Abstract
The initiation of signaling in T lymphocytes in response to the binding of the T cell receptor (TCR) to cognate ligands is a key step in the emergence of adaptive immune responses. Conventional models posit that TCR signaling is initiated by the phosphorylation of receptor-associated immune receptor activation motifs (ITAMs). The cytoplasmic tyrosine kinase Zap70 binds to phosphorylated ITAMs, is subsequently activated, and then propagates downstream signaling. While evidence for such models is provided by experiments with cell lines, in vivo, Zap70 is bound to phosphorylated ITAMs in resting T cells. However, Zap70 is activated only upon TCR binding to cognate ligand. We report the results of computational studies of a new model for the initiation of TCR signaling that incorporates these in vivo observations. Importantly, the new model is shown to allow better and faster TCR discrimination between self-ligands and foreign ligands. The new model is consistent with many past experimental observations, and experiments that could further test the model are proposed.
INTRODUCTION
T lymphocytes (T cells) play an important role in coordinating immune responses to infectious pathogens. They express T cell antigen receptor (TCR) molecules on their surfaces, which can recognize peptides bound to major histocompatibility complex (pMHC) molecules that are displayed on the surfaces of infected, or antigen-presenting, cells. Sufficiently strong binding of the TCR to peptide-MHC molecules can initiate TCR signaling, which is a key step in T cell activation and the development of adaptive immune responses.
Peptides derived from both the host proteome and pathogenic proteins can bind to MHC molecules and can be expressed on the surface of a cell. It is critical that productive TCR signaling resulting in T cell activation be initiated when pathogenic pMHC molecules (agonists) are encountered. T cells discriminate between self-peptides and pathogenic peptides with high specificity and are extraordinarily sensitive to minute amounts of agonists (1). Much effort has been devoted to understanding the cellular machinery and topology of the membrane-proximal signaling network that enables T cells to exhibit these properties (2).
Because of processes that occur during development of T cells in the thymus, TCRs expressed on mature T cells bind weakly to some self-peptide-MHC molecules present on peripheral tissues and antigen-presenting cells. These weak interactions generate some signaling necessary for homeostasis and T cell survival but are insufficient to initiate a full activation response by the T cell. Full activation of the T cell requires a more complete and stronger signal to initiate a cellular response. Although the details of the molecular mechanisms differ, most postulated mechanisms for the ability of TCR signaling to discriminate between agonists and self-ligands are variants of Hopfield's kinetic-proofreading idea, which was first adapted for T cells by Hopfield and McKeithan (3, 4). In brief, one posits that a set of biochemical transformations needs to be completed before productive downstream signaling can ensue. The ligand that binds more strongly to the TCR has a higher probability of remaining engaged for the time required to complete these biochemical reactions. The ability to discriminate between ligands that bind with only modestly dissimilar strengths improves as the number of biochemical transformations to be completed increases. Importantly, this mechanism requires that the biochemical transformations be driven out of equilibrium, which is true, as many of the transformations are phosphorylation reactions that consume ATP.
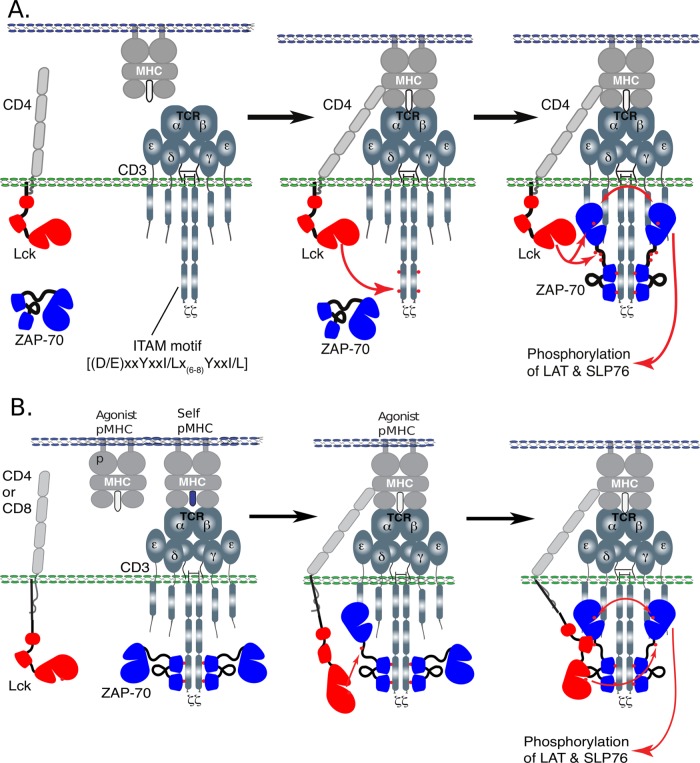
The conventional picture (Fig. 1A) for the earliest steps involved in the initiation of TCR signaling upon receptor engagement are recruitment of a CD4 or CD8 coreceptor-associated kinase, Lck, and subsequent phosphorylation of the tyrosine residues in the cytoplasmic ζ and CD3 chain immunoreceptor tyrosine-based activation motifs (ITAMs). Some models also include a step in which Lck is activated, while experimental work has established that some fraction of coreceptors are associated with already active Lck in the basal state (5–7). Using its tandem SH2 domains, the kinase Zap70 can bind to doubly phosphorylated ITAMs with high affinity and selectivity. Lck phosphorylates and activates Zap70, after which it can transautophosphorylate and activate other vicinal Zap70 molecules. Active Zap70 is required for further downstream signaling events leading to ERK activation and calcium increase (2).
FIG 1.
Models for early steps of TCR signaling. (A) In the conventional model, coreceptor CD4/CD8 binds to MHC when agonist peptide is bound. In the absence of agonist pMHC, ITAM is unphosphorylated, and only in the presence of agonist peptide does ITAM become phosphorylated, leading to Zap70 binding and subsequent activation. (B) In contrast to the conventional model outlined above, it has been shown that even in the absence of agonist pMHC, Zap70 is bound to doubly phosphorylated ITAM but not activated. We suggest a mechanism by which Zap70 can bind to phosphorylated ITAM even in the absence of agonist pMHC, but due to slow subsequent phosphorylation of Tyr319 on Zap70 by Lck and binding of Lck to this residue, full activation is prevented until binding of agonist pMHC.
Previous experimental evidence suggested that in vivo Zap70 is already bound to doubly phosphorylated ITAMs in thymocytes and unstimulated T cells (8). Importantly, however, the Zap70 molecules are not phosphorylated. These in vivo observations suggest that the model described above, as well as its variants, needs modification. Specifically, an appropriate model must describe how continuous weak interactions with self-ligands in vivo (9, 10) allow sufficient ITAM phosphorylation to result in binding of Zap70 but do not allow activation of Zap70 to initiate downstream signaling. It is also important to understand how removal of the kinetic-proofreading steps associated with ITAM phosphorylation and binding of Zap70 impacts ligand discrimination.
In this article, we describe computer simulations carried out to explore a variant of a model we proposed in a recent review article (2) to address these issues. Our results indicate that, indeed, this model is consistent with in vivo observations. Furthermore, the new model is better at discriminating between self- and agonist pMHC ligands than the conventional model. We describe how a specific experiment could test the veracity of the model.
MATERIALS AND METHODS
To study the differences between the new proposed model and the conventional model, we implemented a Gillespie algorithm (11–13) for sampling trajectories of continuous time jump Markov processes. The Gillespie algorithm is a means of sampling from the chemical master equation. We detailed the biochemical reactions that can occur in both models and calculated statistics over many realized stochastic trajectories. For steady-state results, we averaged both temporally (with measurements separated by a sufficiently long burn period) and over many realized trajectories to calculate statistics based on the stationary distribution. Details of all the calculations are outlined in Appendix S4 in the supplemental material.
RESULTS
Proposed signaling model and in silico approach.
Our model (Fig. 1B) is motivated by recent data showing that, after Zap70 binds to a doubly phosphorylated ITAM, Lck must first phosphorylate tyrosines 315 and 319 on Zap70 in order to convert the latter molecule from its inactive to its active conformation (14). We further postulate that Lck can then bind to tyrosine 319 with its SH2 domain, consistent with previous experimental data (15), thus stabilizing the active conformations of both Zap70 and Lck and the entire complex. Active Lck and Zap70 can then further activate other Zap70 molecules, enabling productive downstream signaling. If the rate at which Lck phosphorylates and binds to the Zap70 tyrosine 319 is lower than the rate at which the coreceptor/Lck/TCR-pMHC complex dissociates, then Lck will not activate Zap70 with high probability and productive downstream signaling will not ensue. Even if tyrosine 319 were to be phosphorylated by Lck, it would be transient, as phosphatases would rapidly dephosphorylate the tyrosine if Lck did not bind to it with its SH2 domain. More stable TCR-pMHC bonds, such as with an agonist pMHC, result in a longer lifetime for the coreceptor/Lck/TCR-pMHC complex, enabling activation of Zap70 following the mechanism described above. Note that the proposed model is consistent with experiments showing that the SH2 domain of Lck plays a role in T cell activation and coreceptor function (16–19).
In our computational model, we lump the processes of Lck-mediated tyrosine 319 phosphorylation and subsequent binding via the Lck SH2 domain into one composite step. It is the kinetic rate of these two sequential steps that is important. We have carried out calculations where, upon recruitment to the TCR-pMHC complex, Lck undergoes an activation step (according to some studies), as well as calculations where some fraction of the coreceptors have active Lck associated with them and there is no activation step. The qualitative results for these two cases are the same. The results shown in the text are for the latter model, in which some fraction of coreceptors have active Lck, whereas results for the model including Lck activation are shown in the supplemental material (see Fig. S9 to S12 in the supplemental material).
To simulate the protein interaction networks outlined above and sketched in Fig. 1B, we use the Gillespie algorithm for simulating Markov jump processes (11–13). Details of the simulation, including the simulation volume, the particular rate constants, and the initial copy numbers of all the species used, are provided in the supplemental material (see Appendix S4 in the supplemental material). To compare the properties of the conventional and proposed signaling networks, we use the number of activated Zap70 molecules as a proxy for productive downstream signaling. We estimate steady-state probability distributions of activated Zap70 when the network is stimulated by self-pMHC alone and then when a small percentage (2 to 4%) of agonist pMHC is also present. This is to mimic the fact that a very small number of agonist pMHC molecules can trigger T cell responses (1, 20–26). We carry out calculations over a wide range of values of the ratio of the dissociation rates of the TCR from self-pMHC and agonist (nonself-pMHC) ligands (koff,self/koff,nonself) to assess the ability of the networks to discriminate between self and nonself. Steady-state probability distributions are estimated by simulating long trajectories and recording the number of activated Zap70 molecules periodically (details of the simulation protocol are in Appendix S4 in the supplemental material).
To quantify signal discrimination, we calculate the relative increase in the average number of activated Zap70 molecules upon the introduction of a small quantity of agonist ligands. This is a reasonable metric because, following the intuition of Weber's law, we expect biological systems to respond to the percent change in phosphorylated Zap70 as opposed to the absolute magnitude of the increase. We also compute receiver operating characteristic (ROC) curves, which measure the relative extents of true and false positives (see Appendix S1 in the supplemental material for details) to further examine ligand discrimination by the conventional and new models.
The new model recapitulates in vivo observations.
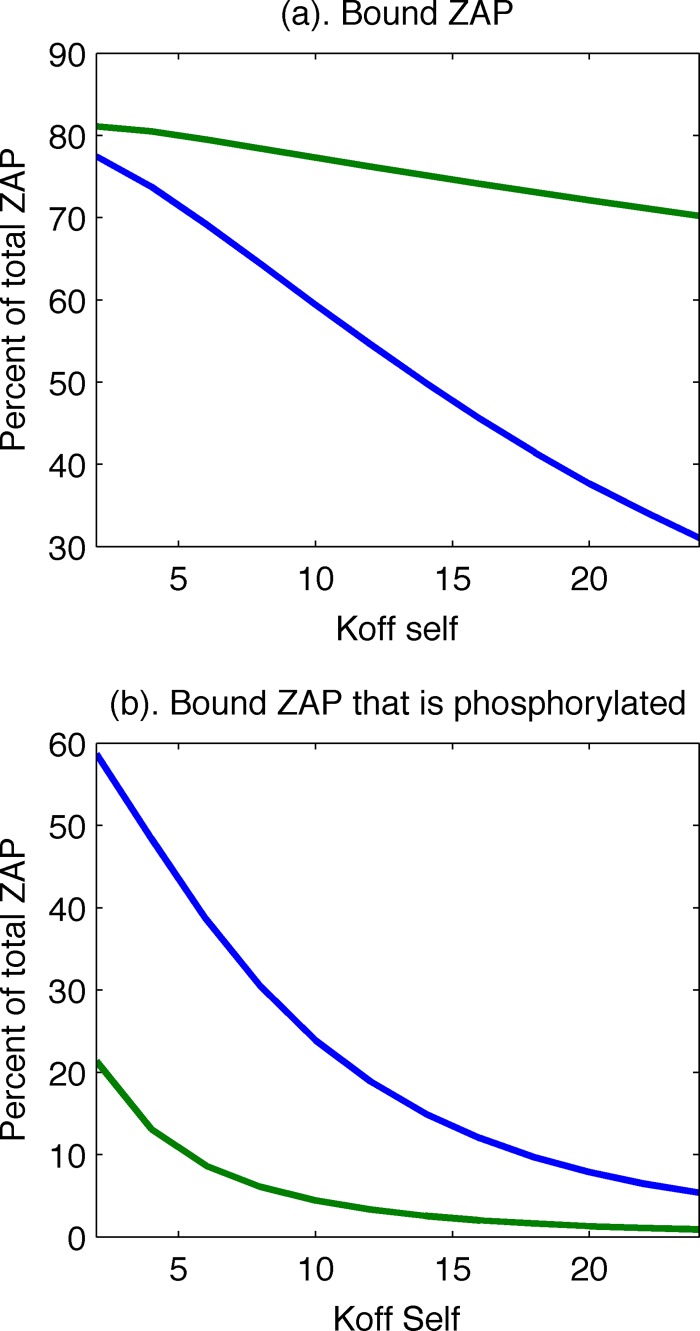
Upon scanning the values of unknown kinetic parameters, we could not find a set of values for which the conventional model could reproduce the in vivo observations that ITAMs are mostly phosphorylated, Zap70 is bound to them, and Zap70 is not phosphorylated. The conventional model can exhibit a high degree of ITAM phosphorylation, with Zap70 bound upon stimulation with self-pMHC alone, but we found that in these circumstances, much of the bound Zap70 is also phosphorylated (Fig. 2). The new model also exhibits a substantial amount of Zap70 bound to phosphorylated ITAMs upon stimulation by self-pMHC alone, but only a negligible fraction of the bound Zap70 molecules are phosphorylated (Fig. 2). Thus, the topology of the new model can recapitulate the in vivo observations, but the conventional model cannot.
FIG 2.
The old model has less Zap70 bound in the absence of agonist pMHC (a), and the percentage of bound Zap70 that is phosphorylated is much higher than in the new model (b). Green lines, new model; blue lines, old model.
An intuitive explanation for these results is that if the parameters are chosen so that stimulation with self-pMHC molecules results in efficient ITAM phosphorylation and binding of Zap70, there is no mechanism to prevent Zap70 from becoming fully activated once it is recruited. The new model is successful in recapitulating the in vivo observation because we introduce a kinetic bottleneck, the phosphorylation of tyrosine 319 on Zap70 followed by binding of Lck to the residue via its SH2 domain, which is necessary for downstream signal propagation. The kinetic-proofreading steps of ITAM phosphorylation and recruitment of Zap70 are replaced by Lck-mediated phosphorylation of tyrosine 319 and binding. Note also that once this sequence of events occurs, it can act like a positive-feedback loop, since Zap70, now held in its active conformation, and localized Lck, whose occupied SH2 domain ensures its continued activity, can phosphorylate other Zap70 molecules and unphosphorylated ITAMs, as well.
As noted above, we have carried out calculations for two variants of the models, one in which Lck is activated upon recruitment to the TCR-pMHC complex and another in which only a certain fraction of coreceptors are associated with active Lck but there is no Lck activation step upon recruitment to the receptor. Figure S9 in the supplemental material shows that the qualitative results are the same for both models.
The new model is better at discriminating between self-ligands and agonist ligands.
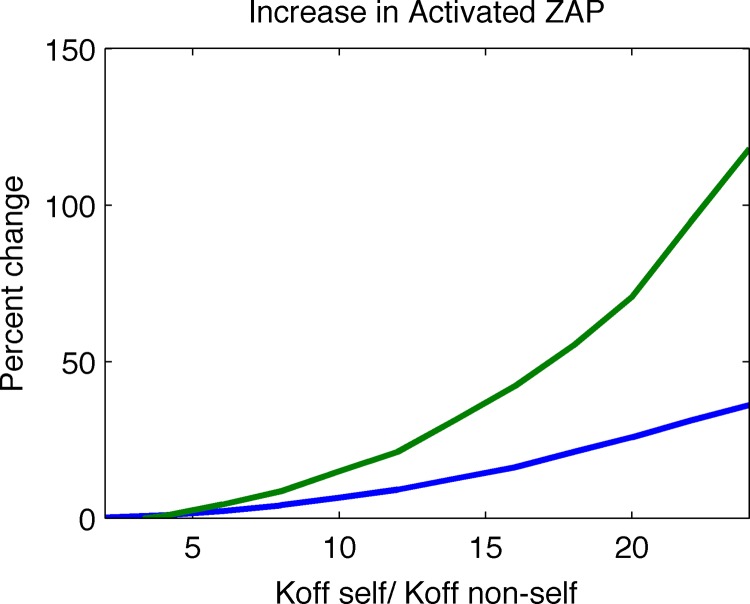
The new model results in a far larger percent increase in activated Zap70 upon introduction of a small fraction of agonists (Fig. 3), indicating that it is better at ligand discrimination.
FIG 3.
The new model exhibits a much greater percent change in activated Zap70 after introducing a small fraction of agonist peptide into the system. The percent change is calculated by allowing the system to reach steady state before and after a small fraction of agonist peptide is added and calculating the average amounts of activated Zap70 in both cases. We calculated this measure of discrimination for various ratios of self/nonself-pMHC-TCR off rates and show that the new model outcompetes the old model for all such ratios. Green line, new model; blue line, old model.
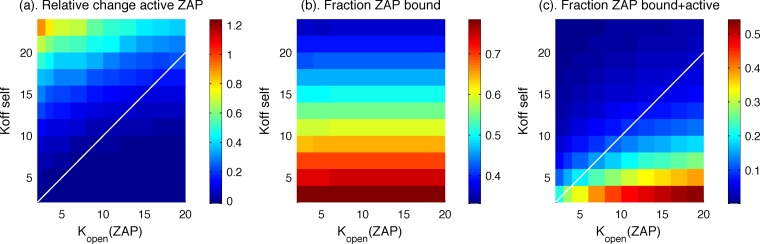
We found that the results shown in Fig. 2 and 3 are robust to variations in the off rate of TCR from self-ligands (koff,self) and the rate at which Lck phosphorylates tyrosine 319 and binds to it (kopen), as long as the latter process is slower than the former. When this condition holds, the new model is better at ligand discrimination (Fig. 4a) and Zap70 molecules bound to ITAMs upon stimulation with self-pMHC are not phosphorylated (Fig. 4c). The results shown in Fig. 4b demonstrate that, as expected, changing the rate kopen does not change the fraction of ZapP70 molecules bound to phosphorylated ITAMs upon stimulation by self-ligands alone. This robustness implies that it is possible to find kinetic parameters such that kopen is less than koff,self and to use experimentally known Zap70 binding kinetics to robustly obtain the results we describe.
FIG 4.
(a) If the TCR-endogenous pMHC off rate (koff self) is greater than the rate at which Lck phosphorylates Tyr319 on Zap70 and binds to it (kopen), there will be a significant increase in the amount of active Zap70 upon stimulation with a small amount of agonist peptide. (b) Changing the rate kopen does not change the fraction of Zap70 that is bound at steady state in the absence of agonist peptide. (c) Negligible amounts of bound Zap70 are active in the regime for which koff self is greater than kopen.
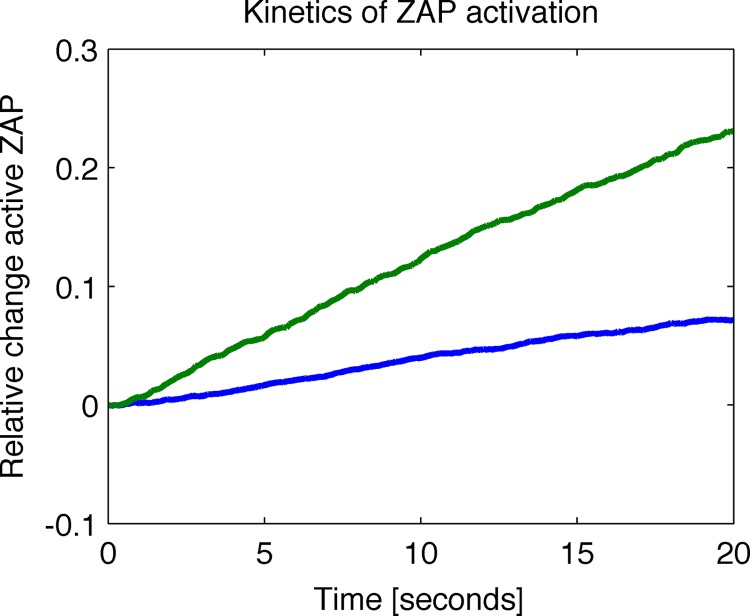
While these results show that at steady state the new model exhibits a larger percent increase in activated Zap70, we also wished to show that the model achieves this response quickly. This is an important metric to consider, because if the new model outperforms the old one at discriminating between ligands but achieves this goal in an unreasonable amount of time, it would not reflect reality (since membrane-proximal signaling occurs quickly [27]). We calculated the average relative change in activated Zap70 as a function of time after the introduction of a small percentage of agonist pMHC. We found that the new model responds faster to cognate ligands (Fig. 5). This is because, upon stimulation by agonists, the kinetic bottleneck is released, and since largely unphosphorylated Zap70 molecules were already bound to the ITAMs, they are rapidly phosphorylated. In the conventional model, in the parameter regime where Zap70 is already bound to ITAMs upon stimulation by self-pMHC, a large fraction of these molecules are already phosphorylated. Therefore, the percentage increase in phosphorylated Zap70 occurs slowly.
FIG 5.
The relative change in active Zap70 as a function of time after stimulation with a small amount (4%) of agonist peptide at time zero shows the new model responds faster to stimulation. The calculations were based on the average of 5,000 trials after a burn time of 500 s with data collected every 0.1 s. Green line, new model; blue line, old model.
The new model is consistent with experiments showing that inhibiting Csk enhances the sensitivity of T cells to weak agonists but not strong agonists.
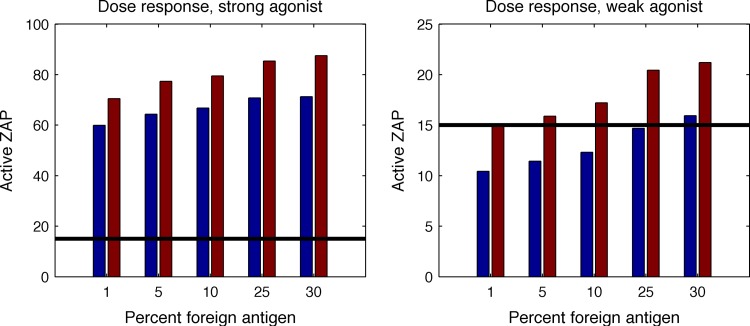
Csk is a cytoplasmic tyrosine kinase that negatively regulates Src family kinases (SFKs), such as Lck, by phosphorylating an inhibitory site in the SFKs. Recent experiments have demonstrated that increasing Lck activity by inhibiting the catalytic function of a PP1 analog-sensitive Csk mutant makes T cells much more sensitive to stimulation by weak agonists but does not significantly alter their sensitivity to strong agonists (28). To explore whether the new model recapitulates this observation, we assume that a threshold number of Zap70 molecules must be phosphorylated for T cell activation. This is because the number of phosphorylated Zap70 molecules is the proxy we are using for productive downstream signaling. Increasing Lck activity by modulating Csk corresponds to increasing the fraction of coreceptors that are bound to active Lck. We found that doubling the fraction of coreceptors with active Lck increases the numbers of phosphorylated Zap70 molecules for all ligands (Fig. 6, left). However, weak agonists can now exceed the threshold number required for T cell activation, whereas the strong agonists already exceed this level of Zap70 phosphorylation with normal levels of Lck activity (Fig. 6, right). Therefore, from the point of view of T cell activation downstream of Zap70, Csk inhibition makes a qualitative difference for stimulation by weak agonists but not for strong agonists. Thus, the predictions of our model are consistent with the recently described experimental findings of Manz et al. (28).
FIG 6.
The new model recapitulates experimental observations with cells with enhanced Lck activation. The blue bars represent average levels of activated Zap70 when the number of coreceptor molecules with active Lck is 133, and the maroon bars correspond to doubling the amount of coreceptor with active Lck. The graphs show dose curves of activated Zap70 as a function of the amount of agonist pMHC in the system. (Left) Dose curve for an agonist-TCR bond with an off rate equal to 0.44 s−1 (a good agonist). (Right) Dose curve for the case where the off rate equals 5.0 s−1 (a weak agonist). The threshold amount of active Zap70 required for T cell activation is shown by the horizontal line. Increasing the Lck activity (by increasing the concentration of active Lck) has no effect on the functional outcome for a good agonist, since all the conditions exceed the threshold. However, for a weak agonist, increasing Lck activity allows activation.
In a model where Lck is activated upon recruitment to the TCR complex, we mimic the effects of inhibiting Csk by increasing the rate of Lck activation. A 5-fold increase has the same qualitative effect as that shown in Fig. 6, right, and allows weaker agonists to exceed the threshold for activation. In Appendix S5 in the supplemental material, we describe calculations demonstrating that the new model also incurs a lower metabolic cost for T cells.
Further experimental tests of the new model.
The necessary condition for the new model to exhibit the properties described here is that kopen must be smaller than the off rate of self-peptide. Excellent estimates for the rates of dissociation of TCR from self-pMHC molecules are available. What is required is a measurement of the rates at which Lck can phosphorylate tyrosine 319 on Zap70 and subsequently bind to it with its SH2 domain (15, 29, 30). The rate of production of Lck bound to tyrosine 319 is encapsulated in kopen.
Another consequence of the new model should be enhanced stabilization of the TCR-CD8-Zap70 complex upon engagement of agonist ligands and productive signaling. Computational and experimental work has shown the kinetics of CD8 to play an important role in T cell discrimination (23, 32). Because CD8 is now effectively bound to the complex, not only through its interaction with the pMHC, but also through the Lck-Zap70 bond, we expect that an important feature of this model is that the off rate of CD8 from a TCR-pMHC will be effectively lowered. It is difficult to determine exactly the extent of stabilization by this mechanism because the binding affinity of Lck's SH2 domain to phosphorylated tyrosine 319 on Zap70 is unknown. We made a conservative estimate of the dissociation rate of the complex by using a discrete-space Markov chain and finding the mean first passage time to dissociation (details are available in Appendix S2 in the supplemental material). The inverse of the mean first passage time is the rate of dissociation. Our rough estimate suggests that the off rate of CD8 from the complex should be reduced by roughly a factor of 4 upon binding of Lck to Zap70 via its SH2 domain.
DISCUSSION
According to conventional models, TCR signaling is initiated by the phosphorylation of ITAMs on ζ-chains. However, in vivo, ITAMs are observed to be phosphorylated in resting T cells. In our proposed model, the steady-state basal activity of Lck is sufficient to allow significant ITAM phosphorylation in vivo, as in this circumstance, transient interactions between coreceptors, TCR, and endogenous pMHC occur continuously. Thus, even though the probability that one such interaction will lead to ITAM phosphorylation can be small, the multitude of events allows a considerable amount of ITAM phosphorylation. Evidence that such basal signaling is ongoing is provided by the observation that stronger interactions with endogenous pMHC allow more ζ-chain phosphorylation and more basal signaling by the TCR (9, 10). Although Zap70 is bound to these phosphorylated ITAMs in vivo, it is not in its active state. Unphosphorylated Zap70 is still autoinhibited as a consequence of the interaction between Tyr319 in interdomain B and the N-terminal catalytic domain. We propose that Lck-mediated phosphorylation of Zap70 at Tyr319 relieves the autoinhibited constraint imposed by Tyr319 on the N lobe of the Zap70 catalytic domain, thereby leading to Zap70 activation. Lck may also bind to Tyr319 via its SH2 domain, further stabilizing the active state of Zap70. In our model, the phosphorylation of Tyr319 and the subsequent binding of Lck to it does not happen upon interactions of the TCR with endogenous peptides, because the rate at which these processes occur is lower than the off rate characterizing TCR-endogenous pMHC bonds. Thus, in the resting state, Zap70 is not activated. However, when longer-lived agonist pMHC-TCR bonds form, these processes can occur, leading to activation of Zap70. Therefore, our model provides an explanation for in vivo observations.
Our computational results show that, compared to the conventional model, the model that we propose is better able to discriminate between endogenous and agonist pMHC ligands under in vivo conditions. This is because in the conventional model, it is not possible to find a set of parameters wherein ITAMs are phosphorylated upon continuous interactions with endogenous pMHC without Zap70 also being activated. Thus, there is only a modest increase in active Zap70 molecules upon introduction of agonists. The new model also leads to faster Zap70 activation when agonist pMHC is expressed on antigen-presenting cells because there are fewer steps to complete. Also, our results show that the new model is sensitive to small amounts of agonists present in a sea of endogenous ligands, as observed in experiments (1, 7, 22, 24). Thus, our proposed model is consistent with in vivo observations and satisfies the key features of extraordinary selectivity and sensitivity that characterize the earliest events in TCR signaling.
The model we propose is also not in conflict with recent computational and experimental studies, which suggest that the half-life of the TCR-agonist pMHC bond required for productive signaling in vitro is set by the time scale associated with a coreceptor-bound active Lck molecule finding a TCR bound to agonist ligand (5). Our new model would posit that in vivo once this happens, the next step is not ITAM phosphorylation, but rather, phosphorylation of Tyr319 on Zap70.
The binding of Lck to Tyr319 on Zap70 could also stabilize the active conformation of Lck, localize it to the TCR-agonist pMHC complex by hindering passive diffusion, and also stabilize the TCR-pMHC-coreceptor complex (as seen in our calculations). Stabilization of the active conformation of Lck promotes its ability to further phosphorylate other Lck molecules and Zap70 molecules associated with vicinal TCRs that are bound to other agonist or endogenous ligands, thereby generating a positive-feedback mechanism that could then overwhelm other, negative-feedback loops that maintain the resting state. By this means, our model is also in harmony with studies that suggest that signaling due to TCR-agonist pMHC ligands is amplified by endogenous pMHC molecules (6, 7, 23). The importance of Lck's SH2 domain for its function has also been emphasized by studies wherein CD4 coreceptor function was reconstituted in a Lck-sufficient antigen-specific hybridoma with CD4-Lck fusion proteins (17).
Tyr493 in the Zap70 activation loop is “preferentially” phosphorylated through transphosphorylation by Zap70 rather than by Lck (30). Thus, the presence of multiple dimeric ITAMs may serve to position pairs of Zap70 molecules across from each other so that they can mediate transautophosphorylation and activation following release from autoinhibition by Lck phosphorylation of Tyr319. Triggering this positive-feedback loop may represent an additional time scale that sets the threshold half-life of the TCR-pMHC bond required for productive downstream signaling.
The importance of Zap70 autoinhibition involving the Tyr319 catalytic domain interaction is highlighted by a recent report of a severe familial autoimmunity syndrome that results from the inheritance of compound heterozygous mutations (31). The two affected children inherited a weak hypermorphic mutation in Zap70, R360P, in the N lobe of the catalytic domain, which is combined with a loss-of-function allele. R360P is predicted to weaken the interaction of Tyr319 with the N lobe. This weak hypermorphic mutation exhibits increased sensitivity to TCR stimulation and downstream signaling to Zap70 substrates in a reconstitution system. This mutation strongly supports the current model and emphasizes the importance of the autoinhibitory mechanism.
While our model is consistent with many old and recent observations, our computational results suggest key experiments that could test it further. A key assumption in the model is that the rate of phosphorylation of Tyr319 on Zap70 by Lck and the subsequent binding of Lck to this residue via Lck's SH2 domain is lower than the off rate characterizing endogenous pMHC-TCR bonds. Thus, a critical test of our model requires measuring the rate at which Lck phosphorylates Tyr319 on Zap70 and subsequently binds to it. We hope that such experiments will be possible in the future.
Supplementary Material
ACKNOWLEDGMENT
This work was supported in part by a grant from the U.S. National Institutes of Health (PO1 AI091580).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/MCB.00165-16.
REFERENCES
- 1.Irvine DJ, Purbhoo MA, Krogsgaard M, Davis MM. 2002. Direct observation of ligand recognition by T cells. Nature 419:845–849. doi: 10.1038/nature01076. [DOI] [PubMed] [Google Scholar]
- 2.Chakraborty AK, Weiss A. 2014. Insights into the initiation of TCR signaling. Nat Immunol 15:798–807. doi: 10.1038/ni.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hopfield JJ. 1974. Kinetic proofreading: a new mechanism for reducing errors in biosynthetic processes requiring high specificity. Proc Natl Acad Sci U S A 71:4135–4139. doi: 10.1073/pnas.71.10.4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McKeithan TW. 1995. Kinetic proofreading in T-cell receptor signal transduction. Proc Natl Acad Sci U S A 92:5042–5046. doi: 10.1073/pnas.92.11.5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stepanek O, Prabhakar A, Osswald C, King C, Bulek A, Naeher D, Beaufils-Hugot M, Abanto M, Galati V, Hausmann B, Lang R, Cole D, Huseby E, Sewell A, Chakraborty AK, Palmer E. 2014. Coreceptor scanning by the T cell receptor provides a mechanism for T cell tolerance. Cell 159:333–345. doi: 10.1016/j.cell.2014.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krogsgaard M, Li Q, Sumen C, Huppa JB, Huse M, Davis MM. 2005. Agonist/endogenous peptide-MHC heterodimers drive T cell activation and sensitivity. Nature 434:238–243. doi: 10.1038/nature03391. [DOI] [PubMed] [Google Scholar]
- 7.Li Q, Dinner AR, Qi S, Irvine DJ, Huppa JB, Davis MM, Chakraborty AK. 2004. CD4 enhances T cell sensitivity to antigen by coordinating Lck accumulation at the immunological synapse. Nat Immunol 8:791–799. [DOI] [PubMed] [Google Scholar]
- 8.Van Oers NS, Killeen N, Weiss A. 1994. ZAP-70 is constitutively associated with tyrosine-phosphorylated TCRζ in murine thymocytes and lymph node T cells. Immunity 1:675–685. doi: 10.1016/1074-7613(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 9.Persuad SP, Parker CR, Lo WL, Weber KS, Allen PM. 2014. Intrinsic CD4+ T cell sensitivity and response to a pathogen are set and sustained by avidity for thymic and peripheral complexes of self peptides and MHC. Nat Immunol 15:266–274. doi: 10.1038/ni.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mandl JN, Monteiro JP, Vrisekoop N, Germain RN. 2013. T cell-positive selection uses self-ligand binding strength to optimize repertoire recognition of foreign antigens. Immunity 38:263–274. doi: 10.1016/j.immuni.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gillespie DT. 1976. A general method for numerically simulating the stochastic time evolution of coupled chemical reactions. J Comput Phys 22:403–434. doi: 10.1016/0021-9991(76)90041-3. [DOI] [Google Scholar]
- 12.Gillespie DT. 1977. Exact stochastic simulation of coupled chemical reactions. J Phys Chem 25:2340–2361. [Google Scholar]
- 13.Gillespie DT. 1992. A rigorous derivation of the chemical master equation. Physica A 188:404–425. doi: 10.1016/0378-4371(92)90283-V. [DOI] [Google Scholar]
- 14.Yan Q, Barros T, Visperas PR, Deindl S, Kadlecek TA, Weiss A, Kuriyan J. 2013. Structural basis for activation of ZAP-70 by phosphorylation of the SH2-kinase linker. Mol Cell Biol 11:2188–2201. doi: 10.1128/MCB.01637-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pelosi M, Di Bartolo V, Mounier V, Mège D, Pascussi J, Dufour E, Blondel A, Acuto O. 1999. Tyrosine 319 in the interdomain B of ZAP-70 is a binding site for the Src homology 2 domain of Lck. J Biol Chem 274:14229–14237. doi: 10.1074/jbc.274.20.14229. [DOI] [PubMed] [Google Scholar]
- 16.Hui E, Vale R. 2014. In vitro membrane reconstitution of the T-cell receptor proximal signaling network. Nat Struct Mol Biol 21:133–142. doi: 10.1038/nsmb.2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu H, Littman DR. 1993. A kinase-independent function of Lck in potentiating antigen-specific T cell activation. Cell 74:633–643. doi: 10.1016/0092-8674(93)90511-N. [DOI] [PubMed] [Google Scholar]
- 18.Straus DB, Chan AC, Patai B, Weiss A. 1996. SH2 domain function is essential for the role of Lck tyrosine kinase in T cell receptor signal transduction. J Biol Chem 271:9976–9981. doi: 10.1074/jbc.271.17.9976. [DOI] [PubMed] [Google Scholar]
- 19.Thome M, Duplay P, Guttinger M, Acuto O. 1995. Syk and ZAP-70 mediate recruitment of p56lck/CD4 to the activated T cell receptor/CD3/zeta complex. J Exp Med 181:1997–2006. doi: 10.1084/jem.181.6.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang J, Brameshuber M, Zeng X, Xie J, Li Q, Chien Y, Valitutti S, Davis M. 2013. A single peptide-major histocompatibility complex ligand triggers digital cytokine secretion in CD4(+) T cells. Immunity 39:846–857. doi: 10.1016/j.immuni.2013.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Purbhoo MA, Irvine DJ, Huppa JB, Davis MM. 2004. T cell killing does not require the formation of a stable mature immunological synapse. Nat Immunol 5:524–530. doi: 10.1038/ni1058. [DOI] [PubMed] [Google Scholar]
- 22.Sykulev Y, Joo M, Vturnia I, Tsomides TJ, Eisen HN. 1996. Evidence that a single peptide-MHC complex on a target cell can elicit a cytolitic T cell response. Immunity 4:565–571. doi: 10.1016/S1074-7613(00)80483-5. [DOI] [PubMed] [Google Scholar]
- 23.Hoerter J, Brzostek J, Artyomov M, Abel S, Casas J, Rybakin V, Ampudia J, Lotz C, Connolly J, Chakraborty AK, Gould K, Gascoigne N. 2013. Coreceptor affinity for MHC defines peptide specificity requirements for TCR interaction with coagonist peptide-MHC. J Exp Med 210:1807–1821. doi: 10.1084/jem.20122528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davis MM, Krogsgaard M, Huse M, Huppa J, Lillemeier BF, Li QJ. 2007. T cells as a self-referential, sensory organ. Annu Rev Immunol 25:681–695. doi: 10.1146/annurev.immunol.24.021605.090600. [DOI] [PubMed] [Google Scholar]
- 25.Holler PD, Kranz DM. 2003. Quantitative analysis of the contribution of TCR/pepMHC affinity and CD8 to T cell activation. Immunity 18:255–264. doi: 10.1016/S1074-7613(03)00019-0. [DOI] [PubMed] [Google Scholar]
- 26.Christinck ER, Luscher MA, Barber BH, Williams DB. 1991. Peptide binding to class I MHC on living cells and quantitation of complexes required for CTL lysis. Nature 352:67–70. doi: 10.1038/352067a0. [DOI] [PubMed] [Google Scholar]
- 27.Huse M, Klein LO, Girvin AT, Faraj JM, Li QJ, Kuhns MS, Davis MM. 2007. Spatial and temporal dynamics of T cell receptor signaling with a photoactivatable agonist. Immunity 27:76–88. doi: 10.1016/j.immuni.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 28.Manz B, Tan YX, Coutney AH, Rutaganira F, Palmer E, Shokat K, Weiss A. 2015. Small molecule inhibition of Csk alters affinity recognition by T cells. eLife 4:e08088. doi: 10.7554/eLife.08088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yan Q, Barros T, Visperas PR, Deindl S, Kadlecek TA, Weiss A, Kuriyan J. 2013. Structural basis for activation of ZAP-70 by phosphorylation of the SH2-kinase linker. Mol Cell Biol 33:2188–2201. doi: 10.1128/MCB.01637-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brdicka T, Kadlecek TA, Roose JP, Pastuszak AW, Weiss A. 2005. Intramolecular regulatory switch in ZAP-70: analogy with receptor tyrosine kinases. Mol Cell Biol 25:4924–4933. doi: 10.1128/MCB.25.12.4924-4933.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsu LY, Tan YX, Xiao Z, Malissen M, Weiss A. 2009. A hypomorphic allele of ZAP-70 reveals a distinct thymic threshold for autoimmune disease versus autoimmune reactivity. J Exp Med 206:2527–2541. doi: 10.1084/jem.20082902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Artyomov MN, Lis M, Devadas S, Davis M, Chakraborty AK. 2010. CD4 and CD8 binding to MHC molecules primarily acts to enhance Lck delivery. Proc Natl Acad Sci U S A 107:16916–16921. doi: 10.1073/pnas.1010568107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.