic cysts in ADPKD have not been established. In our study, no patient with pancreatic cysts had related symptoms or complications. However, only one patient underwent invasive evaluation (ie, cyst aspiration); thus, more complex lesions could have been undetected. In previous reports, investigators have concluded that pancreatic cysts in ADPKD are usually benign and that inflammatory or neoplastic changes that arise from these lesions rarely occur (32,33). In a large cohort from the general population referred with pancreas cysts selected for radiographic surveillance, Gaujoux et al reported a 1% incidence of pancreatic malignancy (34). However, our study was not designed to evaluate the natural history of pancreatic cysts in ADPKD. A systematic assessment of the natural history of pancreatic cysts in ADPKD is warranted to address these issues.
Strengths of this study include the characterization of clinical, laboratory, genetic, and radiologic features of the ADPKD population. ADPKD diagnosis was confirmed in all subjects by results of PKD gene mutation analysis. The control population was matched for several potential clinical and laboratory confounders, including the timing of the MR imaging procedure. Blinding of the investigators to these confounders also limited the potential for bias.
Some aspects of the study design limit the generalization of our findings. The control group was selected retrospectively from a clinical imaging database in a single tertiary care medical center. Both the ADPKD and control groups were predominantly white, and findings may differ in more diverse populations. Although dates of MR imaging testing were matched, we cannot be certain that all methodological confounders were controlled. Although the lower prevalence of pancreatic cysts in the PKD1 and control groups suggests a specific effect of PKD2 mutations in the development of pancreatic cysts in ADPKD, our cross-sectional observational study was not designed to assess mechanisms of pancreas cyst formation. Therefore, our findings should be considered hypothesis generating.
In conclusion, pancreatic cysts were found to be more prevalent in patients with ADPKD than in matched subjects without ADPKD in this cross-sectional study and were associated with the presence of a mutation in the PKD2 gene. Therefore, future studies of the pathogenesis and natural history of pancreatic cysts in ADPKD should be evaluated in the context of the PKD genotype.
Advances in Knowledge
■ Pancreatic cysts are present in 36% of patients with autosomal dominant polycystic kidney disease (ADPKD, 40 of 110) compared with 23% of matched control subjects (25 of 110; P = .027) on two-dimensional single-shot fast spin-echo MR images.
■ Patients with a PKD2 gene mutation were 4.9 times more likely to have pancreatic cysts than the PKD1 group and were 5.9 times more likely to have pancreatic cysts after adjusting for potential confounders.
Implication for Patient Care
■ In ADPKD, the presence of pancreatic cysts favors PKD2 mutation, which tends to have a later onset of renal failure when compared with PKD1.
SUPPLEMENTAL TABLES
Received August 13, 2015; revision requested September 28; revision received December 24; accepted January 13, 2016; final version accepted February 3.
Study supported by National Center for Advancing Translational Sciences (NCATS), National Institutes of Health (UL1RR024143).
Disclosures of Conflicts of Interest: J.K. disclosed no relevant relationships. J.D.B. disclosed no relevant relationships. S.C. disclosed no relevant relationships. S.P.D. disclosed no relevant relationships. N.D.T. disclosed no relevant relationships. W.O.B. disclosed no relevant relationships. S.D. disclosed no relevant relationships. H.E.R. disclosed no relevant relationships. A.Y.T. disclosed no relevant relationships. A.E.G. disclosed no relevant relationships. M.R.P. disclosed no relevant relationships.
Abbreviations:
- ADPKD
- autosomal dominant polycystic kidney disease
- eGFR
- estimated glomerular filtration rate
- OR
- odds ratio
- SSFSE
- single-shot fast spin-echo
- TKV
- total kidney volume
References
- 1.Harris PC, Torres VE. Polycystic kidney disease. Annu Rev Med 2009;60:321–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chapman AB, Devuyst O, Eckardt KU, et al. Autosomal-dominant polycystic kidney disease (ADPKD): executive summary from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int 2015;88(1):17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pirson Y. Extrarenal manifestations of autosomal dominant polycystic kidney disease. Adv Chronic Kidney Dis 2010;17(2):173–180. [DOI] [PubMed] [Google Scholar]
- 4.Ecder T, Schrier RW. Cardiovascular abnormalities in autosomal-dominant polycystic kidney disease. Nat Rev Nephrol 2009;5(4):221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schrier RW, Belz MM, Johnson AM, et al. Repeat imaging for intracranial aneurysms in patients with autosomal dominant polycystic kidney disease with initially negative studies: a prospective ten-year follow-up. J Am Soc Nephrol 2004;15(4):1023–1028. [DOI] [PubMed] [Google Scholar]
- 6.Bae KT, Zhu F, Chapman AB, et al. Magnetic resonance imaging evaluation of hepatic cysts in early autosomal-dominant polycystic kidney disease: the Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease cohort. Clin J Am Soc Nephrol 2006;1(1):64–69. [DOI] [PubMed] [Google Scholar]
- 7.Torra R, Nicolau C, Badenas C, et al. Ultrasonographic study of pancreatic cysts in autosomal dominant polycystic kidney disease. Clin Nephrol 1997;47(1):19–22. [PubMed] [Google Scholar]
- 8.Nicolau C, Torra R, Bianchi L, et al. Abdominal sonographic study of autosomal dominant polycystic kidney disease. J Clin Ultrasound 2000;28(6):277–282. [DOI] [PubMed] [Google Scholar]
- 9.Reig B, Blumenfeld J, Donahue S, Prince MR. Seminal megavesicle in autosomal dominant polycystic kidney disease. Clin Imaging 2015;39(2):289–292. [DOI] [PubMed] [Google Scholar]
- 10.Wu G, Markowitz GS, Li L, et al. Cardiac defects and renal failure in mice with targeted mutations in Pkd2. Nat Genet 2000;24(1):75–78. [DOI] [PubMed] [Google Scholar]
- 11.Lu W, Peissel B, Babakhanlou H, et al. Perinatal lethality with kidney and pancreas defects in mice with a targetted Pkd1 mutation. Nat Genet 1997;17(2):179–181. [DOI] [PubMed] [Google Scholar]
- 12.Boulter C, Mulroy S, Webb S, Fleming S, Brindle K, Sandford R. Cardiovascular, skeletal, and renal defects in mice with a targeted disruption of the Pkd1 gene. Proc Natl Acad Sci U S A 2001;98(21):12174–12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boucher C, Sandford R. Autosomal dominant polycystic kidney disease (ADPKD, MIM 173900, PKD1 and PKD2 genes, protein products known as polycystin-1 and polycystin-2). Eur J Hum Genet 2004;12(5):347–354. [DOI] [PubMed] [Google Scholar]
- 14.Zhang XM, Mitchell DG, Dohke M, Holland GA, Parker L. Pancreatic cysts: depiction on single-shot fast spin-echo MR images. Radiology 2002;223(2):547–553. [DOI] [PubMed] [Google Scholar]
- 15.Macari M, Megibow AJ. Focal cystic pancreatic lesions: variability in radiologists’ recommendations for follow-up imaging. Radiology 2011;259(1):20–23. [DOI] [PubMed] [Google Scholar]
- 16.Kalb B, Sarmiento JM, Kooby DA, Adsay NV, Martin DR. MR imaging of cystic lesions of the pancreas. RadioGraphics 2009;29(6):1749–1765. [DOI] [PubMed] [Google Scholar]
- 17.Lee KS, Sekhar A, Rofsky NM, Pedrosa I. Prevalence of incidental pancreatic cysts in the adult population on MR imaging. Am J Gastroenterol 2010;105(9):2079–2084. [DOI] [PubMed] [Google Scholar]
- 18.Girometti R, Intini S, Brondani G, et al. Incidental pancreatic cysts on 3D turbo spin echo magnetic resonance cholangiopancreatography: prevalence and relation with clinical and imaging features. Abdom Imaging 2011;36(2):196–205. [DOI] [PubMed] [Google Scholar]
- 19.Tan YC, Blumenfeld JD, Anghel R, et al. Novel method for genomic analysis of PKD1 and PKD2 mutations in autosomal dominant polycystic kidney disease. Hum Mutat 2009;30(2):264–273. [DOI] [PubMed] [Google Scholar]
- 20.Tan YC, Michaeel A, Blumenfeld J, et al. A novel long-range PCR sequencing method for genetic analysis of the entire PKD1 gene. J Mol Diagn 2012;14(4):305–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan AY, Michaeel A, Liu G, et al. Molecular diagnosis of autosomal dominant polycystic kidney disease using next-generation sequencing. J Mol Diagn 2014;16(2):216–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pei Y, Watnick T, He N, et al. Somatic PKD2 mutations in individual kidney and liver cysts support a “two-hit” model of cystogenesis in type 2 autosomal dominant polycystic kidney disease. J Am Soc Nephrol 1999;10(7):1524–1529. [DOI] [PubMed] [Google Scholar]
- 23.Harris PC. What is the role of somatic mutation in autosomal dominant polycystic kidney disease? J Am Soc Nephrol 2010;21(7):1073–1076. [DOI] [PubMed] [Google Scholar]
- 24.Kurashige M, Hanaoka K, Imamura M, et al. A comprehensive search for mutations in the PKD1 and PKD2 in Japanese subjects with autosomal dominant polycystic kidney disease. Clin Genet 2015;87(3):266–272. [DOI] [PubMed] [Google Scholar]
- 25.Barua M, Cil O, Paterson AD, et al. Family history of renal disease severity predicts the mutated gene in ADPKD. J Am Soc Nephrol 2009;20(8):1833–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rossetti S, Adeva M, Kubly V, Consugar MB, Torres VE, Harris PC. An Olmsted County population-based study indicates that PKD2 is more common than previously described [abstr]. J Am Soc Nephrol 2007;18:365A. [Google Scholar]
- 27.Dalgaard OZ. Bilateral polycystic disease of the kidneys; a follow-up of two hundred and eighty-four patients and their families. Acta Med Scand Suppl 1957;328:1–255. [PubMed] [Google Scholar]
- 28.Fick GM, Johnson AM, Hammond WS, Gabow PA. Causes of death in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 1995;5(12):2048–2056. [DOI] [PubMed] [Google Scholar]
- 29.Hogan MC, Abebe K, Torres VE, et al. Liver involvement in early autosomal-dominant polycystic kidney disease. Clin Gastroenterol Hepatol 2015;13(1):155–164.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.King BF, Torres VE, Brummer ME, et al. Magnetic resonance measurements of renal blood flow as a marker of disease severity in autosomal-dominant polycystic kidney disease. Kidney Int 2003;64(6):2214–2221. [DOI] [PubMed] [Google Scholar]
- 31.Harris PC, Bae KT, Rossetti S, et al. Cyst number but not the rate of cystic growth is associated with the mutated gene in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 2006;17(11):3013–3019. [DOI] [PubMed] [Google Scholar]
- 32.Sato Y, Mukai M, Sasaki M, et al. Intraductal papillary-mucinous neoplasm of the pancreas associated with polycystic liver and kidney disease. Pathol Int 2009;59(3):201–204. [DOI] [PubMed] [Google Scholar]
- 33.Niv Y, Turani C, Kahan E, Fraser GM. Association between pancreatic cystadenocarcinoma, malignant liver cysts, and polycystic disease of the kidney. Gastroenterology 1997;112(6):2104–2107. [DOI] [PubMed] [Google Scholar]
- 34.Gaujoux S, Brennan MF, Gonen M, et al. Cystic lesions of the pancreas: changes in the presentation and management of 1,424 patients at a single institution over a 15-year time period. J Am Coll Surg 2011;212(4):590–600; discussion 600–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.