Abstract
α-Synuclein, which is present as a small, soluble, cytosolic protein in healthy subjects, is converted to amyloid-like fibrils in diseases such as Parkinson’s disease (PD), dementia with Lewy bodies (DLB), and multiple system atrophy (MSA). Bulk synthesis of purified α-synuclein has made it more convenient to study the nature of the normal protein and the mechanism of its conversion to an abnormal form in vitro and in vivo. Synthetic α-synuclein fibrils and pathological α-synuclein from diseased brains can act as triggers to convert normal α-synuclein to an abnormal form via prion-like mechanisms. In this article, we describe the experimental pathologies of α-synuclein both in vitro and in vivo in human and animal models. Prion-like spreading of abnormal α-synuclein from cell to cell can account for the progression of these α-synucleinopathies.
In vitro and in vivo studies show that prion-like conversion of normal α-synuclein to an abnormal form and cell-to-cell transmission are key events in the progression of α-synucleinopathies (e.g., Parkinson’s).
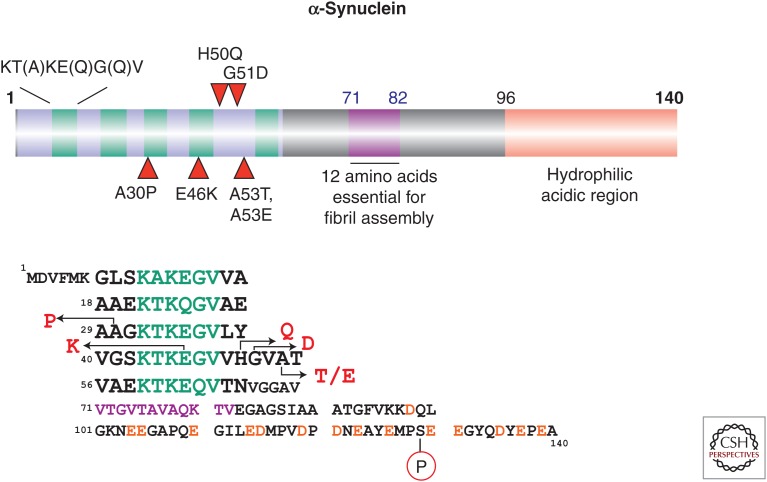
α-Synuclein is a relatively small, cytosolic protein containing 140 amino acid residues. It is abundantly expressed in the brain, where it is located in presynaptic nerve terminals (Maroteaux et al. 1988; Uéda et al. 1993). The N-terminal region (residues 7–66) consists of five imperfect repeats, each 11 amino acids in length, with the consensus sequence KT(A)KE(Q)G(Q)V (Fig. 1). The tandem repeats are continuous, except for a four-amino-acid stretch between repeats 4 and 5. The repeat region has been assumed to form an amphipathic α-helix by binding to phospholipid. The C-terminal region (amino acids 96–140) is negatively charged and hydrophilic (Fig. 1). Expression of α-synuclein has been detected not only in the brain but also in other tissues, including the placenta, lungs, kidneys, and heart. The protein is also abundantly present in blood cells. Its physiological role has not been fully elucidated, but studies with knockout mice suggest that α-synuclein is involved in regulation of dopamine release and transport (Abeliovich et al. 2000; Chandra et al. 2004).
Figure 1.
Schematic illustration of the amino acid sequence of human α-synuclein. Five missense mutations identified in familial forms of Parkinson’s disease (PD) or dementia with Lewy bodies (DLB) and an abnormal phosphorylation site (Ser129) identified in pathological α-synuclein from diseased brains are shown.
The identification of a missense mutation in the α-synuclein gene SNCA in pedigrees of Parkinson’s disease (PD) sheds light on the nature of Lewy bodies (Polymeropoulos et al. 1997), and subsequent immunohistochemical work with anti-α-synuclein antibodies has revealed that α-synuclein is the major component of Lewy bodies (LBs) and Lewy neurites (LNs) in PD and glial cytoplasmic inclusions in multiple system atrophy (MSA) (Spillantini et al. 1997; Baba et al. 1998; Wakabayashi et al. 1998; Spillantini et al. 1998a; Goedert 2001). Thus far, genetic studies indicate that six missense mutations (A30P, E46K, H50Q, G51D, A53T, and A53E) in SNCA are associated with familial forms of PD and dementia with Lewy bodies (DLB) (Fig. 1) (Polymeropoulos et al. 1997; Krüger et al. 1998; Zarranz et al. 2004; Appel-Cresswell et al. 2013; Lesage et al. 2013; Pasanen et al. 2014). In addition, multiplications (duplication and triplication) of SNCA are associated with inherited forms of PD and DLB (Singleton et al. 2003; Chartier-Harlin et al. 2004; Ibáñez et al. 2004), indicating that an increased level of intracellular α-synuclein contributes to onset of familial disease.
Immunohistochemical and ultrastructural studies of α-synuclein in the brains of patients with PD, DLB, MSA, and other neurodegenerative diseases have demonstrated that α-synuclein is deposited as filamentous or fibrous structures of ∼5–10 nm diameter (Spillantini et al. 1998b). Biochemical and protein chemical studies revealed that most of the pathological α-synuclein recovered in a sarkosyl-insoluble fraction is aberrantly phosphorylated at Ser129 (Fig. 1) and also partially ubiquitinated (Fujiwara et al. 2002; Hasegawa et al. 2002; Anderson et al. 2006). Therefore, an antibody to α-synuclein’s phospho-Ser129 (PS129) is widely used to detect the abnormal form of α-synuclein in diseased brains as well as in cellular and animal models. Antibodies to ubiquitin and ubiquitin-binding protein p62 are also useful for co-immunostainings with anti-α-synuclein to distinguish the abnormal from the normal form (Table 1).
Table 1.
Structural and biochemical differences between normal and abnormal α-synuclein in the human brain
| State | Structure | Solubility | Reactions to antibodies | ||
|---|---|---|---|---|---|
| PS129 | Anti-Ub | Anti-p62 | |||
| Healthy (normal) | Unstructured, long-range interactions, ThioS (−) | Buffer-soluble | P129 (−) | Ub (−) | p62 (−) |
| Disease (abnormal) | Filamentous/fibrous, cross-β, ThioS (+) | Sarkosyl-insoluble | P129 (+) | Ub (+) | p62 (+) |
CONFORMATIONAL CHANGES OF α-SYNUCLEIN IN DISEASE CONDITIONS
Circular dichroism and Fourier transform infrared analyses have shown that α-synuclein is a natively unfolded protein with little ordered secondary structure (Weinreb et al. 1996; Uversky et al. 2001). However, nuclear magnetic resonance (NMR) analysis has revealed three intramolecular long-range interactions between (1) the highly hydrophobic region (residues 85–95) and the C terminus (residues 110–130); (2) the C-terminal residues 120–130 and residues 105–115; and (3) the region around residue 120 and the N terminus around residue 20. Additionally, it is noteworthy that parkinsonism-linked mutations greatly perturb these specific tertiary interactions of native α-synuclein (Bertoncini et al. 2005a,b).
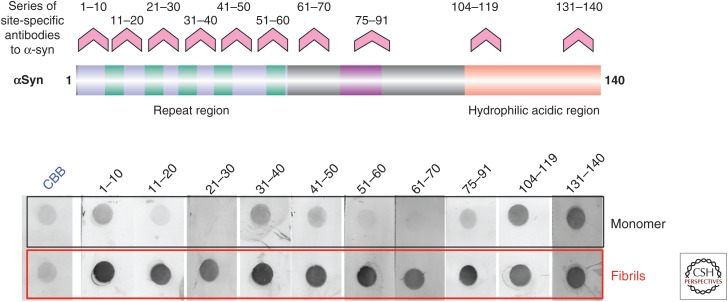
Upon incubation at a high concentration at 37°C with shaking, recombinant α-synuclein in vitro assembles into fibrils that closely resemble those in brains with PD and DLB, whereas other synuclein family proteins (i.e., β-synuclein and γ-synuclein) neither accumulate in the brain (Spillantini et al. 1997, 1998) nor form fibrils (Biere et al. 2000; Serpell et al. 2000). Sequence comparison and in vitro aggregation experiments revealed that the 12 amino acid residues (71–82), where the sequence differs between α- and β-synuclein, are essential for the assembly of α-synuclein fibrils (Fig. 1) (Giasson et al. 2001). During assembly, conformational change from a random coil to a β-sheet structure can be observed. X-ray fiber diffraction and electron diffraction analyses have shown that the transition from a natively unfolded to a cross-β-structure underlies the assembly of α-synuclein into fibrils (Serpell et al. 2000). Both the conformational change of α-synuclein and its inhibition by certain small molecules can be detected by epitope-specific antibodies (Fig. 2) (Masuda et al. 2009; Yonetani et al. 2009). Antibodies to the C-terminal region recognize monomers and fibrils equally, whereas antibodies to the N-terminal region react strongly with fibrils but weakly with monomers (Fig. 2). Under physiological conditions, α-synuclein is known to populate an ensemble of conformations, including conformers that are more compact than expected for a random-coil protein (Syme et al. 2001; Lee et al. 2004; Maiti et al. 2004). The core of the fibril spans approximately residues 30–100 of α-synuclein (Miake et al. 2002; Der-Sarkissian et al. 2003) and is believed to comprise five parallel β-strands that are separated by flexible loops (Vilar et al. 2008).
Figure 2.
Conformational changes detected by site-specific antibodies to α-synuclein. Multiple site-specific antibodies (displayed above peptides corresponding to residues 1–10, 11–20, 21–30, 31–40, 41–50, 51–60, 61–70, 75–91, 104–119, and 131–140 of α-synuclein) (upper panel) were used for dot blot analysis of α-synuclein monomer and fibrils (lower panel). Marked differences in immunoreactivity between the monomer and fibrils can be seen.
IN VITRO MODELS OF PRION-LIKE CONVERSION OF α-SYNUCLEIN
Both the establishment of a recombinant α-synuclein expression system in Escherichia coli and the consequent availability of purified recombinant protein in large amounts (Jakes et al. 1994) greatly promoted in vitro and in vivo studies of fibril formation and prion-like propagation of α-synuclein, which have contributed to elucidation of the pathogenesis of α-synucleinopathies. However, if the original DNA sequence of α-synuclein is used for bacterial expression, ∼20% of the protein is mistranslated, with cysteine at position 136 instead of tyrosine, resulting from a combination of codon usage and sequence context (Masuda et al. 2006). Native α-synuclein has no cysteine among its 140 amino acid residues; therefore, the Y136-TAT construct should be used for bacterial expression of human α-synuclein to avoid potential artifacts. The fact that cysteine-less α-synuclein forms fibrils by self-assembly indicates that the S–S bond is irrelevant to amyloid-like fibril formation, which has also been speculated to be the case for other proteins.
Recombinant α-synuclein readily assembles into amyloid-like fibrils that share many of the morphological, ultrastructural, and biochemical properties of the fibrils present in the human brain. When the protein is incubated with shaking at a high concentration (1–10 mg/mL) at 37°C, most is transformed into fibrils within a few days (Conway et al. 1998; Crowther et al. 1998; El-Agnaf et al. 1998; Serpell et al. 2000). However, at a lower concentration without shaking, little or no fibril formation is observed, and a prolonged period is required for assembly. If preformed fibrils are added to the monomer, fibrillization is promoted, presumably because a nucleation process is no longer required. Thus, the assembly is a nucleation-dependent process. Not only wild-type (WT) but also A53T fibrils have been reported to act as nuclei for fibrillization of WT α-synuclein (Wood et al. 1999).
The A30P mutation has been reported to slow the rate of formation of mature filaments (Giasson et al. 1999; Narhi et al. 1999; Conway et al. 2000; Li et al. 2001) and to promote oligomerization of soluble, nonfibrillar protofibrils (Conway et al. 2000; Goldberg and Lansbury 2000). We investigated seed-dependent fibrillization of α-synuclein and found that the A30P mutant forms fibrils slowly, but the seeding effect is higher than that in the case of WT or other mutant α-synuclein (Yonetani et al. 2009). Furthermore, the A30P-type fibril seeds convert WT α-synuclein into A30P-type fibrils (Yonetani et al. 2009). The results obtained in this in vitro experimental model demonstrate that an abnormal form itself can convert the normal protein into an abnormal form. The results also suggest that there are structural and functional differences among these α-synuclein fibrils. A recent neuropathological study of the brains of patients with the A30P mutation showed widespread occurrence of α-synuclein fibrils, which show similar ultrastructural characteristics to LBs and are recovered in the insoluble fraction (Seidel et al. 2010).
CELLULAR MODELS OF α-SYNUCLEIN PRIONS
Prion-like conversion of intracellular normal proteins into abnormal forms has been demonstrated in cultured cells (Desplats et al. 2009; Emmanouilidou et al. 2010; Nonaka et al. 2010a; Volpicelli-Daley et al. 2011). We found that amyloid-like fibrils of α-synuclein, tau, and TDP-43 can be introduced into cells with transfection reagents, where they convert the corresponding normal proteins into abnormal forms, resulting in phosphorylated and ubiquitinated aggregates in cells (Nonaka et al. 2010a,b, 2013). This method has enabled us to evaluate the nucleation-dependent polymerization of α-synuclein and other intracellular proteins in neurodegenerative diseases.
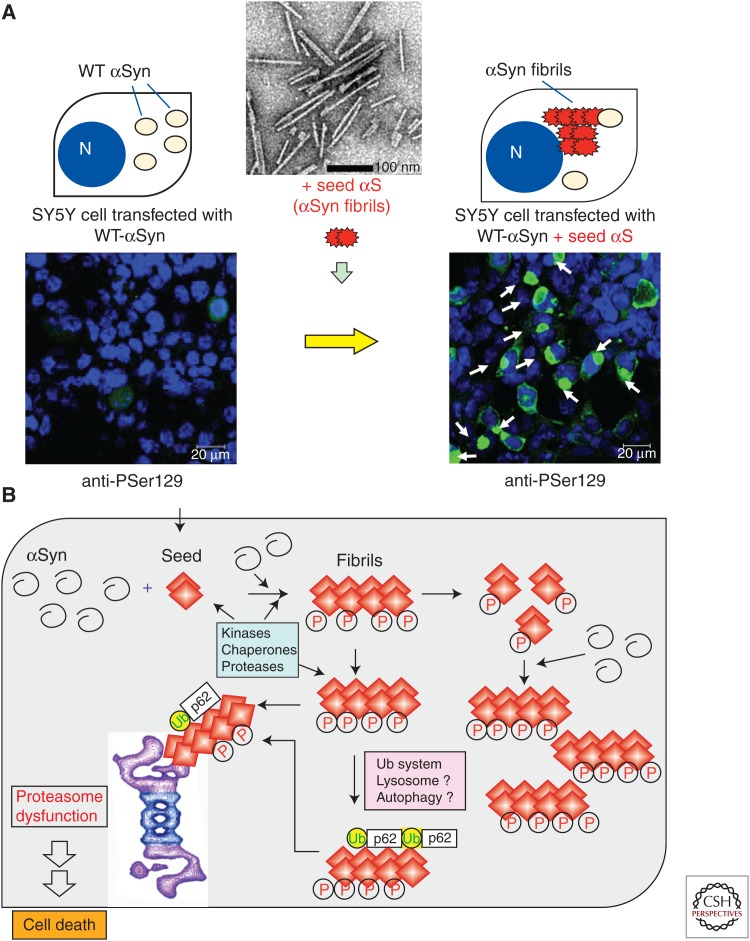
When cultured SH-SY5Y cells were treated with preformed recombinant α-synuclein fibril seeds in the presence of lipofectamine, phosphorylated α-synuclein was detected in the insoluble fractions, indicating that the fibrils were incorporated into the cells. Upon introduction of preformed fibril seeds into cells overexpressing α-synuclein, abundant, highly filamentous α-synuclein-positive inclusions, which are extensively phosphorylated and ubiquitinated, were formed within the cells (Fig. 3A) (Nonaka et al. 2010a). Furthermore, cells with inclusions underwent cell death resulting from proteasome dysfunction, which was monitored by green fluorescent protein with a proteasome degradation signal (Nonaka and Hasegawa 2009; Nonaka et al. 2010a). Similar seed-dependent aggregation was demonstrated for tau and TDP-43 (Nonaka et al. 2010a, 2013). Thus, these models provide evidence of nucleation-dependent and protein-specific polymerization of intracellular amyloid-like proteins in cultured cells.
Figure 3.
Conversion of normal α-synuclein protein to the abnormal form in cultured cells by transduction of amyloid-like protein seeds. (A) Seeded aggregation of α-synuclein in SH-SY5Y cells overexpressing α-synuclein upon transfection with preformed α-synuclein fibrils. Abundant phosphorylated α-synuclein aggregates are formed, which resemble Lewy bodies in the brain, both morphologically and biochemically. (B) Schematic illustration of molecular events associated with the formation of intracellular amyloid-like proteins.
Luk et al. (2009) also reported that α-synuclein monomers and fibrils (but not oligomers) can be introduced into cells by a cationic-liposomal protein transduction reagent. Desplats et al. (2009) reported that α-synuclein is transmitted by endocytosis to neighboring neurons and neuronal precursor cells, forming Lewy-like inclusions. Moreover, they showed that α-synuclein can be transmitted from affected neurons to engrafted neuronal precursor cells in a transgenic (Tg) model of PD-like pathology (Desplats et al. 2009). Volpicelli-Daley et al. (2011) also reported that, in primary neurons treated with preformed α-synuclein fibrils, LN-like pathology first developed in axons and then propagated to form LB-like inclusions in perikarya. Additionally, they reported that accumulation of α-synuclein led to selective decreases in synaptic proteins, progressive impairment of neuronal excitability and connectivity, and eventually neuron death (Volpicelli-Daley et al. 2011).
The results clearly show that nucleation-dependent polymerization of amyloid-like fibrils can occur inside cells, and the formation of intracellular fibrils elicits a variety of cellular reactions, including hyperphosphorylation and compromise of the ubiquitin proteasome system. Although the significance of the hyperphosphorylation of these intracellular pathological proteins remains controversial, it is notable that phosphorylation dramatically increases in soluble fractions when α-synuclein fibrils are introduced. It seems likely that phosphorylation plays an active role in inducing the degradation of these abnormal proteins.
Overexpression of α-synuclein or the mutant itself does not induce amyloid-like fibrils in cells, but if seeds are incorporated into the cells, normal α-synuclein is converted to the abnormal form in the seeds. The fibrils are toxic to cells and are attacked by kinases, chaperones, and proteases that form part of the cellular defense system. However, the fibrils are not degraded, and fragmented fibrils can serve as further template seeds. The cells try to remove the fibrils by recruiting ubiquitin, lysosome, or autophagy systems. However, because of their rigid structure, the fibrils cannot be degraded even by proteasomes, and because of this dysfunction, cells with inclusions undergo cell death (Fig. 3B).
ANIMAL MODELS OF α-SYNUCLEIN PRIONS
The prion-like behavior of α-synuclein in vivo was directly tested by injection of synthetic α-synuclein fibrils or abnormal α-synuclein from diseased brains into brains of Tg mice overexpressing mutant α-synuclein or non-Tg WT mice (Mougenot et al. 2012; Luk et al. 2012a,b; Masuda-Suzukake et al. 2013, 2014; Watts et al. 2013; Recasens et al. 2014). These experiments are similar to others that have been performed to demonstrate the transmission of prion diseases.
Intracerebral Injection of TgM83 Mice Induces α-Synuclein Pathologies
TgM83 homozygous mice, which overexpress human A53T-mutated α-synuclein, develop motor signs with phosphosynuclein pathologies in the brain and spinal cord by ∼240 d. Using TgM83 mice, Mougenot et al. (2012) investigated the possible in vivo transmission of α-synucleinopathy. They prepared brain homogenate from ill TgM83 mice aged ∼360 or ∼540 d, injected it into the brains of TgM83 mice aged ∼56 d, and compared these findings to mice inoculated with brain homogenate from healthy TgM83 mice aged 60 d. The mice inoculated with brain homogenate from an ill mouse showed accelerated pathology and early onset of motor clinical signs compared to mice inoculated with brain homogenate from a healthy or uninjected mouse (Mougenot et al. 2012). Luk et al. (2012a) also reported that when younger mice are intracerebrally inoculated with brain homogenate from older Tg mice showing LB/LN-like inclusions, the formation of pathology and onset of neurological symptoms are accelerated. Furthermore, they showed that injecting synthetic recombinant human α-synuclein fibrils had similar consequences, indicating that α-synuclein fibrils are sufficient to initiate PD-like pathologies and transmit disease in vivo (Luk et al. 2012a). However, these experiments do not rule out the possibility that factors other than abnormal α-synuclein in brain homogenate or synthetic fibrils induce the acceleration of pathologies in TgM83 mice. It is possible that mutant α-synuclein is predisposed to aggregate into LB/LN-like inclusions by other stimuli.
Intracerebral Injection of Non-Tg WT Mice Induces Pathology
Luk et al. (2012b) reported that in WT mice, a single intrastriatal inoculation of synthetic mouse α-synuclein fibrils led to cell-to-cell transmission of pathological α-synuclein and PD-like α-synuclein pathology in anatomically interconnected regions. They also observed progressive loss of dopamine neurons in the substantia nigra (SN) (but not in the adjacent ventral tegmental area) accompanied by motor deficits (Luk et al. 2012a).
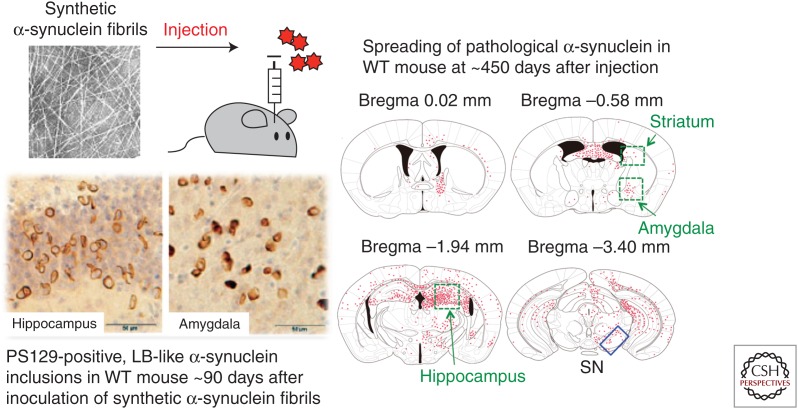
We injected recombinant human α-synuclein fibrils into the SN in the right cerebral hemisphere of healthy C57BL/6J mice. At ∼450 d after inoculation, PS129-positive structures, which were also positive for antiubiquitin (Ub) and p62 antibodies, were observed bilaterally throughout the brain (Fig. 4) (Kuusisto et al. 2001; Masuda-Suzukake et al. 2013). Despite diffusion of exogenous α-synuclein fibrils to bilateral sides of the brain within a few hours after injection, the pathology seems to begin in the injected side of the brain and spread to the noninjected side in a time-dependent manner. In contrast, no PS129-positive pathology was observed in the brains of mice injected with soluble human α-synuclein. Immunoblot analysis with antibodies specific to either human or mouse α-synuclein clearly demonstrated that exogenous human α-synuclein is degraded, which is followed by an accumulation of phosphorylated and ubiquitinated forms of endogenous mouse α-synuclein that are indistinguishable from pathological α-synuclein in the DLB brain ∼90 d after inoculation (Masuda-Suzukake et al. 2013). No such pathology was detected in α-synuclein knockout mice injected with α-synuclein fibrils, providing further evidence that endogenous mouse α-synuclein is converted into an abnormal form via inoculation (Luk et al. 2012a; Masuda-Suzukake et al. 2014).
Figure 4.
Phosphorylated α-synuclein pathology and its distribution in wild-type (WT) mice inoculated into the substantia nigra (SN) with synthetic α-synuclein fibrils at ∼450 d after inoculation. Widespread α-synuclein pathologies were detected with the PS129 antibody. The pathology seems to begin on the injected side of the brain and subsequently spreads to the noninjected side through the neural networks in a time-dependent manner.
Similar pathologies were observed in all of the mice (100%) injected with mouse α-synuclein fibrils, whereas the efficiency of inducing pathology by injecting human α-synuclein fibrils was ∼90% (Masuda-Suzukake et al. 2013). Thus, mouse α-synuclein fibrils showed slightly higher efficiency. It is well known that prion propagation can occur across the species barrier, and the efficiency of propagation depends on the amino acid sequence of the prion protein. In the case of α-synuclein, the healthy mouse and human forms share a 95% amino acid sequence homology. In vitro experiments have also indicated that mouse α-synuclein fibrils promote fibrillization of soluble mouse α-synuclein faster than human α-synuclein fibrils (Masuda-Suzukake et al. 2013).
Luk et al. (2012b) injected mouse α-synuclein fibrils into the striata of mice and observed that the animals developed pathologies in various brain regions together with neuronal loss and motor deficits. When we injected human or mouse α-synuclein fibrils into the SN, our mice developed similar pathologies but did not show significant neuronal loss or motor deficits. To clarify the reason for these differences and also to investigate where α-synuclein pathologies develop and how they propagate, we intracerebrally injected recombinant mouse α-synuclein fibrils into the SN, striatum (STR), and entorhinal cortex (EC) of WT mice and compared the spreading patterns and distribution of pathology ∼30 d after injection (Masuda-Suzukake et al. 2014). In mice injected into the SN, abnormal pathology only appeared in the central nucleus of the amygdala and stria terminalis. The amygdala is connected with the SN, and the stria terminalis serves as a major output pathway of the central nucleus of the amygdala. In contrast, mice injected into the STR developed pathologies in the amygdala, the SN, and a wide range of cortices. The STR has direct projections to the SN and amygdala, and many parts of the neocortex innervate the STR. Injecting fibrils into the EC induced severe phosphorylated α-synuclein pathology in the EC, dentate gyrus, hippocampal CA3 region, and septal nucleus. The dentate gyrus receives projection from the EC via the perforant pathway, and the septal nucleus and fimbria have direct connections with the hippocampus. Fibril-injected mice showed modest motor abnormalities compared to monomer-injected mice at ∼90 d after injection, although the motor deficits in our mice seemed less severe than in those reported by Luk et al. (2012b). These results suggest that propagation of the pathology induced motor clinical signs (Masuda-Suzukake et al. 2014). Comparing the spreading patterns of α-synuclein in mice injected into the STR with those in mice injected into the SN suggests that retrograde transport may be a dominant pathway to spreading α-synuclein pathologies, although the predominant direction that the pathology spreads may depend on cell type or brain area. These results show that the propagation of pathological α-synuclein occurs along neural circuits and involves transsynaptic transport, and that the spread of α-synuclein pathology in different brain regions induces different brain dysfunctions.
Brain Samples from DLB or MSA Patients Also Induced Pathologies
Pathological α-synuclein and brain homogenates prepared from DLB or MSA patients have similar prion-like properties when injected into the brains of healthy mice or monkeys (Masuda-Suzukake et al. 2013; Watts et al. 2013; Recasens et al. 2014). We prepared a sarkosyl-insoluble α-synuclein homogenate from DLB brains and injected it into non-Tg mice. We then investigated the pathology at ∼450 d after injection. In the brains of these mice, PS129-positive pathologies mostly resembled LN-like structures (Masuda-Suzukake et al. 2013). LB-like pathology was detected only in the amygdala and piriform cortex. In the group of mice injected with insoluble α-synuclein from DLB brains, 50% developed phosphorylated α-synuclein pathology in the injected side of the brain, which is less than the percentage of mice injected with recombinant α-synuclein fibrils. Thus, these results demonstrate that inoculating healthy mice with either pure synthetic recombinant α-synuclein fibrils or DLB brain extracts can efficiently and reproducibly induce LB/LN-like phosphorylated α-synuclein pathology.
Watts et al. (2013) inoculated heterozygous TgM83 mice with brain homogenates from two pathologically confirmed MSA cases and found that inoculated mice developed progressive signs of neurological disease with an incubation period of ∼100 d. The brains of MSA-inoculated mice exhibited prominent astrocytic gliosis and microglial activation as well as widespread deposits of phosphorylated α-synuclein that were proteinase K–sensitive, detergent-insoluble, and formic acid–extractable, providing evidence that α-synuclein aggregates formed in the brains of MSA patients are transmissible (Watts et al. 2013; Prusiner et al. 2015). Interestingly, appreciable levels of phosphorylated α-synuclein deposition in oligodendrocytes did not develop within the brains of MSA-inoculated bigenic mice, despite the predilection for oligodendrocytic pathology of α-synuclein in MSA (Watts et al. 2013). Similar results were obtained in WT mice. Injecting sarkosyl-insoluble pellets from MSA brains into the brains of WT mice induced α-synuclein pathologies similar to those in mice injected with DLB samples but not in oligodendrocytes (M Hasegawa and M Masuda-Suzukake, unpubl.).
Why do mice injected with MSA brain homogenate develop neuronal α-synuclein pathology similar to that in PD/DLB? There are two possible explanations. One explanation is that the expression level of α-synuclein in oligodendrocytes is much lower than the expression level in neuronal cells, and the conversion does not occur in glial cells efficiently. If this is so, why does α-synuclein pathology develop in oligodendrocytes in MSA? Does this pathology develop from outside these cells, or is there an overexpression of α-synuclein in oligodendrocytes? Further studies are needed. The second explanation is that inoculated abnormal α-synuclein is incorporated in neuronal cells but not in oligodendrocytes because of the differences in their cellular characteristics. It is reasonable to speculate that the molecular structures of cellular membranes and expression proteins are different between neurons and glial cells. It will be interesting to see whether abnormal α-synuclein is produced in oligodendrocytes and propagated via the oligodendroglial network if it is selectively introduced into oligodendrocytes.
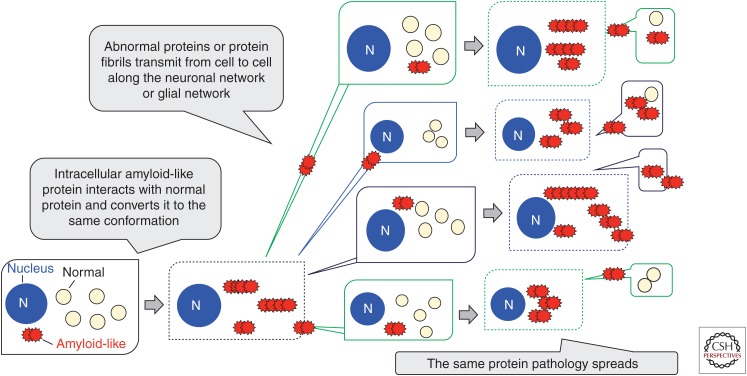
Like the abnormal prion protein, intracellular amyloid-like protein interacts with normal protein and converts it to the abnormal form. The amplified abnormal proteins are transmitted from cell to cell and propagate to various brain regions. As a result, the identical abnormal protein pathology expands. This spreading accounts for disease progression (Fig. 5). These results raise an important question: Are α-synuclein fibrils transmissible among individuals? Sacino et al. (2014) recently reported that intramuscular injection of α-synuclein fibrils accelerated the appearance of α-synuclein pathologies in the brains of TgM83 mice. However, mutant α-synuclein lacking the sequence necessary for fibril formation also accelerated the appearance of pathologies, suggesting that some other factor may be involved in Tg mice. We examined the phenomena by intranasally or intraperitoneally administering synthetic α-synuclein fibrils and the insoluble fraction from DLB brain to WT mice. However, no PS129-positive or p62-positive abnormal structures were detected ∼540 d after administration, even with highly sensitive immunohistochemical staining, suggesting that abnormal α-synuclein cannot pass through the nasal mucosa or that it may take a long time for the pathologies to develop in WT mice (Masuda-Suzukake et al. 2013; M Hasegawa and M Masuda-Suzukake, unpubl.).
Figure 5.
Schematic illustration showing conversion of normal cytosolic protein into the abnormal form and its cell-to-cell propagation along the neuronal or glial network.
CONCLUDING REMARKS
Experimental evidence shows that prion-like conversion of normal α-synuclein to an abnormal form and cell-to-cell transmission of the abnormal form are key events in the spread of α-synuclein pathologies and in the disease progression of α-synucleinopathies. Further studies in vitro and in vivo in human and animal models will be useful to elucidate the progression mechanisms of α-synucleinopathies and to evaluate therapies for these diseases.
ACKNOWLEDGMENTS
This work is supported by a Grant-in-Aid for Scientific Research (A) (Japan Society for the Promotion of Science [JSPS] KAKENHI 23240050 to M.H.) and a Ministry of Health, Labour and Welfare (MHLW) Grant (No. 12946221 to M.H.).
Footnotes
Editor: Stanley B. Prusiner
Additional Perspectives on Prion Diseases available at www.perspectivesinmedicine.org
REFERENCES
- Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A, et al. 2000. Mice lacking α-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron 25: 239–252. [DOI] [PubMed] [Google Scholar]
- Anderson JP, Walker DE, Goldstein JM, de Laat R, Banducci K, Caccavello RJ, Barbour R, Huang J, Kling K, Lee M, et al. 2006. Phosphorylation of Ser-129 is the dominant pathological modification of α-synuclein in familial and sporadic Lewy body disease. J Biol Chem 281: 29739–29752. [DOI] [PubMed] [Google Scholar]
- Appel-Cresswell S, Vilarino-Guell C, Encarnacion M, Sherman H, Yu I, Shah B, Weir D, Thompson C, Szu-Tu C, Trinh J, et al. 2013. α-Synuclein p.H50Q, a novel pathogenic mutation for Parkinson’s disease. Mov Disord 28: 811–813. [DOI] [PubMed] [Google Scholar]
- Baba M, Nakajo S, Tu PH, Tomita T, Nakaya K, Lee VM, Trojanowski JQ, Iwatsubo T. 1998. Aggregation of α-synuclein in Lewy bodies of sporadic Parkinson’s disease and dementia with Lewy bodies. Am J Pathol 152: 879–884. [PMC free article] [PubMed] [Google Scholar]
- Bertoncini CW, Jung YS, Fernandez CO, Hoyer W, Griesinger C, Jovin TM, Zweckstetter M. 2005a. Release of long-range tertiary interactions potentiates aggregation of natively unstructured α-synuclein. Proc Natl Acad Sci 102: 1430–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertoncini CW, Fernandez CO, Griesinger C, Jovin TM, Zweckstetter M. 2005b. Familial mutants of α-synuclein with increased neurotoxicity have a destabilized conformation. J Biol Chem 280: 30649–30652. [DOI] [PubMed] [Google Scholar]
- Biere AL, Wood SJ, Wypych J, Steavenson S, Jiang Y, Anafi D, Jacobsen FW, Jarosinski MA, Wu GM, Louis JC, et al. 2000. Parkinson’s disease-associated α-synuclein is more fibrillogenic than β- and γ-synuclein and cannot cross-seed its homologs. J Biol Chem 275: 34574–34579. [DOI] [PubMed] [Google Scholar]
- Chandra S, Fornai F, Kwon HB, Yazdani U, Atasoy D, Liu X, Hammer RE, Battaglia G, German DC, Castillo PE, et al. 2004. Double-knockout mice for α- and β-synucleins: Effect on synaptic functions. Proc Natl Acad Sci 101: 14966–14971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartier-Harlin MC, Kachergus J, Roumier C, Mouroux V, Douay X, Lincoln S, Levecque C, Larvor L, Andrieux J, Hulihan M, et al. 2004. α-Synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet 364: 1167–1169. [DOI] [PubMed] [Google Scholar]
- Conway KA, Harper JD, Lansbury PT. 1998. Accelerated in vitro fibril formation by a mutant α-synuclein linked to early-onset Parkinson disease. Nat Med 4: 1318–1320. [DOI] [PubMed] [Google Scholar]
- Conway KA, Lee SJ, Rochet JC, Ding TT, Williamson RE, Lansbury PT Jr. 2000. Acceleration of oligomerization, not fibrillization, is a shared property of both α-synuclein mutations linked to early-onset Parkinson's disease: Implications for pathogenesis and therapy. Proc Natl Acad Sci 97: 571–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway KA, Rochet JC, Bieganski RM, Lansbury PT. 2001. Kinetic stabilization of the α-synuclein protofibril by a dopamine–α-synuclein adduct. Science 294: 1346–1349. [DOI] [PubMed] [Google Scholar]
- Crowther RA, Jakes R, Spillantini MG, Goedert M. 1998. Synthetic filaments assembled from C-terminally truncated α-synuclein. FEBS Lett 436: 309–312. [DOI] [PubMed] [Google Scholar]
- Der-Sarkissian A, Jao CC, Chen J, Langen R. 2003. Structural organization of α-synuclein fibril structure studied by site-directed spin labeling. J Biol Chem 278: 24970–24979. [Google Scholar]
- Desplats P, Lee HJ, Bae EJ, Patrick C, Rockenstein E, Crews L, Spencer B, Masliah E, Lee SJ. 2009. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of α-synuclein. Proc Natl Acad Sci 106: 13010–13015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Agnaf OM, Jakes R, Curran MD, Wallace A. 1998. Effects of the mutations Ala30 to Pro and Ala53 to Thr on the physical and morphological properties of α-synuclein protein implicated in Parkinson's disease. FEBS Lett 440: 67–70. [DOI] [PubMed] [Google Scholar]
- Emmanouilidou E, Melachroinou K, Roumeliotis T, Garbis SD, Ntzouni M, Margaritis LH, Stefanis L, Vekrellis K. 2010. Cell-produced α-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J Neurosci 30: 6838–6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara H, Hasegawa M, Dohmae N, Kawashima A, Masliah E, Goldberg MS, Shen J, Takio K, Iwatsubo T. 2002. α-Synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol 4: 160–164. [DOI] [PubMed] [Google Scholar]
- Giasson BI, Uryu K, Trojanowski JQ, Lee VM. 1999. Mutant and wild type human α-synucleins assemble into elongated filaments with distinct morphologies in vitro. J Biol Chem 274: 7619–7622. [DOI] [PubMed] [Google Scholar]
- Giasson BI, Murray IV, Trojanowski JQ, Lee VM. 2001. A hydrophobic stretch of 12 amino acid residues in the middle of α-synuclein is essential for filament assembly. J Biol Chem 276: 2380–2386. [DOI] [PubMed] [Google Scholar]
- Giasson BI, Duda JE, Quinn SM, Zhang B, Trojanowski JQ, Lee VM. 2002. Neuronal α-synucleinopathy with severe movement disorder in mice expressing A53T human α-synuclein. Neuron 34: 521–533. [DOI] [PubMed] [Google Scholar]
- Goedert M. 2001. α-Synuclein and neurodegenerative diseases. Nat Rev Neurosci 2: 492–501. [DOI] [PubMed] [Google Scholar]
- Goldberg MS, Lansbury PT Jr. 2000. Is there a cause-and-effect relationship between α-synuclein fibrillization and Parkinson’s disease? Nat Cell Biol 2: E115–E119. [DOI] [PubMed] [Google Scholar]
- Hasegawa M, Fujiwara H, Nonaka T, Wakabayashi K, Takahashi H, Lee VM, Trojanowski JQ, Mann D, Iwatsubo T. 2002. Phosphorylated α-synuclein is ubiquitinated in α-synucleinopathy lesions. J Biol Chem 277: 49071–49076. [DOI] [PubMed] [Google Scholar]
- Ibáñez P, Bonnet AM, Débarges B, Lohmann E, Tison F, Pollak P, Agid Y, Dürr A, Brice A. 2004. Causal relation between α-synuclein gene duplication and familial Parkinson’s disease. Lancet 364: 1169–1171. [DOI] [PubMed] [Google Scholar]
- Jakes R, Spillantini MG, Goedert M. 1994. Identification of two distinct synucleins from human brain. FEBS Lett 345: 27–32. [DOI] [PubMed] [Google Scholar]
- Krüger R, Kuhn W, Müller T, Woitalla D, Graeber M, Kösel S, Przuntek H, Epplen JT, Schöls L, Riess O. 1998. Ala30Pro mutation in the gene encoding α-synuclein in Parkinson’s disease. Nat Genet 18: 106–108. [DOI] [PubMed] [Google Scholar]
- Kuusisto E, Salminen A, Alafuzoff I. 2001. Ubiquitin-binding protein p62 is present in neuronal and glial inclusions in human tauopathies and synucleinopathies. Neuroreport 12: 2085–2090. [DOI] [PubMed] [Google Scholar]
- Lee JC, Langen R, Hummal PA, Gray HB, Winkler JR. 2004. α-Synuclein structures from fluorescence energy-transfer kinetics: Implications for the role of the protein in Parkinson's disease. Proc Natl Acad Sci 101: 16466–16471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesage S, Anheim M, Letournel F, Bousset L, Honoré A, Rozas N, Pieri L, Madiona K, Dürr A, Melki R, et al. 2013. G51D α-synuclein mutation causes a novel Parkinsonian–pyramidal syndrome. Ann Neurol 73: 459–471. [DOI] [PubMed] [Google Scholar]
- Li J, Uversky VN, Fink AL. 2001. Effect of familial Parkinson's disease point mutations A30P and A53T on the structural properties, aggregation, and fibrillation of human α-synuclein. Biochemistry 40: 11604–11613. [DOI] [PubMed] [Google Scholar]
- Luk KC, Song C, O'Brien P, Stieber A, Branch JR, Brunden KR, Trojanowski JQ, Lee VM. 2009. Exogenous α-synuclein fibrils seed the formation of Lewy body-like intra-cellular inclusions in cultuered cell. Proc Natl Acad Sci 106: 20051–20056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk KC, Kehm VM, Zhang B, O’Brien P, Trojanowski JQ, Lee VM. 2012a. Intracerebral inoculation of pathological α-synuclein initiates a rapidly progressive neurodegenerative α-synucleinopathy in mice. J Exp Med 209: 975–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk KC, Kehm V, Carroll J, Zhang B, O’Brien P, Trojanowski JQ, Lee VM. 2012b. Pathological α-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science 338: 949–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiti NC, Apetrui MM, Zagorski MG, Carey PR, Anderson VR. 2004. Raman spectroscopic characterization of secondary structure in natively unfolded proteins: α-Synuclein. J Am Chem Soc 126: 2399–2408. [DOI] [PubMed] [Google Scholar]
- Maroteaux L, Campanelli JT, Scheller RH. 1988. Synuclein: A neuron-specific protein localized to the nucleus and presynaptic nerve terminal. J Neurosci 8: 2804–2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda M, Dohmae N, Nonaka T, Oikawa T, Hisanaga S, Goedert M, Hasegawa M. 2006a. Cysteine misincorporation in bacterially expressed human α-synuclein. FEBS Lett 580: 1775–1779. [DOI] [PubMed] [Google Scholar]
- Masuda M, Suzuki N, Taniguchi S, Oikawa T, Nonaka T, Iwatsubo T, Hisanaga S, Goedert M, Hasegawa M. 2006b. Small molecule inhibitors of α-synuclein filament assembly. Biochemistry 45: 6085–6094. [DOI] [PubMed] [Google Scholar]
- Masuda M, Hasegawa M, Nonaka T, Oikawa T, Yonetani M, Yamaguchi Y, Kato K, Hisanaga S, Goedert M. 2009. Inhibition of α-synuclein fibril assembly by small molecules: Analysis using epitope-specific antibodies. FEBS Lett 583: 787–791. [DOI] [PubMed] [Google Scholar]
- Masuda-Suzukake M, Nonaka T, Hosokawa M, Oikawa T, Arai T, Akiyama H, Mann DM, Hasegawa M. 2013. Prion-like spreading of pathological α-synuclein in brain. Brain 136: 1128–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda-Suzukake M, Nonaka T, Hosokawa M, Kubo M, Shimozawa A, Akiyama H, Hasegawa M. 2014. Pathological α-synuclein propagates through neural networks. Acta Neuropathol Commun 2: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miake H, Mizusawa H, Iwatsubo T, Hasegawa M. 2002. Biochemical characterization of the core structure of α-synuclein filaments. J Biol Chem 277: 19213–19219. [DOI] [PubMed] [Google Scholar]
- Mougenot AL, Nicot S, Bencsik A, Morignat E, Verchère J, Lakhdar L, Legastelois S, Baron T. 2012. Prion-like acceleration of α-synucleinopathy in a transgenic mouse model. Neurobiol Aging 33: 2225–2228. [DOI] [PubMed] [Google Scholar]
- Narhi L, Wood SJ, Steavenson S, Jiang Y, Wu GM, Anafi D, Kaufman SA, Martin F, Sitney K, Denis P, et al. 1999. Both familial Parkinson's disease mutations accelerate α-synuclein aggregation. J Biol Chem 274: 9843–9846. [DOI] [PubMed] [Google Scholar]
- Nonaka T, Kametani F, Arai T, Akiyama H, Hasegawa M. 2009. Truncation and pathogenic mutations facilitate the formation of intracellular aggregates of TDP-43. Hum Mol Genet 18: 3353–3364. [DOI] [PubMed] [Google Scholar]
- Nonaka T, Watanabe ST, Iwatsubo T, Hasegawa M. 2010a. Seeded aggregation and toxicity of α-synuclein and tau: Cellular models of neurodegenerative diseases. J Biol Chem 285: 34885–34898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka T, Watanabe S, Masuda M, Hasegawa M. 2010b. Cell into which protein, which can serve as polymerization nucleus of protein polymer, or polymer thereof is introduced, and method for production of the cell. U.S. Patent Application No. 20100047826, February 25, 2010.
- Nonaka T, Masuda-Suzukake M, Arai T, Hasegawa Y, Akatsu H, Obi T, Yoshida M, Murayama S, Mann DM, Akiyama H, Hasegawa M. 2013. Prion-like properties of pathological TDP-43 aggregates from diseased brains. Cell Rep 4: 124–134. [DOI] [PubMed] [Google Scholar]
- Pasanen P, Myllykangas L, Siitonen M, Raunio A, Kaakkola S, Lyytinen J, Tienari PJ, Pöyhönen M, Paetau A. 2014. Novel α-synuclein mutation A53E associated with atypical multiple system atrophy and Parkinson's disease-type pathology. Neurobiol Aging 35: 2180.e1–5. [DOI] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, et al. 1997. Mutation in the α-synuclein gene identified in families with Parkinson’s disease. Science 276: 2045–2047. [DOI] [PubMed] [Google Scholar]
- Prusiner SB, Woerman AL, Mordes DA, Watts JC, Rampersaud R, Berry DB, Patel S, Oehler A, Lowe JK, Kravitz SN, et al. 2015. Evidence for α-synuclein prions causing multiple system atrophy in humans with Parkinsonism. Proc Natl Acad Sci 112: E5308–E5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recasens A, Dehay B, Bove J, Carballo-Carbajal I, Dovero S, Perez-Villalba A, Fernagut PO, Blesa J, Parent A, Perier C, et al. 2014. Lewy body extracts from Parkinson disease brains trigger α-synuclein pathology and neurodegeneration in mice and monkeys. Ann Neurol 75: 351–362. [DOI] [PubMed] [Google Scholar]
- Sacino AN, Brooks M, Thomas MA, McKinney AB, Lee S, Regenhardt RW, McGarvey NH, Ayers JI, Notterpek L, Borchelt DR, et al. 2014. Intramuscular injection of α-synuclein induces CNS α-synuclein pathology and a rapid-onset motor phenotype in transgenic mice. Proc Natl Acad Sci 111: 10732–10737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidel K, Schöls L, Nuber S, Petrasch-Parwez E, Gierga K, Wszolek Z, Dickson D, Gai WP, Bornemann A, Riess O, et al. 2010. First appraisal of brain pathology owing to A30P mutant α-synuclein. Ann Neurol 67: 684–689. [DOI] [PubMed] [Google Scholar]
- Serpell LC, Berriman J, Jakes R, Goedert M, Crowther RA. 2000. Fiber diffraction of synthetic α-synuclein filaments shows amyloid-like cross-β conformation. Proc Natl Acad Sci 97: 4897–4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, et al. 2003. α-Synuclein locus triplication causes Parkinson’s disease. Science 302: 841. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. 1997. α-Synuclein in Lewy bodies. Nature 388: 839–840. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Crowther RA, Jakes R, Cairns NJ, Lantos PL, Goedert M. 1998a. Filamentous α-synuclein inclusions link multiple system atrophy with Parkinson's disease and dementia with Lewy bodies. Neurosci Lett 251: 205–208. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M. 1998b. α-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with Lewy bodies. Proc Natl Acad Sci 95: 6469–6473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syme CD, Blanch EW, Holt C, Jakes R, Goedert M, Hecht L, Barron LD. 2001. A Raman optical activity study of rheomorphism in caseins, synucleins and tau: New insight into the structure and behaviour of natively unfolded proteins. Eur J Biochem 269: 148–156. [DOI] [PubMed] [Google Scholar]
- Uéda K, Fukushima H, Masliah E, Xia Y, Iwai A, Yoshimoto M, Otero DA, Kondo J, Ihara Y, Saitoh T. 1993. Molecular cloning of cDNA encoding an unrecognized component of amyloid in Alzheimer disease. Proc Natl Acad Sci 90: 11282–11286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uversky VN, Li J, Fink AL. 2001. Evidence for a partially folded intermediate in α-synuclein fibril formation. J Biol Chem 276: 10737–10744. [DOI] [PubMed] [Google Scholar]
- Vilar M, Chou HT, Lührs T, Maji SK, Riek-Loher D, Verel R, Manning G, Stahlberg H, Riek R. 2008. The fold of α-synuclein fibrils. Proc Natl Acad Sci 105: 8637–8642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpicelli-Daley LA, Luk KC, Patel TP, Tanik SA, Riddle DM, Stieber A, Meaney DF, Trojanowski JQ, Lee VM. 2011. Exogenous α-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron 72: 57–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi K, Yoshimoto M, Tsuji S, Takahashi H. 1998. α-Synuclein immunoreactivity in glial cytoplasmic inclusions in multiple system atrophy. Neurosci Lett 249: 180–182. [DOI] [PubMed] [Google Scholar]
- Watts JC, Giles K, Oehler A, Middleton L, Dexter DT, Gentleman SM, DeArmond SJ, Prusiner SB. 2013. Transmission of multiple system atrophy prions to transgenic mice. Proc Natl Acad Sci 110: 19555–19560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinreb PH, Zhen W, Poon AW, Conway KA, Lansbury PT Jr. 1996. NACP, a protein implicated in Alzheimer’s disease and learning, is natively unfolded. Biochemistry 35: 13709–13715. [DOI] [PubMed] [Google Scholar]
- Wood SJ, Wypych J, Steavenson S, Louis JC, Citron M, Biere AL. 1999. α-Synuclein fibrillogenesis is nucleation-dependent. Implications for the pathogenesis of Parkinson’s disease. J Biol Chem 274: 19509–19512. [DOI] [PubMed] [Google Scholar]
- Yonetani M, Nonaka T, Masuda M, Inukai Y, Oikawa T, Hisanaga S, Hasegawa M. 2009. Conversion of wild-type α-synuclein into mutant-type fibrils and its propagation in the presence of A30P mutant. J Biol Chem 284: 7940–7950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarranz JJ, Alegre J, Gómez-Esteban JC, Lezcano E, Ros R, Ampuero I, Vidal L, Hoenicka J, Rodriguez O, Atarés B, et al. 2004. The new mutation, E46K, of α-synuclein causes Parkinson and Lewy body dementia. Ann Neurol 55: 164–173. [DOI] [PubMed] [Google Scholar]