Abstract
Yin Yang 1 (YY1) regulates both gene expression and protein modifications, and has shown a proliferative role in cancers. In this study, we demonstrate that YY1 promotes AKT phosphorylation at S473, a marker of AKT activation. YY1 expression positively correlated with AKT(S473) phosphorylation in a tissue microarray and cultured cells of breast cancer, but negatively associated with the distant metastasis-free survival of 166 breast cancer patients. YY1 promotes AKT phosphorylation at S473 through direct interaction with AKT, and the AKT-binding site is mapped to the residues G201–S226 on YY1. These residues are also involved in YY1 interaction with Mdm2, Ezh2, and E1A, and thus are designated as the oncogene protein binding (OPB) domain. YY1-promoted AKT phosphorylation relies on the OPB domain but is independent of either transcriptional activity of YY1 or the activity of phosphoinositide-3-kinases. We also determine that YY1-promoted mTORC2 access to AKT leads to its phosphorylation at S473. Importantly, a peptide based on the OPB domain blocks YY1 interaction with AKT and reduces AKT phosphorylation and cell proliferation. Thus, we demonstrate for the first time that YY1 promotes mTORC2-mediated AKT activation and disrupting YY1–AKT interaction by OPB domain-based peptide may represent a potential strategy for cancer therapy.
Keywords: Yin Yang 1, AKT, OPB domain, phosphorylation, oncoproteins, breast cancer
Introduction
Although discovered as a transcription factor (Shi et al., 1991), Yin Yang 1 (YY1) has been found present in both nucleus and cytoplasm in cultured cells (Palko et al., 2004; Rizkallah and Hurt, 2009; de Nigris et al., 2010) as well as tumor tissues (de Nigris et al., 2010; Wan et al., 2012), which suggests multiple functions of YY1 beyond its transcriptional activity. For instance, YY1 promotes p53 and p27 ubiquitination independently of its transcriptional or DNA-binding activity (Gronroos et al., 2004; Sui et al., 2004; Wan et al., 2012).
The YY1 gene locus at the chromosome 14q encodes six transcript isoforms and two (7.5 and 2.9 kb) of them are overexpressed (Chinnappan et al., 2009). Previous studies demonstrated genetic alterations of YY1 in cancers. Recurrent somatic YY1(T372R) mutation was determined in insulinoma, a major type of pancreatic neuroendocrine tumors (PNETs) (Cao et al., 2013). YY1 gene fusion with Ewing sarcoma breakpoint region 1 (EWSR1) was identified in mesothelioma (Panagopoulos et al., 2013). Recently, YY1 was shown to play an oncogenic role in cancer studies (Zhang et al., 2011), which was consistent with the finding that the YY1 promoter contains G-quadruplex structures, a signature of many oncogenes including c-Myc and Bcl-2 (Huang et al., 2012). On the one hand, YY1 promotes growth, migration, invasion, and morphological changes of nontumorigenic breast cells. On the other hand, YY1 contributes to maintaining the tumorigenicity of breast cancer cells (Wan et al., 2012).
In breast cancer, YY1 promotes multiple proliferative signals involved in mammary oncogenesis. YY1 activates the expression of breast cancer oncogene ERBB2 (Begon et al., 2005; Allouche et al., 2008), but an inverse correlation between YY1 and ERBB2 proteins was also shown (Powe et al., 2009). YY1 antagonizes p53 (Sui et al., 2004; Yakovleva et al., 2004), with its role in p53-deficient breast cancer unclear. YY1 recruits Ezh2 for gene silencing (Wilkinson et al., 2006), while disrupted YY1–Ezh2 interaction did not affect global histone H3K27 methylation (Basu et al., 2010). Therefore, additional mechanisms may take place for YY1, in particular cytoplasmic YY1, in cancer cells to exert its proliferative and oncogenic activity (Krippner-Heidenreich et al., 2005; Seligson et al., 2005; Wan et al., 2012).
As an oncogene, AKT transmits external proliferative signals and promotes numerous cell survival pathways. Fully activated AKT requires phosphorylation of both S473 and T308, pAKT(S473) and pAKT(T308), catalyzed by mTORC2 and PDK1, respectively (Manning and Cantley, 2007). Phosphoinositide-3-kinases (PI3Ks) produce phosphatidyl-inositol-3,4,5-trisphosphate (PIP3) that recruits AKT to the membrane through binding to its Pleckstrin homology (PH) domain, which is essential for AKT activation. AKT deactivation is mediated by two phosphatases, PHLPP2 and PP2A, that remove the phosphate groups on S473 and T308 of AKT, respectively (Brognard et al., 2007).
In this study, we demonstrate that YY1 directly interacts with AKT and promotes mTORC2-mediated AKT phosphorylation at S473 independent of either YY1-mediated transcription or PI3K activity. The residues 201–226 on YY1 were named as REPO based on its role in recruiting polycomb group proteins to YY1-targeted promoters (Wilkinson et al., 2006, 2010). Since this region is involved in the interaction between YY1 and multiple oncogene products, including Mdm2, Ezh2, E1A, and AKT (presented in this study), we designated it as the oncogene protein binding (OPB) domain of YY1. Importantly, we show that blocking YY1 interaction with the oncoproteins reduces breast cancer cell proliferation, suggesting its potential for therapeutic target.
Results
YY1 expression positively correlates with AKT phosphorylation
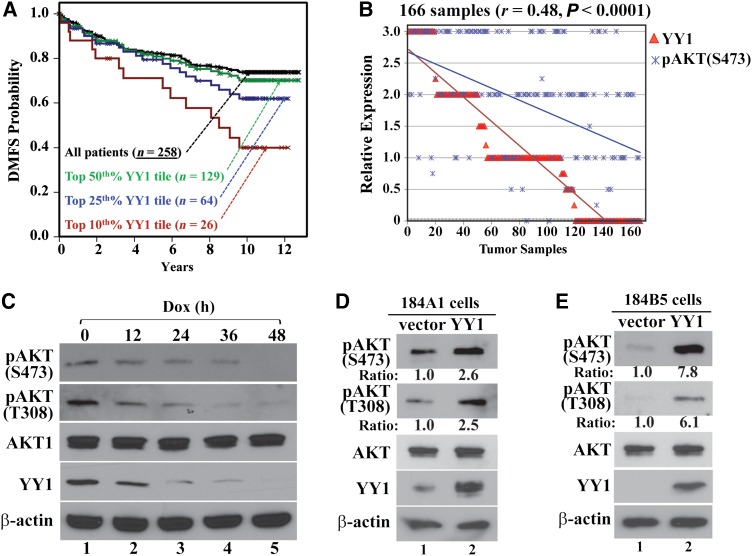
We recently reported that YY1 depletion reduced both proliferation and xenograft tumor growth of breast cancer cells (Wan et al., 2012). To evaluate whether YY1 associates with clinical outcomes, we analyzed a gene array dataset consisting of samples from 258 breast cancer patients (Miller et al., 2005). YY1 levels of all samples and three top YY1 expression tiles (50th%, 25th%, and 10th%) correlated monotonically with the decreasing distant metastasis-free survival (DMFS) of the patients (Figure 1A), suggesting the potential of YY1 as a prognostic marker for breast cancer patients.
Figure 1.
Correlations between YY1 expression and AKT phosphorylation. (A) The correlation between YY1 expression and distant metastasis-free survival (DMFS) in 258 breast cancer patients (Miller et al., 2005). YY1 expression levels in all samples were analyzed by three probes (Wan et al., 2012) and shown as three top YY1 tiles (50th%, 25th%, and 10th%). P = 0.047 by Cox proportional hazards regression (hazard ratio = 2.74 with 95% confidence interval of 1.03–7.29). (B) The correlation between YY1 and pAKT(S473) levels in samples from 166 breast cancer patients. (C) YY1 knockdown by Dox-induced shYY1-1 decreased AKT phosphorylation in MDA-MB-231 cells. Dox-treated cells were collected at the indicated time points, and cell lysates were analyzed by western blot for pAKT(S473), pAKT(T308), AKT1, YY1, and β-actin. (D and E) Ectopic YY1 expression increased AKT phosphorylation. Both 184A1 (D) and 184B5 (E) cells were infected with lentivirus carrying an empty vector or expressing YY1. The cells were analyzed for AKT phosphorylation 3 days post infection.
Since AKT is a key regulator of multiple cell proliferation and survival pathways (Dummler et al., 2006), we asked whether YY1 is involved in AKT activation. We studied YY1 expression and AKT(S473) phosphorylation, a marker of its activation, in a tissue microarray derived from 166 breast cancer patients (Wan et al., 2012), and observed a significant positive correlation between YY1 and pAKT(S473) immunostaining (r = 0.48; P < 0.0001) (Figure 1B). Estrogen receptor (ER) status was available for 147 samples (80 ER+ and 67 ER−); the correlation between YY1 and pAKT(S473) levels was more pronounced in ER− (r = 0.51; P = 0.0203) than ER+ (r = 0.46, P < 0.0001) samples (Supplementary Figure S1A and B). Progesterone receptor (PR) status was available for 146 samples (63 PR+ and 83 PR−); YY1 and pAKT(S473) correlation was higher in PR− (r = 0.56; P < 0.0001) than PR+ (r = 0.39, P = 0.0015) samples (Supplementary Figure S1C and D).
To determine whether YY1 is necessary for AKT phosphorylation in breast cancer cells, we studied AKT phosphorylation in MDA-MB-231 cells with YY1 depletion. When YY1 was individually knocked down by three different YY1 shRNAs (shYY1-1, shYY1-2, and shYY1-3, Supplementary Figure S2A), both pAKT(S473) and pAKT(T308) levels decreased (Figure 1C and Supplementary Figure S3A and B). Same results were observed in other breast cancer cell lines, including ZR-75-1, BT-474, and SK-BR-3 (Supplementary Figure S3C). Conversely, ectopically expressing YY1 in nontumorigenic 184A1 and 184B5 cells markedly increased pAKT(S473) and pAKT(T308) levels (Figure 1D and E). In these experiments, total AKT levels remained unchanged, suggesting that YY1 stimulated AKT phosphorylation.
YY1 colocalizes with phosphorylated AKT
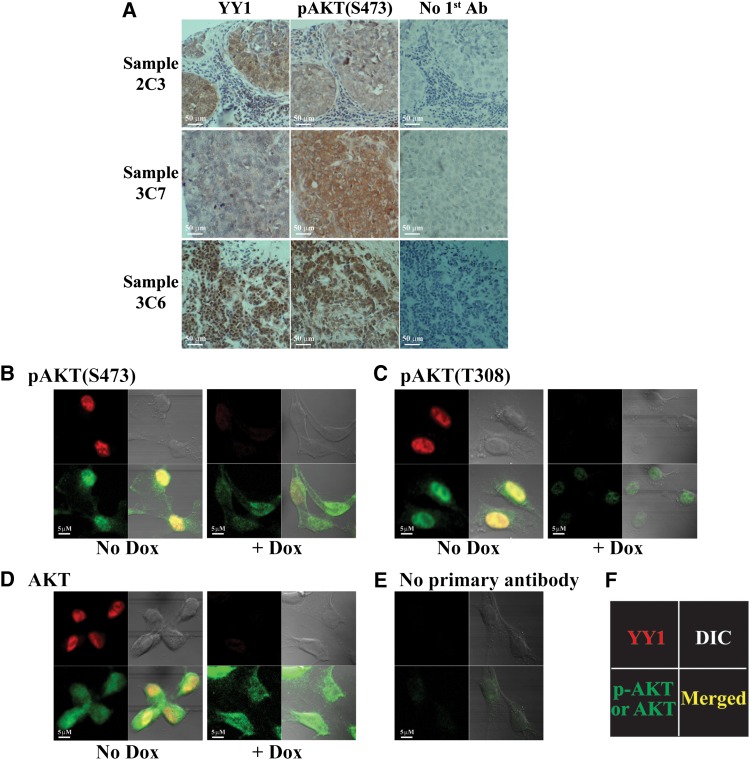
Among YY1-positive staining samples, ∼16% showed cytoplasmic YY1 staining, 35% showed nuclear staining, and the rest showed both cytoplasmic and nuclear YY1 signals. Our observation of YY1 cytoplasmic presence in breast cancer tissues is consistent with previous studies (Palko et al., 2004; Rizkallah and Hurt, 2009; Wan et al., 2012). AKT phosphorylation or activation has been reported in both cytoplasm and nucleus (Wang and Brattain, 2006). In the breast cancer samples tested, YY1 staining correlated with pAKT(S473) signal (Figure 2A). In cultured tumor cells, YY1 predominantly localizes in nuclei, unless cells undergo mitosis (Krippner-Heidenreich et al., 2005; Rizkallah and Hurt, 2009). We co-stained YY1 with phosphorylated or total AKT in MDA-MB-231 cells expressing doxycycline (Dox)-induced shYY1-1. Phosphorylated AKT was predominantly stained in nuclei, while total AKT was detected in both nuclei and cytoplasm (Figure 2B–D). Without YY1 knockdown (no Dox), YY1 signal generally overlapped with both pAKT(S473) and pAKT(T308) staining in nuclei (Figure 2B and C). With YY1 knockdown (+Dox), phospho-AKT, but not total AKT, became generally weaker, consistent with the aforementioned western blot results. No significant signal was detected in cells without primary antibody treatment (Figure 2E). These data suggest a regulatory role of YY1 in AKT phosphorylation. After fractionating nuclear and cytoplasmic portions of MDA-MB-231 cells, we detected signals for pAKT(S473), pAKT(T308), and YY1 in both nucleus and cytoplasm (Supplementary Figure S3D).
Figure 2.
Immunostaining of YY1 and pAKT in breast cancer tissues and cells. (A) Examples of YY1 and pAKT(S473) staining in breast cancer tissue samples. Top and middle panels are two samples with cytoplasmic YY1 staining, while the bottom panel is a sample with nuclear YY1 staining. (B–D) Immunostaining of YY1, pAKT(S473), pAKT(T308), and total AKT in MDA-MB-231 cells without and with Dox-induced YY1 knockdown. (E) Immunostaining without primary antibodies as background control. (F) The orientation of the antibodies used. DIC, differential interference contrast.
YY1 directly interacts with AKT
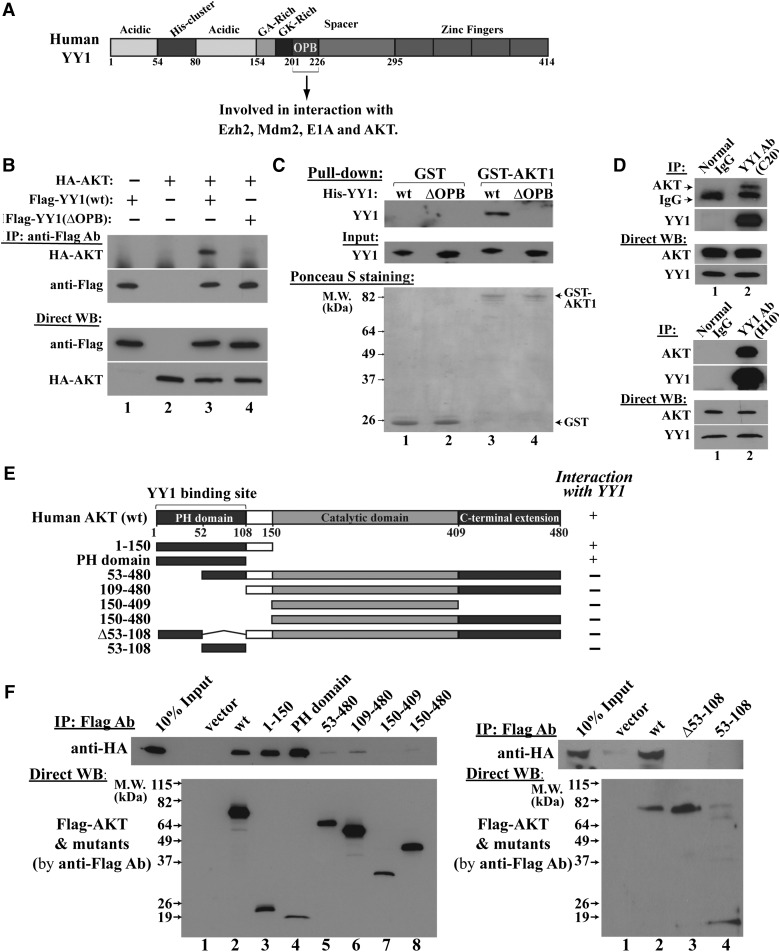
The residues G201–S226 on YY1 are involved in binding to Mdm2, Ezh2, and E1A (Lee et al., 1995; Lewis et al., 1995; Sui et al., 2004). Based on our previous study (Sui et al., 2004), we further defined that the G201–S226 region of YY1 is the binding site for Mdm2 and essential for its activity in promoting Mdm2-mediated p53 ubiquitination (Supplementary Figure S4A–C). Due to its interaction with the oncogene products, we named the G201–S226 region of YY1 as OPB domain (Figure 3A). We found that deletion of OPB domain did not alter YY1-mediated expression of Cdc6, a validated YY1 target gene (Schlisio et al., 2002; Deng et al., 2007) (Supplementary Figure S4D). These data, together with that wild-type (wt) YY1 and YY1(ΔOPB) equally bound to p53 (Supplementary Figure S4B), suggest that the OPB domain deletion did not dramatically distort YY1 protein structure. We then tested whether the YY1 OPB domain is required for AKT binding. In co-immunoprecipitation (co-IP) studies, Flag-YY1(wt), but not Flag-YY1(ΔOPB), brought down HA-AKT (Figure 3B), suggesting that the OPB domain is essential to YY1–AKT association. Furthermore, purified GST-AKT interacted with His-YY1(wt), but not His-YY1(ΔOPB) (Figure 3C), indicating a direct AKT binding to the YY1 OPB domain. Importantly, endogenous YY1 and AKT in MDA-MB-231 cells interacted in a co-IP study using two different YY1 antibodies (Figure 3D). Thus, our protein binding experiments indicate that AKT directly binds to the YY1 OPB domain and this binding unlikely requires a phosphorylation status of AKT, because the proteins expressed and purified from bacteria can interact.
Figure 3.
AKT and YY1 have direct interaction. (A) Schematic domain structure of YY1 protein. The OPB domain interacts with Ezh2, Mdm2, E1A, and AKT. (B) Co-IP studies in HeLa cells co-transfected with HA-AKT and Flag-YY1(wt) or Flag-YY1(ΔOPB). The cell lysates were IPed by Flag antibody and analyzed by western blot using the indicated antibodies. (C) In vitro protein binding assay for purified GST or GST-AKT and His-YY1 or His-YY1(ΔOPB), followed by western blot using a YY1 antibody (H-10). Ponceau S-stained membrane shows relative amounts of GST and GST-AKT. (D) The interaction of endogenous YY1 and AKT in MDA-MB-231 cells. Top panel, IPed by YY1 (C-20, rabbit IgG) and western blot for AKT and YY1 (H-10). Bottom panel, IPed by YY1 (H-10, mouse IgG) and western blot for AKT and YY1 (H-414, rabbit IgG). Positions of YY1-IPed AKT and IgG heavy chain are indicated. (E) Schematic domain structure of AKT wt and mutants, with their interactions with HA-YY1 in co-IP studies shown at the right. (F) Western blots of co-IP samples from cells co-transfected with HA-YY1 plasmid with Flag-AKT constructs (shown in E) using the indicated antibodies.
To determine YY1-binding site on AKT protein, we generated multiple AKT mutants (Figure 3E) with a Flag tag driven by the CMV promoter. These Flag-AKT constructs were co-transfected with pcDNA3/HA-YY1 into HeLa cells and the cell lysates were co- immunoprecipitated (co-IPed) by Flag antibody-conjugated agarose, followed by western blot analyses using antibodies against Flag and HA epitopes. As shown in Figure 3F left panel, AKT mutants (PH domain, or 1–108) and (1–150) are sufficient to bring down HA-YY1, while deletion of the first 52 amino acids, i.e. AKT mutant (53–480), abolished the interaction. To determine whether the residues 53–108 of the PH domain are necessary for YY1 binding, we generated AKT mutants (Δ53–108) and (53–108) for co-IP study. Deletion of 53–108 residues abolished AKT binding to YY1, but AKT(53–108) did not interact with YY1 either (Figure 3F, right panel). These data suggest that the intact PH domain is necessary for AKT interaction with YY1.
The OPB domain of YY1 is essential to YY1-mediated AKT phosphorylation
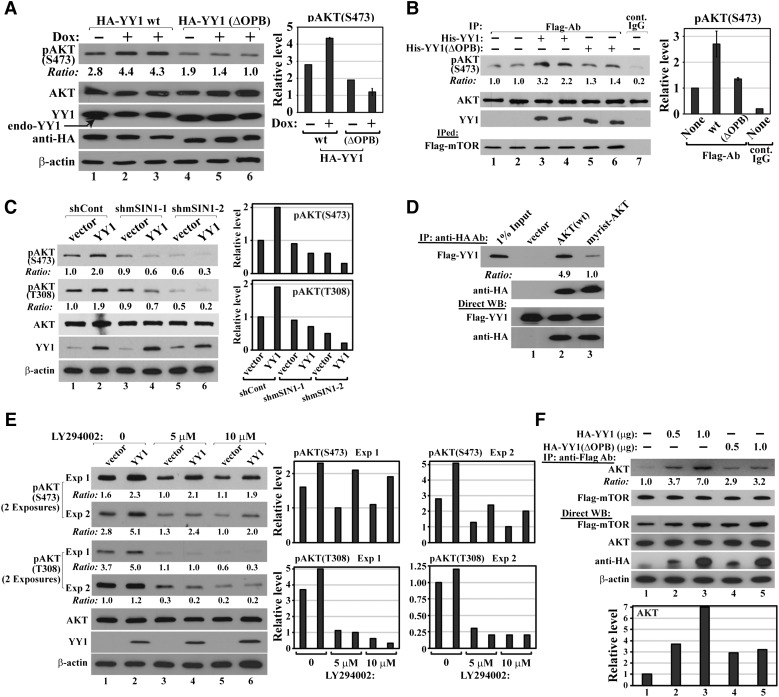
To determine whether YY1–AKT interaction is important for AKT phosphorylation, we expressed HA-YY1 wt or ΔOPB by lentiviral infection in MDA-MB-231 cells with simultaneous knockdown of endogenous YY1 by Dox-induced shYY1-2 or shYY1-3, which targets the YY1 mRNA 3′-UTR and thus silences endogenous YY1 but not ectopic YY1 (Supplementary Figure S2A and B). Cells expressing HA-YY1(ΔOPB) showed lower pAKT(S473) levels than cells expressing HA-YY1(wt) with endogenous YY1 silenced by either shYY1-2 (Figure 4A) or shYY1-3 (Supplementary Figure S5A), suggesting that the OPB domain is essential for YY1-mediated AKT phosphorylation. Interestingly, the coexistence of both endogenous YY1 and HA-YY1 led to less AKT phosphorylation than HA-YY1 alone (Figure 4A, lane 1 vs. lanes 2–3), suggesting that a further YY1 increase in breast cancer cells already expressing high levels of endogenous YY1 is unfavorable to AKT phosphorylation. This may explain the apparent tumor suppressive effects of YY1 when its levels are further increased in breast cancer cells (Ishii et al., 2012; Lee et al., 2012).
Figure 4.
YY1 binding promotes AKT phosphorylation. (A) YY1(wt) but not YY1(ΔOPB) increases AKT phosphorylation. MDA-MB-231 cells with Dox-inducible shYY1-2 were infected by lentiviruses generated with pSL5/HA-YY1 wt or ΔOPB, and cultured in the absence or presence of Dox. Cell lysates were analyzed by western blot using the indicated antibodies. Duplicated samples: 2–3 and 5–6. (B) His-YY1 wt, but not ΔOPB, markedly enhances mTOR-mediated pAKT(S473) in vitro. Flag-mTOR was IPed in a CHAPS-containing buffer from HeLa cells transfected with pcDNA3/Flag-mTOR and incubated with recombinant GST-AKT and His-YY1(wt) or His-YY1(ΔOPB). Duplicated samples: 1–2, 3–4, and 5–6. Samples were analyzed for pAKT(S473), AKT, YY1, and Flag-mTOR. (C) mSIN1 knockdown blocks YY1-promoted AKT phosphorylation. Nontumorigenic 184B5 cells were infected by lentivirus carrying control or mSIN1 shRNAs. Five days post infection and puromycin selection, cell lysates were analyzed by the indicated antibodies. (D) AKT myristoylation decreases its interaction with YY1. Myrist, myristoylation. Constructs of empty vector, HA-AKT(wt), or HA-myrist-AKT were co-transfected into HeLa cells with Flag-YY1-expressing plasmid. HA antibody-conjugated agarose was used in co-IP assays with the indicated antibodies. (E) YY1 increases AKT phosphorylation in the presence of PI3K inhibitor. 184B5 cells infected by lentivirus carrying an empty vector or expressing YY1 were treated with two different concentrations of LY294002 for 16 h. Cell lysates were analyzed by the indicated antibodies. (F) YY1 enhances AKT and mTOR interaction. HA-YY1(wt) or HA-YY1(ΔOPB) plasmid was co-transfected with Flag-mTOR and AKT constructs. Flag antibody-conjugated agarose beads were used in co-IP studies with the indicated antibodies. In A, B, C, E, and F, densitometry analyses of western blots were shown at the right.
YY1 promotes mTORC2-mediated AKT phosphorylation
To determine whether YY1 directly promotes AKT phosphorylation, we studied its effects on AKT phosphorylation in reconstituted systems. We first observed that recombinant GST-AKT was phosphorylated at S473 by Flag-mTOR complex IPed from transfected HeLa cells (Figure 4B, lanes 1–2 vs. lane 7). This effect was enhanced over 2-fold by recombinant His-YY1(wt) but reduced to a lesser extent (1.4-fold) by recombinant His-YY1(ΔOPB) (lanes 3–4 and 5–6 vs. lanes 1–2). However, in a similar in vitro assay, Flag-PDK1 IPed from the lysates of transfected HeLa cells promoted GST-AKT phosphorylation at T308, which was not significantly promoted by YY1 (Supplementary Figure S5B). In both in vitro studies, neither IPed Flag-mTOR complex caused T308 phosphorylation nor Flag-PDK1 promoted S473 phosphorylation for recombinant GST-AKT (data not shown). Consistently, YY1 interacts with mTOR, but not PDK1 (Supplementary Figure S5C). These results suggest that YY1 directly enhances AKT phosphorylation at S473, but not T308, which is independent of its transcriptional activity. This was confirmed by the observation that YY1 zinc finger chimeras 13 and 17, deficient in binding to the YY1 consensus site (Galvin and Shi, 1997), also increased pAKT(S473) levels (Supplementary Figure S5D). To further dissect the mechanism of YY1-mediated AKT phosphorylation, we ectopically expressed YY1 in 184B5 cells with silenced mSIN1, a unique component of mTORC2 that phosphorylates AKT at S473 (Frias et al., 2006; Jacinto et al., 2006). As shown in Figure 4C, the YY1-induced AKT phosphorylation at both S473 and T308 was abolished with mSIN1 individually knocked down by two different mSIN1 shRNAs (shmSIN1-1 and shmSIN1-2, Supplementary Figure S5E). These data, together with the in vitro phosphorylation results, suggest that YY1 specifically promotes mTORC2-mediated AKT phosphorylation at S473 and its effect on pAKT(T308) is likely indirect, facilitated by S473 phosphorylation. This model is further corroborated by the lack of YY1 stimulation to T308 phosphorylation of AKT(S473A) mutant (Supplementary Figure S5F).
We also tested whether YY1 affects AKT dephosphorylation in two in vitro dephosphorylation assays (Chan et al., 2011). Dephosphorylation of AKT S473 and T308 is mediated by two phosphatases, PHLPP2 and PP2A, respectively. As presented in Supplementary Figure S6A–C, YY1 does not change the kinetics of AKT dephosphorylation at S473 and T308.
YY1-promoted AKT phosphorylation is independent of PI3K activity
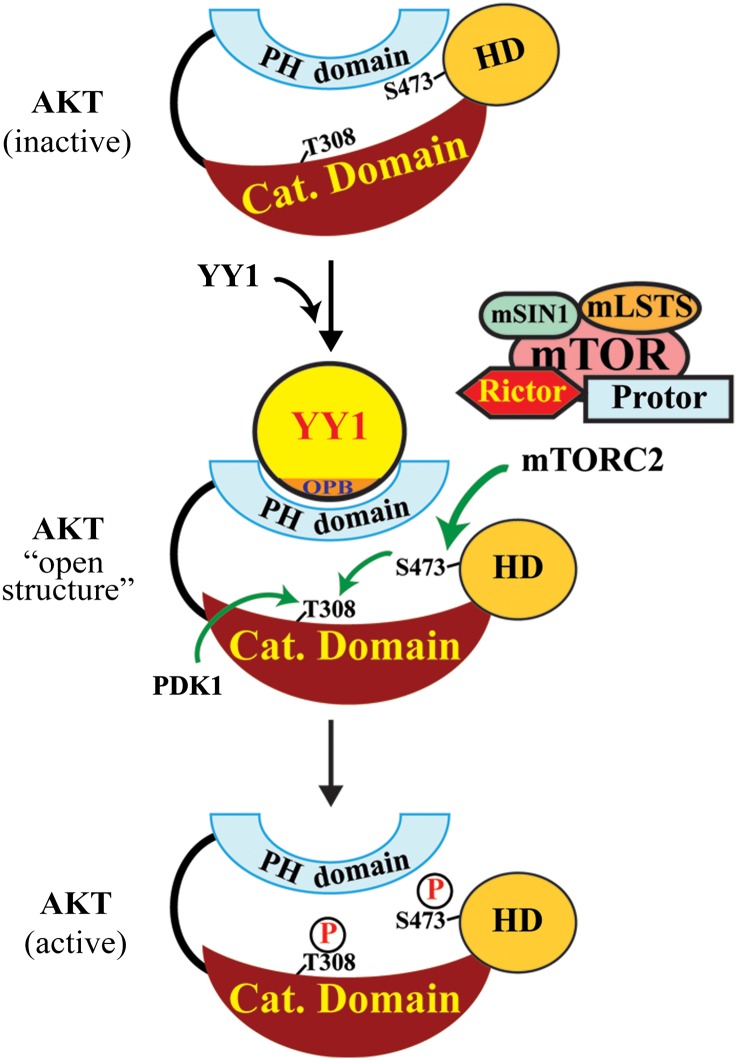
The classical model of AKT activation proposes that PH domain binding to PIP3 in membrane leads to AKT conformational changes that facilitate S473 and T308 phosphorylation. As we have mapped YY1 binding site on AKT to the PH domain (Figure 3E and F), we predicted that YY1 and PIP3 may compete in binding to AKT. This prediction was borne out by our observation that YY1 showed reduced interaction with AKT tagged by a myristoylation (myrist) sequence that promotes AKT association with PIP3/membrane (Kulik et al., 1997) (Figure 4D). Based on these data, YY1 binding to the PH domain may represent an unrecognized mechanism of AKT phosphorylation, similar to but independent of that mediated by PIP3. We conducted additional experiments to confirm this prediction. First, we tested the dependence of YY1-promoted AKT phosphorylation on PIP2–PIP3 conversion. We infected 184B5 cells by pSL2 or pSL2/YY1 lentivirus and treated the cells with a PI3K inhibitor, LY294002, for 16 h to block PIP3 synthesis. The inhibition of PI3Ks did not deprive the ability of YY1 in promoting AKT(S473) phosphorylation, although it diminished T308 phosphorylation and reduced overall pAKT(S473) levels (Figure 4E). The observed pAKT(T308) decrease was likely attributed to the lack of PDK1 membrane recruitment and reduced overall pAKT(S473) levels caused by LY294002-mediated PIP3 depletion. Second, we determined whether YY1 affects mTORC2–AKT association. In co-IP assays, HA-YY1(wt) promoted AKT and Flag-mTOR interaction, which was markedly compromised when HA-YY1(ΔOPB) was used (Figure 4F). However, in a similar experiment, neither HA-YY1(wt) nor HA-YY1(ΔOPB) could affect AKT–PDK1 interaction (Supplementary Figure S7). Thus, our data suggest a novel mechanism of AKT phosphorylation promoted by YY1. As illustrated in Figure 5, we propose that YY1 binding to the PH domain causes a conformational change of AKT that exposes S473 and allows the access of mTORC2 to phosphorylate S473. The S473 phosphorylation stabilizes the open structure of AKT and enables its binding to PDK1 to facilitate PDK1-mediated T308 phosphorylation, leading to full activation of AKT. YY1-promoted AKT phosphorylation may likely occur in both cytoplasm and nucleus, because both nuclear export/localization signal (NES/ NLS)-tagged YY1 could increase pAKT(S473) levels (Supplementary Figure S8A and B).
Figure 5.
A schematic model of YY1-promoted AKT phosphorylation. YY1 binds AKT PH domain and imposes its conformational change to expose S473, which allows mTORC2 access to phosphorylate S473. This phosphorylation stabilizes the conformation leading to T308 phosphorylation and consequently full AKT activation.
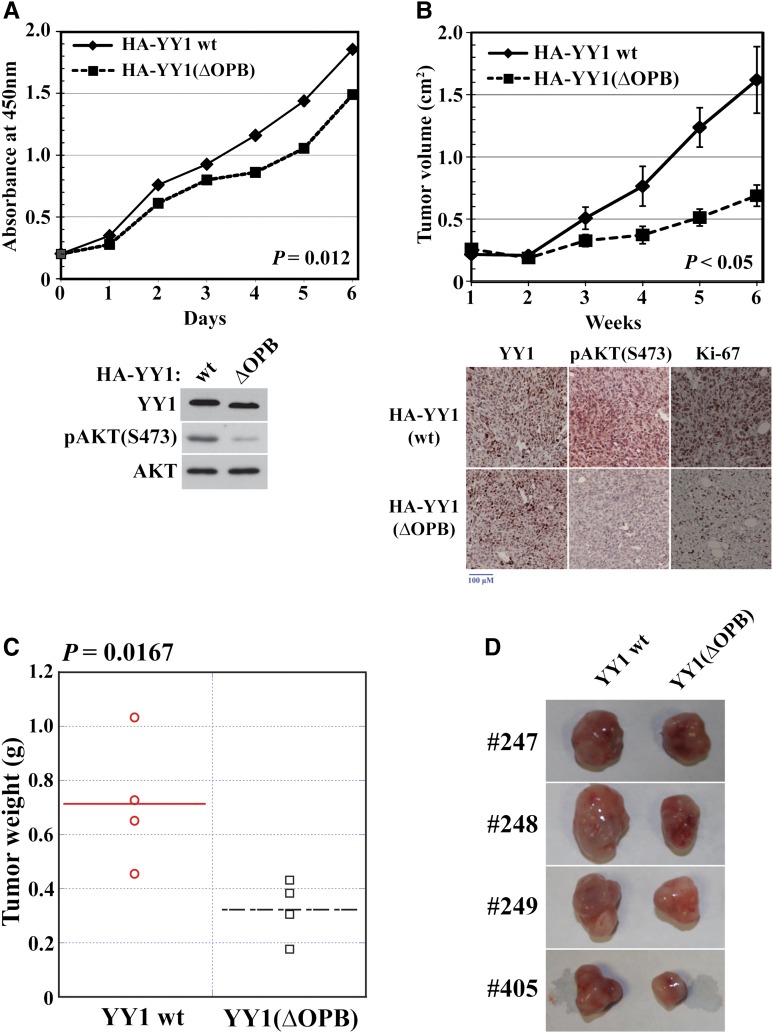
To determine the biological function of the OPB domain, we studied the proliferation and tumor formation of MDA-MB-231 cells expressing HA-YY1(wt) and HA-YY1(ΔOPB) with simultaneously silenced endogenous YY1. HA-YY1(ΔOPB) significantly decreased cell proliferation compared with HA-YY1(wt) (Figure 6A). Consistently, xenografted cells expressing HA-YY1(ΔOPB) showed significantly reduced tumor sizes and weights compared with cells expressing HA-YY1(wt) (Figure 6B–D). The tumors expressing HA-YY1(ΔOPB) exhibited decreased pAKT(S473) and Ki-67 staining compared with tumors expressing HA-YY1(wt) (Figure 6B, bottom panel), suggesting the need of the OPB domain in YY1-promoted AKT phosphorylation and tumor formation.
Figure 6.
YY1 OPB domain is necessary for breast cancer cell proliferation and tumor formation. MDA-MB-231 cells were infected by pSL5/HA-YY1 wt or ΔOPB, with endogenous YY1 simultaneously silenced by shYY1-2, and tested by WST-1 assay (A) and xenograft tumor formation experiment (B–D). (A) Cell proliferation data were derived from samples in triplicates for three times. Bottom panel, MDA-MB-231 cells for WST-1 assays were analyzed by western blot. (B–D) Xenograft tumor volume (B), weight (C), and image (D) are presented. Bottom panel, representative immunohistochemical analyses of xenograft tumors by using YY1, pAKT(S473), and Ki-67 antibodies.
Peptides based on the YY1 OPB domain inhibit breast cancer cell proliferation
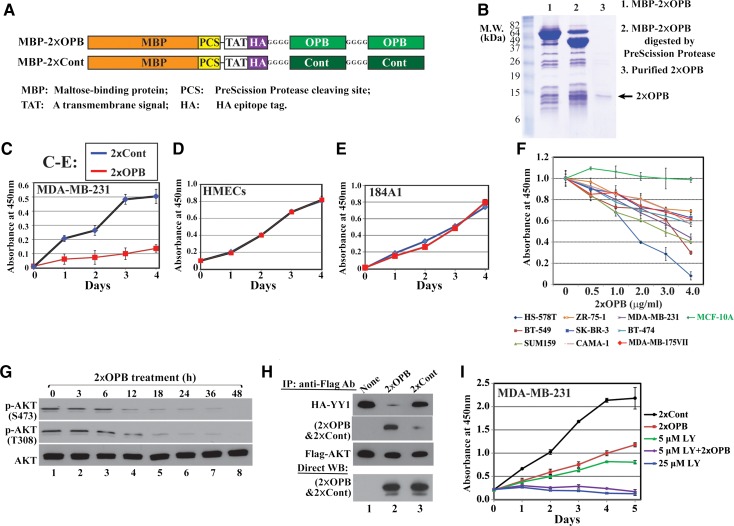
We generated constructs expressing a 2×OPB peptide and its scrambled control peptide (2×Cont). The double-repeated peptides are fused to the maltose binding protein (MBP) with a PreScission Protease (PSP) cleaving site, a transmembrane signal (TAT), and a HA epitope tag sequence (Figure 7A). The fusion proteins were purified by amylose resin, and the peptides were released by PSP (Figure 7B with 2×OPB as an example). In comparison with 2×Cont, 2×OPB inhibited the proliferation of MDA-MB-231 cells but not that of non-tumorigenic human mammary epithelial cells (HMECs) and 184A1 cells (Figure 7C–E). Additionally, 2×OPB showed inhibitory effects on all tested breast cancer cell lines in a dosage-dependent manner, but not on nontumorigenic MCF-10A cells (Figure 7F). Importantly, MDA-MB-231 cells treated with the 2×OPB peptide exhibited decreased p-AKT(S473) and p-AKT(T308) (Figure 7G), but the 2×Cont peptide did not have such effect (not shown). Furthermore, the 2×OPB, but not 2×Cont, competed with HA-YY1 in binding to Flag-AKT (Figure 7H). These results suggest that 2×OPB attenuates AKT activation and cell growth through blocking YY1–AKT interaction. If YY1 and PI3K mediate AKT phosphorylation through different pathways, 2×OPB and PI3K inhibitor should have additive inhibitory effects on cell proliferation. This was corroborated by the studies in Figure 7I; the 2×OPB and LY294002 co-treatment more dramatically inhibited cell proliferation than each molecule alone did.
Figure 7.
OPB-based peptide inhibits breast cancer cell proliferation and disrupts YY1–AKT interaction. (A) Schematic diagrams of MBP-2×OPB and MBP-2×Cont fusion proteins. The peptides with N-terminal TAT-HA can be released by PreScission Protease (PSP). (B) Coomassie blue staining of SDS-PAGE for 2×OPB before and after PSP digestion and after purification. (C–F) In WST-1 assays, 2×OPB peptide (2 µg/ml) inhibited the proliferation of tumor cells (C), but not nontumorigenic cells (D and E). Cell proliferation data were derived from samples in triplicates for three times. (F) 2×OPB peptide (2 µg/ml) inhibited the proliferation of other breast cancer cell lines, except for nontumorigenic MCF-10A cells. (G) 2×OPB reduced AKT phosphorylation at S473 and T308 in MDA-MB-231 cells. (H) 2×OPB, but not 2×Cont, competed with HA-YY1 in binding to Flag-AKT. (I) In WST-1 assays, co-treatment of 2×OPB (2 µg/ml) and LY294002 (LY, 5 μM) additively inhibited cell proliferation compared with individual treatment. 2×Cont (2 µg/ml) and 25 μM LY294002 were used as negative and positive controls, respectively.
Discussion
In the current study, we discovered a novel activity of YY1 in promoting AKT phosphorylation at S473 through direct protein interaction. This activity of YY1 is independent of either its transcriptional activity or PI3K–PIP3–membrane signaling. It is noteworthy that our finding by no means challenges the well characterized canonical model of AKT activation through the signaling cascade consisting of growth stimuli, tyrosine kinase receptors and PI3Ks. Thus, depleting endogenous YY1 and concurrently expressing YY1(ΔOPB) would not totally abolish AKT phosphorylation, as shown in Figure 4A. Although currently we cannot exclude that YY1 may be involved in other AKT activation mechanisms, we propose that YY1-promoted AKT phosphorylation represents one of alternative mechanisms contributing to AKT activation independently from PI3K. Importantly, this regulation is likely clinically relevant, because YY1 levels correlated with pAKT(S473) in our tissue microarray studies (Figure 1B). Additionally, the higher correlation coefficients between YY1 and pAKT(S473) in the hormone receptor-negative breast cancer samples suggest that YY1 increase may be an indication of poor prognoses for breast cancer patients. This is corroborated by our analyses in a cohort consisting of 258 breast cancer patients (Miller et al., 2005), in which we observed a positive correlation between YY1 and metastatic potential (Figure 1A). Our data suggest that YY1 binding to the PH domain of AKT likely alters its conformation to that favoring mTORC2-mediated S473 phosphorylation. Future studies are needed to characterize this predicted conformational changes of AKT upon YY1 binding and validate our proposed model using technologies in structural and cellular biology, such as fluorescence resonance energy transfer (FRET). Nevertheless, our data strongly support an oncogenic role of YY1 in breast cancer, at least partially through mediating AKT phosphorylation.
Since both YY1 and PIP3 bind to the PH domain, AKT phosphorylation mediated by YY1 and PI3K–PIP3 may interfere with each other; thus, AKT–membrane association reduced YY1 binding to AKT (Figure 4D). We predict that YY1-regulated AKT phosphorylation can be very dynamic. YY1 binding can cause AKT conformational change to increase the access of mTORC2 leading to S473 phosphorylation. During AKT activation process, YY1 may remain its association with AKT or act in a ‘hit and run’ fashion. Interestingly, YY1 directly binds to mTOR (Supplementary Figure S5C; Cunningham et al., 2007), but whether their interaction plays a role in YY1-regulated AKT phosphorylation needs further investigation. Multiple studies suggested the presence and regulatory role of active AKT in nucleus during oncogenesis (Martelli et al., 2012). Consistently, most commercial antibodies for AKT S473 and T308 phosphorylation detect dominant signals in nucleus, which is consistent with our data in Figure 2. YY1 is present in both nucleus and cytoplasm, especially in cancers (Palko et al., 2004; Rizkallah and Hurt, 2009; Wan et al., 2012). Thus, we predict that YY1 promotes AKT phosphorylation through interacting with AKT in both cytoplasm and nucleus, which is supported by our observation that forced YY1 presence in either cytoplasm or nucleus led to enhanced AKT phosphorylation (Supplementary Figure S8). Most cancer cell lines exhibit exclusively nuclear YY1 signal in immunostaining studies, but tumor tissues may show YY1 presence in both nucleus and cytoplasm. Although our immunostaining experiments showed colocalization of YY1 and phosphorylated AKT mostly in the nuclei of MDA-MB-231 cells, YY1 and AKT may colocalize in both nucleus and cytoplasm in actual tumors, as suggested by Figure 2A. Most previous studies concerned on the role of nuclear YY1; to our knowledge, this study provides the first evidence suggesting a functional role of cytoplasmic YY1. In nucleus, YY1 may recruit AKT to its targeted promoters, which can extend AKT regulation to gene transcription. Consistent with the observation above, we detected pAKT(S473), pAKT(T308), and YY1 signal in both cytoplasm and nucleus from fractionated MDA-MB-231 cells (Supplementary Figure S3D). Interestingly, while phosphorylated AKT is quite significant in nucleus compared with cytoplasm, total nuclear AKT showed much lower signal than cytoplasmic AKT, suggesting that the majority of cytoplasmic AKT is dephosphorylated whereas most nuclear AKT is phosphorylated or activated in breast cancer cells.
AKT activation was observed in >38% of human invasive breast cancers and associated with poor clinical outcomes (Perez-Tenorio and Stal, 2002; Bose et al., 2006); thus, it contributes to breast cancer pathogenesis and is a rational therapeutic target. The tumor suppressor PTEN negatively regulates PI3K–PIP3-promoted AKT activation through converting PIP3 to PIP2 (Di Cristofano and Pandolfi, 2000); thus, PTEN deficiency causes AKT activation due to increased PIP3 distribution in plasma membrane. However, AKT could be aberrantly activated in tumors without detectable PI3K or PTEN alterations (Sun et al., 2001; Mahajan and Mahajan, 2012), and AKT activation did not always correlate with PTEN loss (Bose et al., 2006), suggesting that AKT can be activated by alternative mechanisms independent of PI3K–PIP3. Consistently, multiple reports suggested that mTORC2 and PDK1 are not the only kinases mediating AKT phosphorylation at S473 and T308 (Mahajan and Mahajan, 2012). Thus, YY1-regulated AKT phosphorylation independent of PI3K–PIP3 signaling can potentially involve other kinases. We proposed that the positive effect of YY1 on phospho-T308 is indirect, likely through promoting S473 phosphorylation, which was supported by the data that YY1 failed in stimulating T308 phosphorylation of AKT(S473A) mutant (Supplementary Figure S5F). This prediction is also reinforced by a recently reported AKT activation mechanism that S473 phosphorylation enables AKT–PDK1 interaction to further phosphorylate T308 (Najafov et al., 2012). When co-expressing ectopic YY1 and AKT(S473A), we detected reduced T308 phosphorylation signal for both endogenous AKT and AKT(S473A) (Supplementary Figure S5F). The reason to cause this phenomenon is likely that ectopic YY1 competed with PIP3 in binding to AKT PH domain and thus reduced AKT recruitment to plasma membrane, which consequently attenuated PDK1-mediated T308 phosphorylation. Actually, PDK1 is also a PH domain-containing kinase, and its recruitment by PIP3 to plasma membrane facilitates its activity to phosphorylate AKT at T308 (Guertin and Sabatini, 2007). Thus, it is possible that YY1-mediated AKT(S473) phosphorylation and the consequent conformational changes, which do not require PIP3 recruitment, allow other kinases, such as IKBKE and TBK1 (Mahajan and Mahajan, 2012), to access to phosphorylate T308.
The OPB domain of YY1 directly interacts with multiple endogenous oncogene products, including Ezh2, Mdm2, and AKT. We attempted to discriminate their binding sites on YY1 through individually mutating residues in the OPB domain; however, our data indicate that the three oncoproteins likely share the interacting site(s) on YY1 (data not shown). Thus, the deficiencies of the YY1(ΔOPB) mutant in supporting cell proliferation and tumor formation compared with YY1(wt) (Figure 6) should represent the effects of attenuating all three oncogenic pathways, suggesting the essential function of the YY1 OPB domain in oncogenesis.
Our model of YY1-promoted AKT phosphorylation reinforces the oncogenic role of YY1 and its potential as therapeutic target. Since YY1 is overexpressed in most cancers and ubiquitously present in cells (Zhang et al., 2011), its activity of promoting AKT phosphorylation brings the expediency of AKT activation to stimulate a variety of cell signaling pathways that contribute to oncogenesis. There should be a boundary of YY1 level, beyond which YY1 does not function in promoting AKT phosphorylation. This boundary must be physiologically relevant to cancer development, because we and others observed that further YY1 increase in cancer cells expressing high level of YY1 adversely affected cell proliferation (Sui et al., 2004; Ishii et al., 2012; Lee et al., 2012). Our current understanding of YY1 function is still insufficient to provide a mechanistic explanation for this boundary.
The peptides based on the OPB sequence of YY1 decreased AKT phosphorylation and competed with YY1 in binding to AKT (Figure 7). This suggests that 2×OPB binding to AKT is insufficient to cause the AKT conformational change favorable for mTORC2-mediated phosphorylation, but instead blocks YY1–AKT interaction, leading to reduced AKT phosphorylation. 2×OPB only affects the proliferation of breast cancer cells but not nontumorigenic mammary cells, correlated with the high and low YY1 expression in these cells, respectively (Wan et al., 2012). This implies that blocking the interaction of YY1 with its target oncoproteins using molecules mimicking the OPB domain may represent a novel therapeutic strategy in breast cancer treatment. As YY1-promoted AKT phosphorylation is independent of PIP3, overexpressed YY1 may circumvent the PI3K inhibitor resistance in breast cancer patients by promoting AKT phosphorylation and activation in the absence of PI3K activity. Consistently, simultaneous inhibition of PI3K and YY1 activity by LY294002 and the 2×OPB peptide additively decreased breast cancer cell proliferation. Thus, our study also provides insights into developing individualized therapies. For breast cancer patients with high levels of YY1 expression, both PI3Ks and YY1 should be targeted to improve therapeutic efficacies.
Materials and methods
Antibodies, DNA, and vectors
Antibodies used include YY1 (H-10, sc-7341; C-20, sc-281; H-414, sc-1703, Santa Cruz Biotechnology), β-actin (MAB1501, Chemicon International Inc.), GAPDH (10R-G109A, Fitzgerald Industries International), Phospho-AKT at Thr308 (cat#9275S, Cell Signaling) and Phospho-AKT at S473 (cat#4051S, Cell Signaling), AKT (cat#9272, Cell Signaling), Ezh2 (AC22, Cell Signaling), Flag (M2, Sigma-Aldrich), and HA (32-6700, Invitrogen). Oligonucleotides for PCR and DNA sequencing were synthesized by Integrated DNA Technologies, Inc.
Three shRNAs (shYY1-1, shYY1-2, and shYY1-3) targeting YY1 were designed as previously described (Sui et al., 2002; Sui and Shi, 2005). shYY1-1 (GGGAGCAGAAGCAGGTGCAGAT) (Sui et al., 2004) targets the coding region and shYY1-2 (GCTCACCTGTTGCTTACAATT) and shYY1-3 (GATGCTGATGTTCAGTGTAATT) target the 3′-UTR of YY1 mRNA (Supplementary Figure S2A). The efficiencies of shYY1-1 for silencing both ectopic and endogenous YY1 and shYY1-2 and shYY1-3 for selectively silencing endogenous YY1 are demonstrated in Supplementary Figure S2B.
Tissue microarray study
TMA slides processed by IHC using an YY1 (H-414) antibody were examined by two observers and analyzed for the intensity and percentage of stained tumor cells. The intensity score was determined based on a scoring range from 0 to 2+; 0 is a negative staining and 2+ is an intense staining. This is accomplished by first analyzing a series of randomly selected breast cancer tissues for YY1 expression using our standardized method. Specific blocks were selected as standards for 0, 1+, and 2+ staining intensity and included in the TMA analysis. At the time of analysis, researchers did not have any information pertaining to the patients. Each core was assigned an identification number corresponding to the patient's information to allow unbiased analysis of the tissues. By visual comparison, the intensities in the TMA samples were determined. The percent of tumor was defined in units of 0–10%, 11%–50%, 51%–75%, and 76%–100% breast tumor cell staining. A mean score was obtained by multiplying the intensity score with the percent of tumor cells stained in corresponding intensity and adding together. This overall score was averaged by the number of cores studied for the patient. If there was no tumor, no score was given.
Cell culture, lentiviral production and infection
Nontumorigenic breast cell lines 184A1 and 184B5 were provided by Dr Stampfer and cultured as described (Walen and Stampfer, 1989). All other mammary cell lines were cultured according to the protocols of ATCC. Lentivirus production and infection were performed as previously described (Stovall et al., 2012).
In vitro AKT phosphorylation
A previous protocol was followed with modifications (Sarbassov et al., 2005). HeLa cells were transfected with pcDNA3/Flag-mTOR. Immunoprecipitated (IPed) Flag-mTOR by Flag antibody-conjugated agarose (Sigma) was incubated with recombinant GST-AKT and purified recombinant His-YY1 wt or ΔOPB mutant in a kinase buffer (25 mM HEPES, pH 7.5, 100 mM KAc, 1 mM MgCl2) at 37°C for 20 min. The samples were then analyzed by western blots for pAKT(S473) levels.
In vitro AKT dephosphorylation
A reported protocol was followed to determine in vitro AKT dephosphorylation (Chan et al., 2011). Cells were treated in an AKT phosphorylation solution (100 μM of both hydrogen peroxide and sodium orthovanadate) for 15 min to maximally activate AKT prior to collection. The cell lysates were incubated at 30°C for different time periods and collected for western blot analyses using phos-AKT antibodies.
Breast cancer xenograft study
The study in a mouse model was performed under the protocol approved by the IACUC of Wake Forest School of Medicine (Stovall et al., 2012).
Peptide expression and purification
Constructs expressing the OPB-based peptide and its control, both in fusion with the maltose binding protein (MBP), were expressed in BL21(DE3) Tuner bacteria. MBP fusion proteins were purified by amylose resin (New England Bio-Labs), and the peptides were released by the PreScission Protease (PSP).
Protein interaction studies
Immunoprecipitation and in vitro protein binding studies were carried out as previously described (Deng et al., 2007).
Statistical analysis
Data in reporter assays and WST-1 assays are presented as mean ± SD. Comparisons between two groups on a single parameter were conducted using Student's t-test. Statistical analyses were performed using SigmaStat (Systat Software Inc.). The criterion for statistical significance was set at P < 0.05 and indicated by asterisks (*) in the figures.
Supplementary material
Supplementary material is available at Journal of Molecular Cell Biology online.
Funding
This work was supported by grants from the American Cancer Society (RSG-09-082-01-MGO) and the National Natural Science Foundation of China (81472635) to G.S., R21 CA182248 to G.S. and G.K., and SRG grant 407071502154 from Alfaisal University to G.K.
Conflict of interest: none declared.
Supplementary Material
Acknowledgements
We thank Drs Doug Lyles, Thomas Hollis, and Yong Q. Chen at Wake Forest School of Medicine for productive discussions. We thank Mr James Lovato at Wake Forest School of Medicine for statistical analyses.
References
- Allouche A., Nolens G., Tancredi A. et al. (2008). The combined immunodetection of AP-2alpha and YY1 transcription factors is associated with ERBB2 gene overexpression in primary breast tumors. Breast Cancer Res. 10, R9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu A., Hodawadekar S., Andrews O. et al. (2010). YY1 PcG function as a potential cancer therapeutic target. For. Immunopathol. Dis. Therap. 1, 31–50. [Google Scholar]
- Begon D.Y., Delacroix L., Vernimmen D. et al. (2005). Yin Yang 1 cooperates with activator protein 2 to stimulate ERBB2 gene expression in mammary cancer cells. J. Biol. Chem. 280, 24428–24434. [DOI] [PubMed] [Google Scholar]
- Bose S., Chandran S., Mirocha J.M. et al. (2006). The Akt pathway in human breast cancer: a tissue-array-based analysis. Mod. Pathol. 19, 238–245. [DOI] [PubMed] [Google Scholar]
- Brognard J., Sierecki E., Gao T. et al. (2007). PHLPP and a second isoform, PHLPP2, differentially attenuate the amplitude of Akt signaling by regulating distinct Akt isoforms. Mol. Cell 25, 917–931. [DOI] [PubMed] [Google Scholar]
- Cao Y., Gao Z., Li L. et al. (2013). Whole exome sequencing of insulinoma reveals recurrent T372R mutations in YY1. Nat. Commun. 4, 2810. [DOI] [PubMed] [Google Scholar]
- Chan T.O., Zhang J., Rodeck U. et al. (2011). Resistance of Akt kinases to dephosphorylation through ATP-dependent conformational plasticity. Proc. Natl Acad. Sci. USA 108, E1120–E1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnappan D., Xiao D., Ratnasari A. et al. (2009). Transcription factor YY1 expression in human gastrointestinal cancer cells. Int. J. Oncol. 34, 1417–1423. [PubMed] [Google Scholar]
- Cunningham J.T., Rodgers J.T., Arlow D.H. et al. (2007). mTOR controls mitochondrial oxidative function through a YY1-PGC-1alpha transcriptional complex. Nature 450, 736–740. [DOI] [PubMed] [Google Scholar]
- de Nigris F., Crudele V., Giovane A. et al. (2010). CXCR4/YY1 inhibition impairs VEGF network and angiogenesis during malignancy. Proc. Natl Acad. Sci. USA 107, 14484–14489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Z., Wan M., Sui G. (2007). PIASy-mediated sumoylation of Yin Yang 1 depends on their interaction but not the RING finger. Mol. Cell. Biol. 27, 3780–3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cristofano A., Pandolfi P.P. (2000). The multiple roles of PTEN in tumor suppression. Cell 100, 387–390. [DOI] [PubMed] [Google Scholar]
- Dummler B., Tschopp O., Hynx D. et al. (2006). Life with a single isoform of Akt: mice lacking Akt2 and Akt3 are viable but display impaired glucose homeostasis and growth deficiencies. Mol. Cell. Biol. 26, 8042–8051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frias M.A., Thoreen C.C., Jaffe J.D. et al. (2006). mSin1 is necessary for Akt/PKB phosphorylation, and its isoforms define three distinct mTORC2s. Curr. Biol. 16, 1865–1870. [DOI] [PubMed] [Google Scholar]
- Galvin K.M., Shi Y. (1997). Multiple mechanisms of transcriptional repression by YY1. Mol. Cell. Biol. 17, 3723–3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronroos E., Terentiev A.A., Punga T. et al. (2004). YY1 inhibits the activation of the p53 tumor suppressor in response to genotoxic stress. Proc. Natl Acad. Sci. USA 101, 12165–12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guertin D.A., Sabatini D.M. (2007). Defining the role of mTOR in cancer. Cancer Cell 12, 9–22. [DOI] [PubMed] [Google Scholar]
- Huang W., Smaldino P.J., Zhang Q. et al. (2012). Yin Yang 1 contains G-quadruplex structures in its promoter and 5′-UTR and its expression is modulated by G4 resolvase 1. Nucleic Acids Res. 40, 1033–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii H., Hulett M.D., Li J.M. et al. (2012). Yin Yang-1 inhibits tumor cell growth and inhibits p21WAF1/Cip1 complex formation with cdk4 and cyclin D1. Int. J. Oncol. 40, 1575–1580. [DOI] [PubMed] [Google Scholar]
- Jacinto E., Facchinetti V., Liu D. et al. (2006). SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell 127, 125–137. [DOI] [PubMed] [Google Scholar]
- Krippner-Heidenreich A., Walsemann G., Beyrouthy M.J. et al. (2005). Caspase-dependent regulation and subcellular redistribution of the transcriptional modulator YY1 during apoptosis. Mol. Cell. Biol. 25, 3704–3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulik G., Klippel A., Weber M.J. (1997). Antiapoptotic signalling by the insulin-like growth factor I receptor, phosphatidylinositol 3-kinase, and Akt. Mol. Cell. Biol. 17, 1595–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.S., See R.H., Galvin K.M. et al. (1995). Functional interactions between YY1 and adenovirus E1A. Nucleic Acids Res. 23, 925–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M.H., Lahusen T., Wang R.H. et al. (2012). Yin Yang 1 positively regulates BRCA1 and inhibits mammary cancer formation. Oncogene 31, 116–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis B.A., Tullis G., Seto E. et al. (1995). Adenovirus E1A proteins interact with the cellular YY1 transcription factor. J. Virol. 69, 1628–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan K., Mahajan N.P. (2012). PI3K-independent AKT activation in cancers: a treasure trove for novel therapeutics. J. Cell. Physiol. 227, 3178–3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning B.D., Cantley L.C. (2007). AKT/PKB signaling: navigating downstream. Cell 129, 1261–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martelli A.M., Tabellini G., Bressanin D. et al. (2012). The emerging multiple roles of nuclear Akt. Biochim. Biophys. Acta 1823, 2168–2178. [DOI] [PubMed] [Google Scholar]
- Miller L.D., Smeds J., George J. et al. (2005). An expression signature for p53 status in human breast cancer predicts mutation status, transcriptional effects, and patient survival. Proc. Natl Acad. Sci. USA 102, 13550–13555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najafov A., Shpiro N., Alessi D.R. (2012). Akt is efficiently activated by PIF-pocket- and PtdIns(3,4,5)P3-dependent mechanisms leading to resistance to PDK1 inhibitors. Biochem. J. 448, 285–295. [DOI] [PubMed] [Google Scholar]
- Palko L., Bass H.W., Beyrouthy M.J. et al. (2004). The Yin Yang-1 (YY1) protein undergoes a DNA-replication-associated switch in localization from the cytoplasm to the nucleus at the onset of S phase. J. Cell Sci. 117, 465–476. [DOI] [PubMed] [Google Scholar]
- Panagopoulos I., Thorsen J., Gorunova L. et al. (2013). RNA sequencing identifies fusion of the EWSR1 and YY1 genes in mesothelioma with t(14;22)(q32;q12). Genes Chromosomes Cancer 52, 733–740. [DOI] [PubMed] [Google Scholar]
- Perez-Tenorio G., Stal O. (2002). Activation of AKT/PKB in breast cancer predicts a worse outcome among endocrine treated patients. Br. J. Cancer 86, 540–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powe D.G., Akhtar G., Onsy Habashy H. et al. (2009). Investigating AP-2 and YY1 protein expression as a cause of high HER2 gene transcription in breast cancers with discordant HER2 gene amplification. Breast Cancer Res. 11, R90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizkallah R., Hurt M.M. (2009). Regulation of the transcription factor YY1 in mitosis through phosphorylation of its DNA-binding domain. Mol. Biol. Cell 20, 4766–4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarbassov D.D., Guertin D.A., Ali S.M. et al. (2005). Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307, 1098–1101. [DOI] [PubMed] [Google Scholar]
- Schlisio S., Halperin T., Vidal M. et al. (2002). Interaction of YY1 with E2Fs, mediated by RYBP, provides a mechanism for specificity of E2F function. EMBO J. 21, 5775–5786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seligson D., Horvath S., Huerta-Yepez S. et al. (2005). Expression of transcription factor Yin Yang 1 in prostate cancer. Int. J. Oncol. 27, 131–141. [PubMed] [Google Scholar]
- Shi Y., Seto E., Chang L.S. et al. (1991). Transcriptional repression by YY1, a human GLI-Kruppel-related protein, and relief of repression by adenovirus E1A protein. Cell 67, 377–388. [DOI] [PubMed] [Google Scholar]
- Stovall D.B., Wan M., Zhang Q. et al. (2012). DNA vector-based RNA interference to study gene function in cancer. J. Vis. Exp. e4129. [DOI] [PMC free article] [PubMed]
- Sui G., Shi Y. (2005). Gene silencing by a DNA vector-based RNAi technology. Methods Mol. Biol. 309, 205–218. [DOI] [PubMed] [Google Scholar]
- Sui G., Soohoo C., Affarel B. et al. (2002). A DNA vector-based RNAi technology to suppress gene expression in mammalian cells. Proc. Natl Acad. Sci. USA 99, 5515–5520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui G., Affarel B., Shi Y. et al. (2004). Yin Yang 1 is a negative regulator of p53. Cell 117, 859–872. [DOI] [PubMed] [Google Scholar]
- Sun M., Wang G., Paciga J.E. et al. (2001). AKT1/PKBalpha kinase is frequently elevated in human cancers and its constitutive activation is required for oncogenic transformation in NIH3T3 cells. Am. J. Pathol. 159, 431–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walen K.H., Stampfer M.R. (1989). Chromosome analyses of human mammary epithelial cells at stages of chemical-induced transformation progression to immortality. Cancer Genet. Cytogenet. 37, 249–261. [DOI] [PubMed] [Google Scholar]
- Wan M., Huang W., Kute T.E. et al. (2012). Yin Yang 1 plays an essential role in breast cancer and negatively regulates p27. Am. J. Pathol. 180, 2120–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R., Brattain M.G. (2006). AKT can be activated in the nucleus. Cell. Signal. 18, 1722–1731. [DOI] [PubMed] [Google Scholar]
- Wilkinson F., Park K., Atchison M.L. (2006). Polycomb recruitment to DNA in vivo by the YY1 REPO domain. Proc. Natl Acad. Sci. USA 103, 19296–19301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson F., Pratt H., Atchison M.L. (2010). PcG recruitment by the YY1 REPO domain can be mediated by Yaf2. J. Cell. Biochem. 109, 478–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakovleva T., Kolesnikova L., Vukojevic V. et al. (2004). YY1 binding to a subset of p53 DNA-target sites regulates p53-dependent transcription. Biochem. Biophys. Res. Commun. 318, 615–624. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Stovall D.B., Inoue K. et al. (2011). The oncogenic role of Yin Yang 1. Crit. Rev. Oncog. 16, 163–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.