Abstract
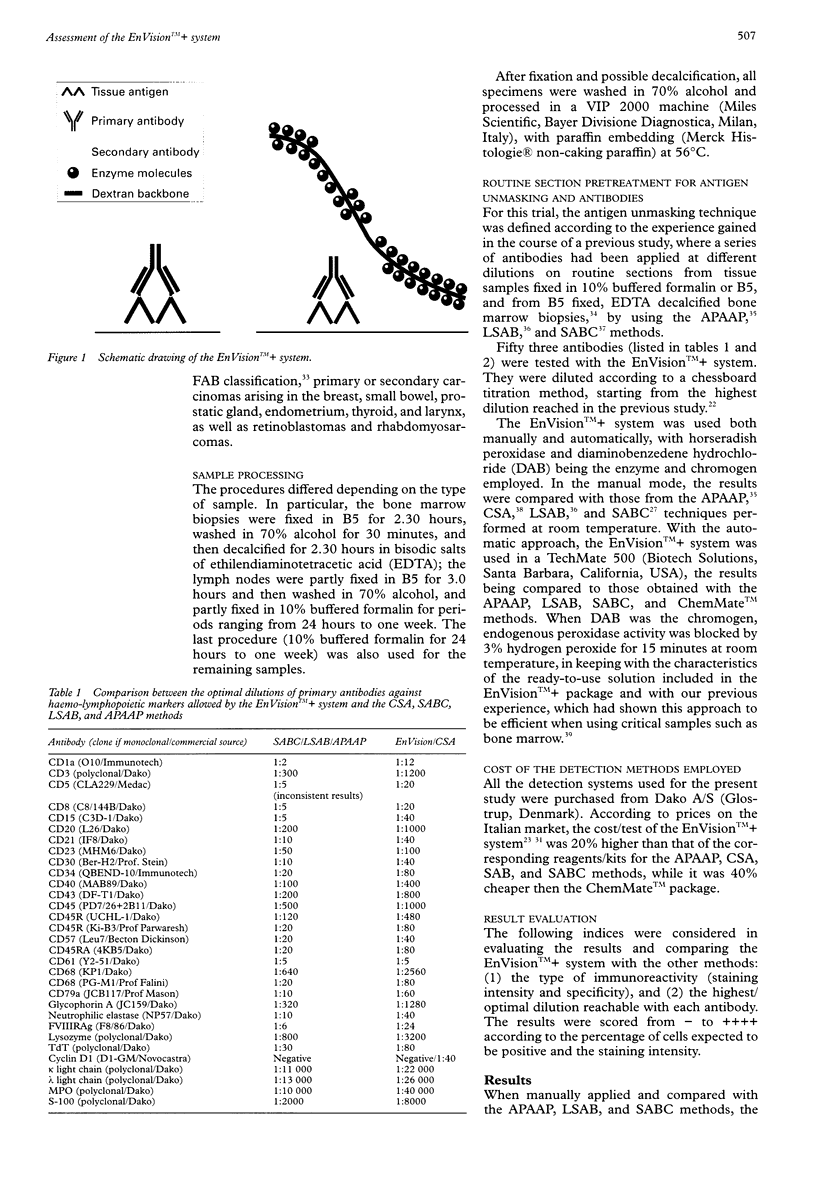
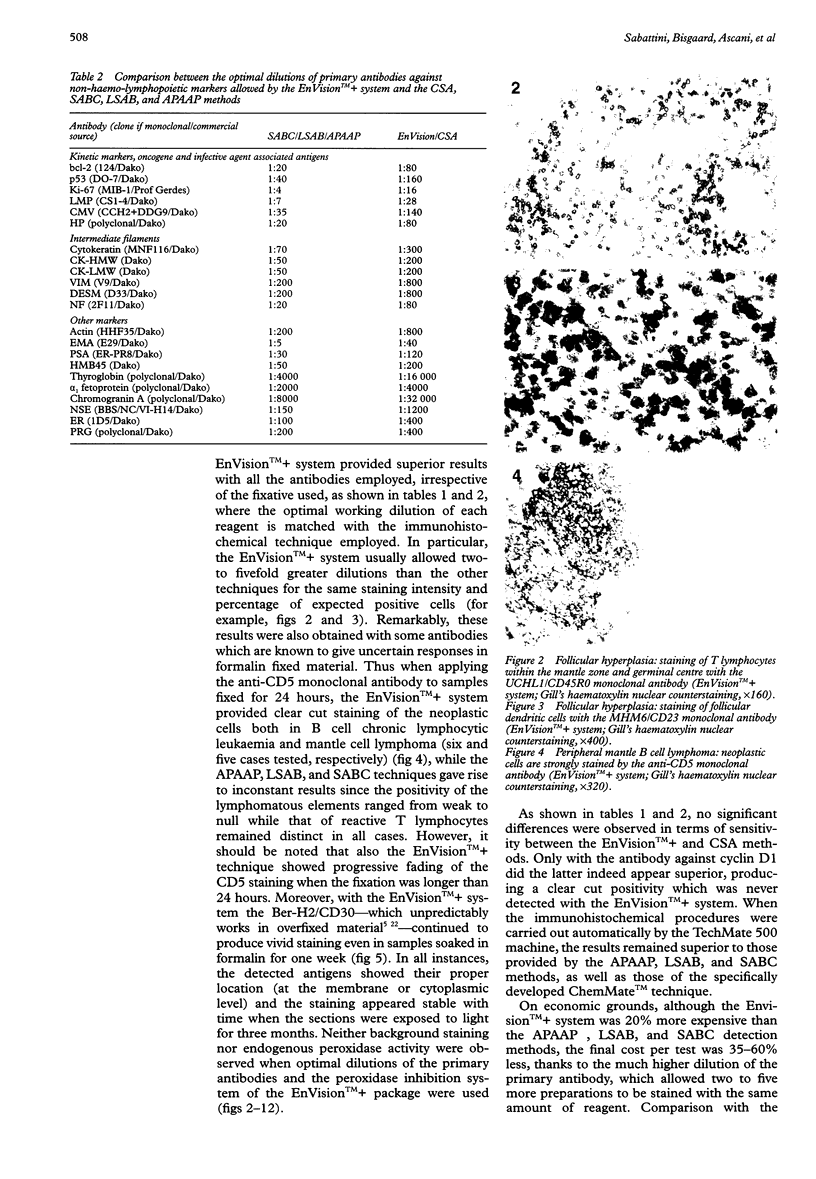
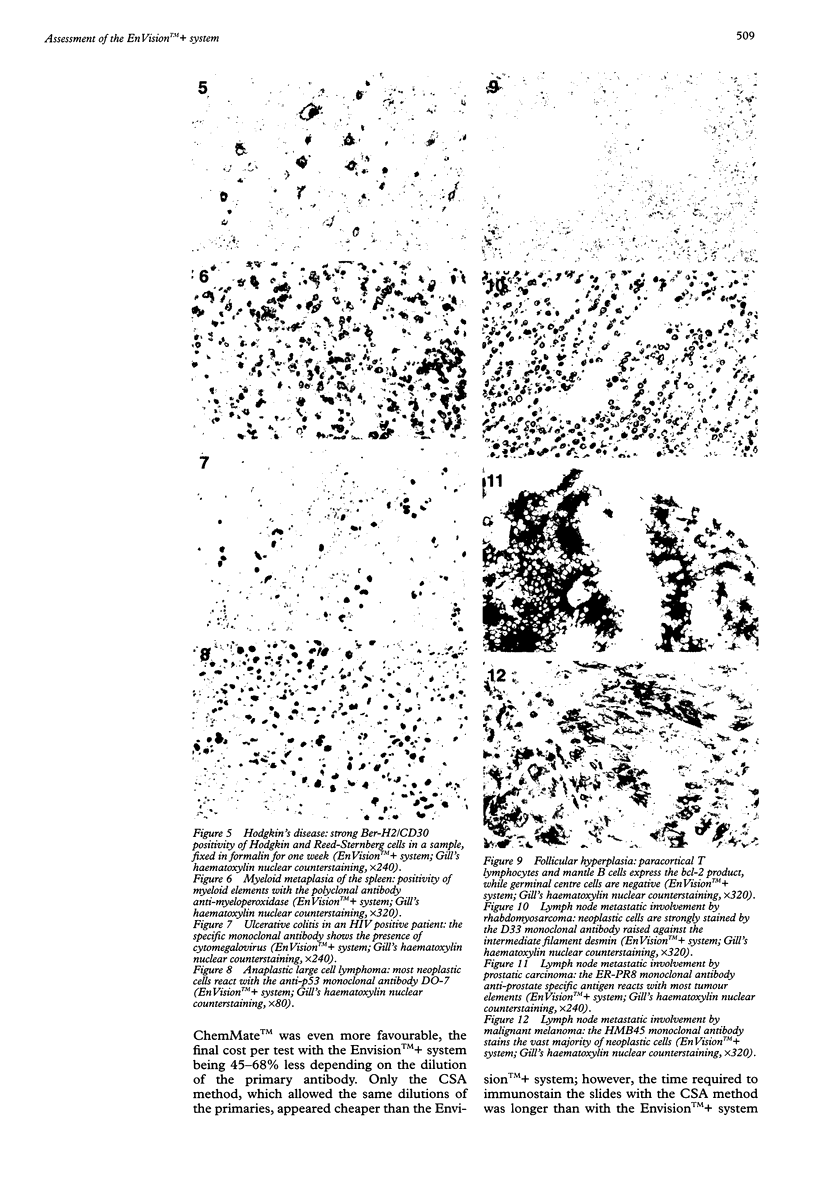
AIM: To assess a newly developed immunohistochemical detection system, the EnVision++. METHODS: A large series of differently processed normal and pathological samples and 53 relevant monoclonal antibodies were chosen. A chessboard titration assay was used to compare the results provided by the EnVision++ system with those of the APAAP, CSA, LSAB, SABC, and ChemMate methods, when applied either manually or in a TechMate 500 immunostainer. RESULTS: With the vast majority of the antibodies, EnVision++ allowed two- to fivefold higher dilutions than the APAAP, LSAB, SABC, and ChemMate techniques, the staining intensity and percentage of expected positive cells being the same. With some critical antibodies (such as the anti-CD5), it turned out to be superior in that it achieved consistently reproducible results with differently fixed or overfixed samples. Only the CSA method, which includes tyramide based enhancement, allowed the same dilutions as the EnVision++ system, and in one instance (with the anti-cyclin D1 antibody) represented the gold standard. CONCLUSIONS: The EnVision++ is an easy to use system, which avoids the possibility of disturbing endogenous biotin and lowers the cost per test by increasing the dilutions of the primary antibodies. Being a two step procedure, it reduces both the assay time and the workload.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett J. M., Catovsky D., Daniel M. T., Flandrin G., Galton D. A., Gralnick H. R., Sultan C. Proposals for the classification of the acute leukaemias. French-American-British (FAB) co-operative group. Br J Haematol. 1976 Aug;33(4):451–458. doi: 10.1111/j.1365-2141.1976.tb03563.x. [DOI] [PubMed] [Google Scholar]
- Bobrow M. N., Harris T. D., Shaughnessy K. J., Litt G. J. Catalyzed reporter deposition, a novel method of signal amplification. Application to immunoassays. J Immunol Methods. 1989 Dec 20;125(1-2):279–285. doi: 10.1016/0022-1759(89)90104-x. [DOI] [PubMed] [Google Scholar]
- Cattoretti G., Becker M. H., Key G., Duchrow M., Schlüter C., Galle J., Gerdes J. Monoclonal antibodies against recombinant parts of the Ki-67 antigen (MIB 1 and MIB 3) detect proliferating cells in microwave-processed formalin-fixed paraffin sections. J Pathol. 1992 Dec;168(4):357–363. doi: 10.1002/path.1711680404. [DOI] [PubMed] [Google Scholar]
- Cattoretti G., Pileri S., Parravicini C., Becker M. H., Poggi S., Bifulco C., Key G., D'Amato L., Sabattini E., Feudale E. Antigen unmasking on formalin-fixed, paraffin-embedded tissue sections. J Pathol. 1993 Oct;171(2):83–98. doi: 10.1002/path.1711710205. [DOI] [PubMed] [Google Scholar]
- Chilosi M., Lestani M., Pedron S., Montagna L., Benedetti A., Pizzolo G., Menestrina F. A rapid immunostaining method for frozen sections. Biotech Histochem. 1994 Jul;69(4):235–239. doi: 10.3109/10520299409106292. [DOI] [PubMed] [Google Scholar]
- Cordell J. L., Falini B., Erber W. N., Ghosh A. K., Abdulaziz Z., MacDonald S., Pulford K. A., Stein H., Mason D. Y. Immunoenzymatic labeling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase (APAAP complexes). J Histochem Cytochem. 1984 Feb;32(2):219–229. doi: 10.1177/32.2.6198355. [DOI] [PubMed] [Google Scholar]
- Falini B., Pileri S., Martelli M. F. Histological and immunohistological analysis of human lymphomas. Crit Rev Oncol Hematol. 1989;9(4):351–419. doi: 10.1016/s1040-8428(89)80018-6. [DOI] [PubMed] [Google Scholar]
- Giorno R. A comparison of two immunoperoxidase staining methods based on the avidin-biotin interaction. Diagn Immunol. 1984;2(3):161–166. [PubMed] [Google Scholar]
- Harris N. L., Jaffe E. S., Stein H., Banks P. M., Chan J. K., Cleary M. L., Delsol G., De Wolf-Peeters C., Falini B., Gatter K. C. A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood. 1994 Sep 1;84(5):1361–1392. [PubMed] [Google Scholar]
- Mason J. T., O'Leary T. J. Effects of formaldehyde fixation on protein secondary structure: a calorimetric and infrared spectroscopic investigation. J Histochem Cytochem. 1991 Feb;39(2):225–229. doi: 10.1177/39.2.1987266. [DOI] [PubMed] [Google Scholar]
- Morgan J. M., Navabi H., Schmid K. W., Jasani B. Possible role of tissue-bound calcium ions in citrate-mediated high-temperature antigen retrieval. J Pathol. 1994 Dec;174(4):301–307. doi: 10.1002/path.1711740410. [DOI] [PubMed] [Google Scholar]
- Norton A. J., Jordan S., Yeomans P. Brief, high-temperature heat denaturation (pressure cooking): a simple and effective method of antigen retrieval for routinely processed tissues. J Pathol. 1994 Aug;173(4):371–379. doi: 10.1002/path.1711730413. [DOI] [PubMed] [Google Scholar]
- Pileri S. A., Roncador G., Ceccarelli C., Piccioli M., Briskomatis A., Sabattini E., Ascani S., Santini D., Piccaluga P. P., Leone O. Antigen retrieval techniques in immunohistochemistry: comparison of different methods. J Pathol. 1997 Sep;183(1):116–123. doi: 10.1002/(SICI)1096-9896(199709)183:1<116::AID-PATH1087>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Pileri S. A., Roncador G., Ceccarelli C., Piccioli M., Sabattini E., Briskomatis A., Santini D., Leone O., Piccaluga P. P., Leoncini L. Immunohistochemistry of bone-marrow biopsy. Leuk Lymphoma. 1997 Dec;26 (Suppl 1):69–75. doi: 10.3109/10428199709058602. [DOI] [PubMed] [Google Scholar]
- Pileri S., Falini B., Sabattini E., Bigerna B., Gherlinzoni F., Tazzari P. L. Immunohistochemistry of malignant lymphomas. Advantages and limitations of the new monoclonal antibodies working in paraffin sections. Haematologica. 1991 May-Jun;76(3):226–234. [PubMed] [Google Scholar]
- Pileri S., Serra L., Martinelli G. The use of pronase enhances sensitivity of the PAP method in the detection of intracytoplasmic immunoglobulins. Basic Appl Histochem. 1980;24(3):203–207. [PubMed] [Google Scholar]
- Suurmeijer A. J., Boon M. E. Notes on the application of microwaves for antigen retrieval in paraffin and plastic tissue sections. Eur J Morphol. 1993 Mar-Jun;31(1-2):144–150. [PubMed] [Google Scholar]
- Taylor C. R., Shi S. R., Chaiwun B., Young L., Imam S. A., Cote R. J. Strategies for improving the immunohistochemical staining of various intranuclear prognostic markers in formalin-paraffin sections: androgen receptor, estrogen receptor, progesterone receptor, p53 protein, proliferating cell nuclear antigen, and Ki-67 antigen revealed by antigen retrieval techniques. Hum Pathol. 1994 Mar;25(3):263–270. doi: 10.1016/0046-8177(94)90198-8. [DOI] [PubMed] [Google Scholar]
- Tsutsumi Y., Serizawa A., Kawai K. Enhanced polymer one-step staining (EPOS) for proliferating cell nuclear antigen (PCNA) and Ki-67 antigen: application to intra-operative frozen diagnosis. Pathol Int. 1995 Feb;45(2):108–115. doi: 10.1111/j.1440-1827.1995.tb03430.x. [DOI] [PubMed] [Google Scholar]
- Van der Loos C. M., Naruko T., Becker A. E. The use of enhanced polymer one-step staining reagents for immunoenzyme double-labelling. Histochem J. 1996 Oct;28(10):709–714. doi: 10.1007/BF02409008. [DOI] [PubMed] [Google Scholar]














