Abstract
Borrelia miyamotoi is a relapsing fever spirochete in Ixodes ticks that has been recently identified as a human pathogen causing hard tick-borne relapsing fever (HTBRF) across the Northern hemisphere. No validated serologic test exists and current serologic assays have low sensitivity in early HTBRF. To examine the humoral immune response against B. miyamotoi, we infected C3H/HeN mice with B. miyamotoi strain LB-2001 expressing variable small protein 1 (Vsp1) and demonstrated that spirochetemia was cleared after 3 days - coinciding with anti-Vsp1 IgM production. Clearance was also observed after passive transfer of immune sera to infected SCID mice. Next, we showed that anti-Vsp1 IgG eliminates Vsp1-expressing B. miyamotoi, selecting for spirochetes expressing a variable large protein (VlpC2) resistant to anti-Vsp1. The viability of Asian isolate B. miyamotoi HT31, expressing Vlp15/16 and Vlp18, was also unaffected by anti-Vsp1. Finally, in nine HTBRF patients, we demonstrated IgM reactivity to Vsp1 in two and against Vlp15/16 in four around one week after these patients tested positive for B. miyamotoi by PCR. Our data show that B. miyamotoi is able to express various variable major proteins (VMPs) to evade humoral immunity and that VMPs are antigenic in humans. We propose that serologic tests based on VMPs are of additional value in diagnosing HTBRF.
Introduction
Borrelia miyamotoi is a tick-borne relapsing fever (TBRF) spirochete that is present in several Ixodes tick species across the Northern hemisphere (1, 2). While its existence has been recognized since 1994, B. miyamotoi’s potential to infect humans was not discovered until 2011, when patients suffering from a nonspecific (viral-like) febrile illness were found to be infected with B. miyamotoi (3). Since then, various reports have described clinical cases of B. miyamotoi-infected patients in Europe, the United States and Japan, confirming the clinical presentation of high fever, chills, severe headache, myalgia, and/or arthralgia (4–7). Chronic central nervous system infections have been described In two immunocompromised patients receiving B-cell depleting therapy (8, 9). The disease entity caused by B. miyamotoi has been termed B. miyamotoi disease (BMD) (7) or hard tick-borne relapsing fever (HTBRF) (10), of which we use the latter description throughout the manuscript. Recently, B. miyamotoi has been successfully propagated in culture using two different culture media, both of which utilize MKP medium with addition of bovine or human serum (11, 12). Although peak concentrations of B. miyamotoi remain relatively low, these methods, combined with whole-genome sequencing (13), may contribute to the discovery of new serological markers and aid the understanding of the disease pathogenesis.
Currently, HTBRF is diagnosed by PCR on blood during acute illness, while serodiagnosis has been performed using the GlpQ antigen, which is present in TBRF Borrelia species, but not in B. burgdorferi s.l. (7, 14–17). A recent paper showed that 11% of HTBRF patients had IgM reactivity in a rGlpQ enzyme immunoassay (EIA) upon presentation, while 64% demonstrated IgM seroconversion to GlpQ in convalescent sera (7). These findings underscore the need for additional early seromarkers to support a diagnosis of B. miyamotoi infection at disease onset or after antibiotic treatment, when (q)PCR might be negative. A study in The Netherlands revealed higher prevalence of GlpQ antibodies among forestry workers, Lyme disease patients and those suspected to have human granulocytic anaplasmosis (HGA), suggesting they had been infected with B. miyamotoi (17). However, there have not been any studies investigating which B. miyamotoi proteins are most antigenic.
B. miyamotoi was reported to express vsp genes two decades ago (18), and a recent study confirmed the presence of B. miyamotoi genes coding for variable major proteins (VMPs), also revealing several variable large proteins (Vlps) (19). TBRF spirochetes are able to switch serotypes by non-reciprocal gene transfer of these immunogenic VMPs, thereby evading the host antibody response and enabling relapses to occur (20–24). This system has been extensively studied in B. hermsii, where 59 genes coding for VMPs have been identified on archival plasmids in non-expression loci, consisting of immunogenic variable small proteins (Vsps, approximately 22 kDa) and Vlps (approximately 37 kDa) (16, 25–28). The dimeric Vsps have a different protein structure compared to the monomeric Vlps and only a remote evolutionary relationship (26, 29). At each moment in time, only one of these serotype-defining VMPs is expressed by a spirochete, namely when this vsp/vlp gene has been copied into the expression site that is located on a linear plasmid. However, populations can consist of several serotypes, and serotype switching can also occur spontaneously in a small fraction of spirochetes, with an estimated frequency of 10−3 to 10−4 per spirochete per generation (30). Thus, infected hosts will clear TBRF spirochetes by IgM directed against one or a few dominant VMPs, leaving outlier spirochetes that express different VMPs to replicate and cause a relapse of spirochetemia (30, 31). Borrelia turicatae Vsps have been described to have a conserved core and a variable exposed dome, explaining why IgM raised against one Vsp is less likely to bind another, and they have been shown to exert different tissue tropisms (27, 32–35). For B. miyamotoi, this system is yet to be unraveled. Based on the presence of vmp genes B. miyamotoi and of the involvement of VMPs in TBRF pathogenesis, it could be expected that B. miyamotoi VMPs are variably expressed, immunogenic and involved in immune evasion.
In this study we experimentally infected mice with in vitro-cultured B. miyamotoi LB-2001, a tick isolate from Connecticut, United States, and used the evolving humoral immune response to identify novel B. miyamotoi antigens expressed in early infection. We identified Vsp1 as a dominant antigenic target and show that antibodies to this antigen are capable of eliminating most spirochetes in B. miyamotoi LB-2001 infected SCID mice after passive transfer and in cultured B. miyamotoi LB-2001 in vitro. Surviving spirochetes expressed a different VMP and were resistant to anti-Vsp1 antibody-mediated killing. Finally, we show that VMP-specific antibodies can be detected in HTBRF patient sera, providing insight into HTBRF pathogenesis and revealing B. miyamotoi VMPs as additional early serodiagnostic markers.
Materials and Methods
Infection, passive transfer and immunizations with Borrelia lysates
Stocks of a P4 passage of Borrelia miyamotoi LB-2001 were cultured in MKP-F medium from −80°C glycerol stocks (12). Seven-day cultures of 5th passage spirochetes were counted using a Petroff-Hauser counting chamber and 6–8-week old female C3H/HeN mice (Charles River) were infected by i.p. injection with 107 spirochetes in 200µl PBS. 5–7 week old CB17 FoxChase SCID mice were similarly infected using 105 spirochetes in PBS. Passive transfer was performed by i.p. injection of 250µl plasma from C3H/HeN mice infected 5 or 14 days earlier, after syringe-filtering and confirming the absence of spirochetes by dark-field microscopy. Borrelia miyamotoi LB-2001 and Borrelia burgdorferi 297 were cultured for 7 days in MKP-F medium or BSK (Sigma, St. Louis, MO, USA) at 33°C, washed four times in sterile PBS and heat-inactivated at 56°C for 20 minutes, followed by sonication (6 times 15 seconds). Groups of five C3H/HeN mice were immunized with 5µg of either lysate emulsified with complete (first immunization) or incomplete Freund’s adjuvant (two boosters after 2 and 4 weeks), and serum was obtained after 6 weeks. Passive transfer of these antisera was performed similar to the method used for naturally immune sera. For analysis of Vsp1 expression in culture, Borrelia miyamotoi LB-2001 was passaged 50 times, and glycerol stocks of P5, P10, P20, P30, P40 and P50 cultures were inoculated in triplicate at 33°C from −80°C for 7 days until a mean concentration of 1.3×107/ml to 2.6×107/ml, followed by generation of lysates as described above which were run on 4–20% SDS gel and stained by Coomassie blue.
Protein identification
B. miyamotoi LB-2001 was cultured in MKP-F medium at 33°C from a P4 −80°C glycerol stock for 7 days, after which spirochetes were washed four times by centrifuging (10.000×G 7 minutes) and resuspended in sterile PBS. Suspensions were heat-inactivated for 20 minutes in a water bath at 56°C and sonicated 6 times for 15 seconds (Branson, Danbury, CT, USA). A total of 7.5µg was loaded in a mini-protean 4–20% SDS gel (Bio-rad, Hercules, CA, USA) together with B. burgdorferi 297 lysate (cultured for 7 days in BSK medium). Gel bands around 23–25 kDa, 37–39 kDa and 59–63 kDa were sent to the Yale School of Medicine Keck proteomics laboratory for trypsin digestion followed by LC –MS/MS on a LTQ Orbitrap (Thermo Scientific, Waltham, MA, USA) followed by a BLAST on the MASCOT database. A 37 kDa band from B. miyamotoi HT31 and a 35 kDa band from B. miyamotoi LB-2001 after Vsp1 IgG challenge were analyzed by York University department of biology, where tryptic peptides were analyzed by MALDI-MS and MS/MS using a Bruker ultraflex III TOF/TOF.
In-silico analysis
The amino acid sequences of B. miyamotoi (Genbank: AGS80212.1): and B. turicatae Vsp1 (Genbank: AAB65089.1) were aligned using AlignX software (Invitrogen, Carlsbad, CA, USA) and visualized using Boxshade 3.2 web-based software (Expasy, Swiss Institute of Bioinformatics). The signal sequence was predicted using a model for spirochetal lipoprotein signal sequence prediction (36). Protein structure prediction of B. miyamotoi Vsp1 was performed using the online homology modeling server Phyre2 (Protein Homology/analogY Recognition Engine V 2.0, Imperial College, London, UK) and visualized using the PyMOL Molecular Graphics System, Version 1.7.4 Schrödinger, LLC. Three annotated crystal structures of B. burgdorferi OspC and one B. turicatae Vsp1 structure yielded 100% confident structural homologies of residues 45–211 (44% i.d., PDB 1F1M, template d1f1ma), 45–214 (42% i.d., PDB 1G5Z, template d1g5za), 45–210 (47% i.d., PDB 1GGQ, template d1ggqqa) and 51–208 (57% i.d., PDB 1YJG, template c1yjgE), respectively. The predicted Vsp1 monomer has been aligned to the B. turicatae Vsp1 dimer crystal structure (PDB 2GA0) to predict its dimeric structure.
Recombinant protein generation
DNA was extracted from B. miyamotoi LB-2001 and HT31 (cultured to passage 5) using a Blood and tissue kit (Qiagen). A PCR was performed with Phusion high fidelity mix (New England Biolabs, Ipswich, MA, USA) and forward primer AAAAGCTAGCTGTGGAAGTGGGG, and using reverse primers AAAACTCGAGTGAAGATTGACCAGC for LB-2001 and HT31. After 98°C 30 seconds, 25 cycles of 98°C 10 seconds, 62°C 30 seconds and 72°C 30 seconds were performed and PCR products were ligated into the pet21b vector using Nhe1 and Xho1 restriction sites. In both B. miyamotoi isolates an identical Vsp1 sequence was identified, with consistently two base point mutations compared with the annotated sequence identified by whole-genome sequencing (Genbank KF031441): nucleotide 223 and 287 (both guanine instead of adenosine), leading to a N75→D75 substitution and a D96→G96 substitution, respectively compared to the annotated sequence. The LB-2001 construct was transformed into BL21 (DE3) cells (Novagen, Madison, WI, USA) and 300 ml LB/ampicillin cultures were induced with IPTG at OD 0.5–0.8 and incubated overnight at 30°C. Cells were resuspended in sterile PBS, 1mg/ml lysozyme (Sigma-Aldrich) was added and samples were sonicated 6 times for 15 seconds. Next, 1% triton-X100 was added and samples were incubated for 30 minutes at 4°C, centrifuged and filtered using a 0.22µm membrane. EDTA-free protease inhibitor (Roche) was added, samples were run over Ni-NTA columns using manufacturer instructions, and eluted using 300µM imidazole. Pooled fractions were dialyzed four times using 9kDa Amicon ultra centrifugal filters (Merck Millipore). Recombinant Vlp15/16 (WP_025444482.1) was similarly generated using primers AAAAGCTAGCTGTAATAATGGAGGAGGGG and AAAACTCGAGCTTCTGTGCACTAGTTGTTAC; rVlp18 (WP_025444235.1) was cloned using AAAAGCTAGCTGTGGTCAACAAACAGAAGG and AAAACTCGAGCTCTGCTGTTTTTGAGTTTCTTG, both from B. miyamotoi HT31 DNA. Protein and antibody concentrations were determined using a DC protein assay (Bio-rad).
ELISAs
To measure IgM and IgG directed against recombinant proteins, high-binding half-surface plates Greiner) were coated overnight at 4°C with 50nM of recombinant protein. Next, plates were washed in PBS-tween 0.05% and incubated for 2 hours with blocking buffer (PBS + 1% BSA) at room temperature. Plates were subsequently washed and incubated for one hour at room temperature with 1:1000 C3H/HeN mouse sera in blocking buffer. Plates were washed and incubated for 30 minutes with 1:1000 anti-mouse IgG-HRP (Cell signaling, Danvers, MA, USA) or 1:2000 anti-mouse IgM (Southern Biotech, Birmingham, AL, USA) in blocking buffer. Finally, plates were washed and developed using TMB substrate, and absorbance was read at 450nm (Bio-Tek, Winooski, VT, USA). In the case of rabbit anti-Vsp1 antisera, sera were diluted 1:10.000 and 1:4.000 anti-rabbit IgG-HRP (Cell signaling) was used as a secondary antibody. IgM against B. miyamotoi LB-2001 or B. burgdorferi 297 lysates was similarly performed using 1µg/ml lysate in PBS as coating antigen, 1:100 diluted mouse sera as primary antibody and 1:2000 anti-mouse IgM (Life technologies, Carlsbad, CA, USA) as a secondary antibody.
Western blots
Western blots were performed by loading 3µg of Borrelia lysate on mini-protean 4–20% SDS gels (Bio-rad), blotting to PVDF membranes and blocking overnight in blocking buffer (TBS with 0.05% tween and 5% skim milk powder) at 4°C. The next day, blots were incubated for one hour in mouse serum diluted 1:100 (5 and 7 days after infection) or 1:200 (14 days after infection) in TBS-T with 2.5% skim milk powder. Membranes were washed 3× in TBS-T and incubated 1:10.000 with Donkey/goat anti-mouse IgM / IgG 800CW (Li-Cor) for 30 minutes, followed by three washes and developed on a Li-Cor Odyssey infrared imager (Li-Cor, Lincoln, NE, USA). Western blots comparing Borrelia miyamotoi isolates LB-2001 and HT31 were performed on 250 ng of lysates made from P4 passages from a −80°C stock cultured for 7 days at 33°C. Membranes were incubated with 1:500 diluted sera from four individual mice 14 days after infection, anti-mouse IgG-HRP (Cell signaling, Beverly, MA, USA) as a secondary antibody, and developed on an ImageQuant LAS4000 (GE healthcare biosciences, Pittsburgh, PA, USA). Western blots on recombinant proteins were performed by electrophoresis of 500ng recombinant proteins, incubation with 1:1000 pooled sera for 1 hour at room temperature, and 1:10.000 anti-mouse IgM-HRP (Southern Biotech, Birmingham, AL, USA) or anti-mouse IgG-HRP (Cell signaling) for 30 minutes. Western blots on B. miyamotoi cultured after Vsp1/OVA antibody challenge were performed using 500ng lysates and 1:4000 rabbit anti-Vsp1, followed by 1:10.000 anti-rabbit IgG-HRP (Cell signaling), or using 1:1000 pooled mouse sera (n=4) from C3H/HeN mice (naïve/5 days after B. miyamotoi infection/14 days after infection), and 1:10.000 HRP-linked anti-mouse IgM or IgG. Western blots on human sera were performed similarly, using 500 ng of recombinant proteins and incubation in human sera diluted 1:500 followed by 1:10.000 anti-human IgM-HRP (Southern Biotech) or IgG-HRP (Bio-rad). Human sera were collected from Russian HTBRF patients (3, 37) where diagnosis was confirmed by PCR and sequencing. All HTBRF patients developed a fever >38°C axillary temperature. No relapses were reported in these patients, as all patients were antibiotically treated shortly after disease onset. Sera from Russian patients hospitalized for 8–15 days in a hospital for infectious diseases with a typical erythema migrans and a history of tick bite were used. Sera from healthy blood donors were used as controls.
Dark-field spirochete counts
Blood samples (30µl) were obtained by tail nick in Microvette Lithium-Heparin plasma tubes (Sarstedt, Nümbrecht, Germany). 2.5µl of blood was added to 47.5µl of Sidestep lysis and stabilization buffer (Agilent, Santa Clara, CA, USA), and the rest of the blood was centrifuged at 500×G for 5 minutes as described elsewhere to obtain plasma (38). Plasma samples were counted under dark-field microscope using a Petroff-Hauser counting chamber, using a 1mm2 counting square according to manufacturer instructions. When no spirochetes were observed, 8 additional squares of 1mm2 were counted to obtain a detection limit of 5.555 spirochetes/ml, and total spirochete concentrations were calculated based on 9mm2 observed surface, or given the detection limit when negative.
Anti-Vsp1 antibody generation and killing assays
Two rabbits were immunized with 200µg or rVsp1 subcutaneously in complete Freunds adjuvant (t=0) and incomplete Freunds adjuvant (t = 14, 28 and 56 days) (Eurogentec, Liège, Belgium), and sacrificed at t=65 days. Final pooled sera were purified using the Zeba™ Desalt Spin Column (Life technologies) and Melon™ Gel IgG Spin Purification Kit (Life technologies) and filter-sterilized using a 0.22µm filter. A titration killing assay was performed using various concentrations of polyclonal anti-Vsp1 IgG (0 to 1000µg/ml) in 5µl PBS added to V-shaped sterile microtiter plates. Borrelia miyamotoi LB-2001 at a concentration of 3×106/ml was added (32.5µl/well) together with 12.5 µl pooled normal human serum (NHS) at a final concentration of 25%. As a control, heat-inactivated human serum (NHS incubated at 56°C for 30 minutes) was added instead at the same concentration. Wells were resuspended and the plate was incubated at 37°C for 1 hour followed by blinded dark-field microscopy scoring of 100 spirochetes per well (12), using immobilization as a marker for spirochete mortality (39–42). B. miyamotoi HT31 and B. burgdorferi 297 P4 strains cultured from −80°C stocks were equalized to 3×106/ml using MKP-F medium and subjected to a final concentration of 100µg/ml anti-Vsp1 IgG. The remaining 45µl per well in the B. miyamotoi LB-2001 suspensions subjected to anti-Vsp1 or anti-OVA IgG (43) was inoculated into 1.6 ml of MKP-F in 2 ml screwcap tubes, and after one week at 33°C they were passaged at the same concentrations into 7ml MKP-F followed by two 7-day passages at 33°C and storage at −80°C in 4% glycerolpeptone, from which they were cultured to generate lysates.
PCR for B. miyamotoi LB-2001 expression site on Vsp1 and VlpC2
DNA was extracted from a P3 passage of B. miyamotoi LB-2001 isolates 1 (expressing Vsp1) and 2 (anti-Vsp1 exposed) using a DNeasy blood &tissue kit (Qiagen). We designed a forward primer based on the “-35” promoter element of B. miyamotoi LB-2001 which was located upstream of the annotated Vsp1 gene as described above: GAATTTGAAAAGTAAGATTCTTGCAC. To identify the sequence for the B. miyamotoi LB-2001 35 kDa band which is dominantly expressed by a population surviving anti-Vsp1 IgG challenge, a PCR was performed with Phusion high fidelity PCR master mix (New England Biolabs) using the forward “-35” promoter element primer and reverse primer TTATTTACTTTTAGCTTCAGAGGTCTTATTAT, which was based on the gene coding for a protein to which 3 peptides of the mass-spectrometry of this band had matched, i.e. B. miyamotoi Fr64b Vlp5. PCR conditions were 98°C 30 seconds followed by 30 cycles of 98°C 10 seconds, 60°C 30 seconds and 72°C 30 seconds, and a final 10-minute extension step at 72°C. The PCR product was purified and sequenced, and a partial gene was annotated sharing >99% identity with the B. miyamotoi gene VlpC2. Furthermore, a reverse primer GCACTTTTTGCATGAGCATC was designed to identify Vsp1 in the expression site, and reverse primer TTACCTGCTTCACCATCACC (with a similar Tm) was designed based on the VlpC2 sequence. A PCR was performed using Phusion high fidelity PCR master mix using 1µl DNA, 2.5µl of both primers (10µM), 19µl H2O and 25µl of Phusion master mix. A 98°C 30 second denaturation was followed by 30 cycles of 98°C 10 seconds, 57.5°C 30 seconds and 72°C 15 seconds, followed by 10 minutes at 72°C.
Ethics statement
Animals were housed and handled under the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The animal research protocols were approved by Yale University's Institutional Animal Care & Use Committee (IACUC) (protocol number 2014-07941) and by the Academic Medical Center’s Ethical Committee for Animal Research (DEC) protocol number DIX103058).
Statistics
One-way ANOVA was performed on ELISA results using GraphPad Prism version 5.00 for Windows, GraphPad Software, San Diego California USA, www.graphpad.com. When p<0.05, individual columns were compared using a student’s t-test. Killing assay motility percentages were compared with the control (PBS or anti-OVA) using a Mann-Whitney test. A Chi squared test was performed in a case when all replicates were similar (0), and indicated with #.
Results
B. miyamotoi LB-2001 expresses Vsp1, which induces a dominant antibody response in C3H/HeN mice
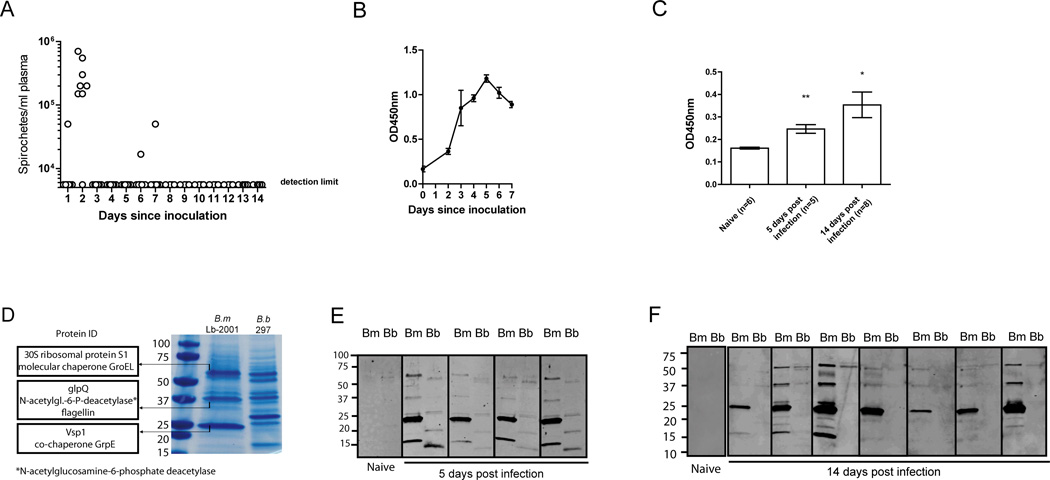
We set up a mouse model where 107 B. miyamotoi LB-2001 spirochetes (passaged up to four times in MKP-F medium) were inoculated in 200µl PBS intraperitoneally (i.p.) into C3H/HeN mice. Plasma was examined by dark-field microscopy for spirochetes daily, and a peak in spirochetemia was observed after 2 days, followed by a low-grade spirochetemia in 3 out of 8 mice after 5 or 6 days, one of which was at the detection limit at day 5 (Fig. 1A). In most mice however, the pathogen remained below detection limit from day 3, coinciding with a rise in B. miyamotoi-specific IgM (Fig. 1B). Infected mice developed detectable anti-B. miyamotoi IgG at day 5 that increased at day 14 after inoculation (Fig. 1C). Although B. miyamotoi and B. burgdorferi s.l. are spirochetes of the same genus in the same tick vector, they have a different protein expression profile (Fig. 1D). We performed mass spectrometry on the three dominant protein bands corresponding to molecular weights of ~60 kDa, ~39 kDa, and 23 kDa in B. miyamotoi LB-2001 lysate. This revealed that the ~60 kDa band was comprised of mostly 30S ribosomal protein S1 (Genbank accession number WP_020954521.1, predicted molecular weight 62.6 kDa) and GroEL (WP_020955008.1, 58.9 kDa), while the ~39 kDa (reactive) band contained GlpQ (WP_020954631.1, 38.7 kDa) in the highest abundancy, followed by N-acetylglucosamine-6-phosphate deacetylase (WP_020955113.1, 39.4 kDa) and flagellin (WP_020954538.1, 35.4 kDa) (Fig. 1D). The prominent 23 kDa band was found to contain variable small protein 1 (Vsp1) (AGS80212.1; 23.3 kDa, Supplemental Fig. 1A) and co-chaperone GrpE (WP_020954892.1; 21.3 kDa). Western blots using 5-day and 14-day infected mouse sera showed that the IgM and IgG responses were mainly directed against the 23 kDa B. miyamotoi protein band, and did not react with any 23 kDa protein in B. burgdorferi lysate that was run in adjacent lanes as a control (Fig. 1E, 1F). Because of its dominant expression, and because VMPs are immunogenic in other TBRF species, we hypothesized that Vsp1 was responsible for the reactivity against the 23 kDa protein.
FIGURE 1. Borrelia miyamotoi infection induces a robust antibody response against a 23 kDa protein.
(A) Dark-field microscopy on plasma from eight C3H/HeN mice after intraperitoneal (i.p.) inoculation with B. miyamotoi LB2001 spirochetes. Horizontal lines represent mean concentrations, negatives represent the detection limit. (B) ELISA detecting IgM against B. miyamotoi LB-2001 whole-cell lysate. (C) IgG response to infection demonstrated by ELISA using sera from mice infected 5 or 14 days post-infection. (D) SDS-PAGE of B. miyamotoi whole-cell lysate stained by Coomassie brilliant blue, revealing abundant protein bands at ~23 kDa, ~39 kDa and ~60 kDa. These bands were analyzed by LC–MS/MS and the most abundant proteins are depicted on the left. For comparison, B. burgdorferi lysate (B.b 297) was run in the right lane. (E, F) Western blot analysis of nitrocellulose strips loaded with B. miyamotoi LB-2001 (Bm) and B. burgdorferi 297 (Bb) whole-cell lysates, detecting IgM in sera of four mice 5 days after B. miyamotoi infection (E) or IgG 14 days after infection in seven mice (F). Protein size in kDa is depicted on the left. * p<0.05, ** p<0.01. Error bars illustrate mean ± S.E.M.
Vsp1 in-silico analysis
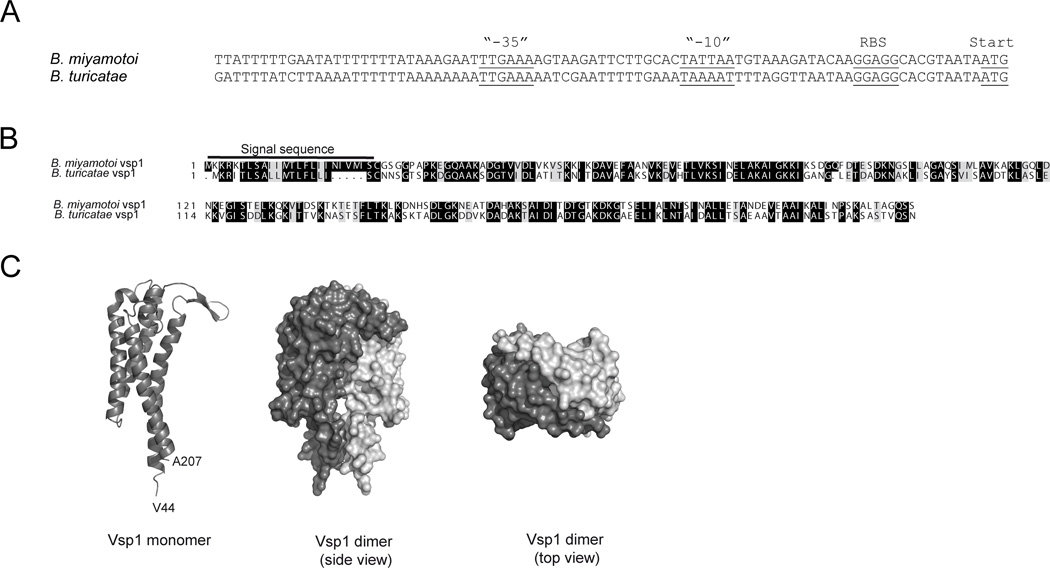
Vsp1 appeared to be the dominantly expressed VMP in this B. miyamotoi population, with its gene being located on a 26 kb plasmid fragment behind a σ70-type prokaryotic promoter. A comparison of the upstream sequence of the expressed Vsp1 sequence with the vsp/vlp promoter region of B. turicatae revealed homology at the “-35” element, which was flanked by AT-rich inverted repeats, as well as an identical ribosomal binding site site (Fig. 2A). Next, we were interested whether the Vsp1 protein structure was similar to the previously described B. turicatae Vsp1. First, we aligned the Vsp1 amino acid sequence with that of B. turicatae Vsp1, the only Vsp for which a crystal structure has been described (RCSB protein databank accession 2GA0_A)(32). In silico analyses revealed 55% identity and 70% consensus positions with the amino acid sequence of B. turicatae Vsp1 (Fig. 2B). A homology model with 100% confidence of the B. miyamotoi Vsp1 structure was generated in silico using the Phyre2 webserver (44) (Fig. 2C). The predicted structure of B. miyamotoi Vsp1 consists of four α-helices forming a bundle with two small β-strands pointing outwards, and hydrophobic residues line the core of the α-helical bundle.
FIGURE 2. Borrelia miyamotoi Vsp1 promoter region and predicted protein structure.
(A) Alignment of the promoter regions of the B. miyamotoi and B. turicatae expression sites, based on B. miyamotoi Vsp1 (KF031441.1) and B. turicatae VspB (AF049852) sequences. AT-rich sequences surround the “-35” promoter element. RBS: ribosomal binding site. Start: start codon. (B) Alignment of B. miyamotoi Vsp1 and B. turicatae Vsp1 amino acid sequences, including their respective signal sequences, conserved amino acids (grey background) and identical residues (black background). (C) Structure prediction based on annotated crystal structures of B. turicatae Vsp1 and B. burgdorferi OspC using the Phyre2 webportal. Vsp1 and OspC are known to form dimers, and in this figure a predicted monomer (dark gray) has been aligned to a B. turicatae Vsp1 dimer crystal structure (PDB 2GA0), consisting of 2 B. miyamotoi Vsp1 monomers (light and dark grey, respectively).
Verification of Vsp1 antibodies in mice
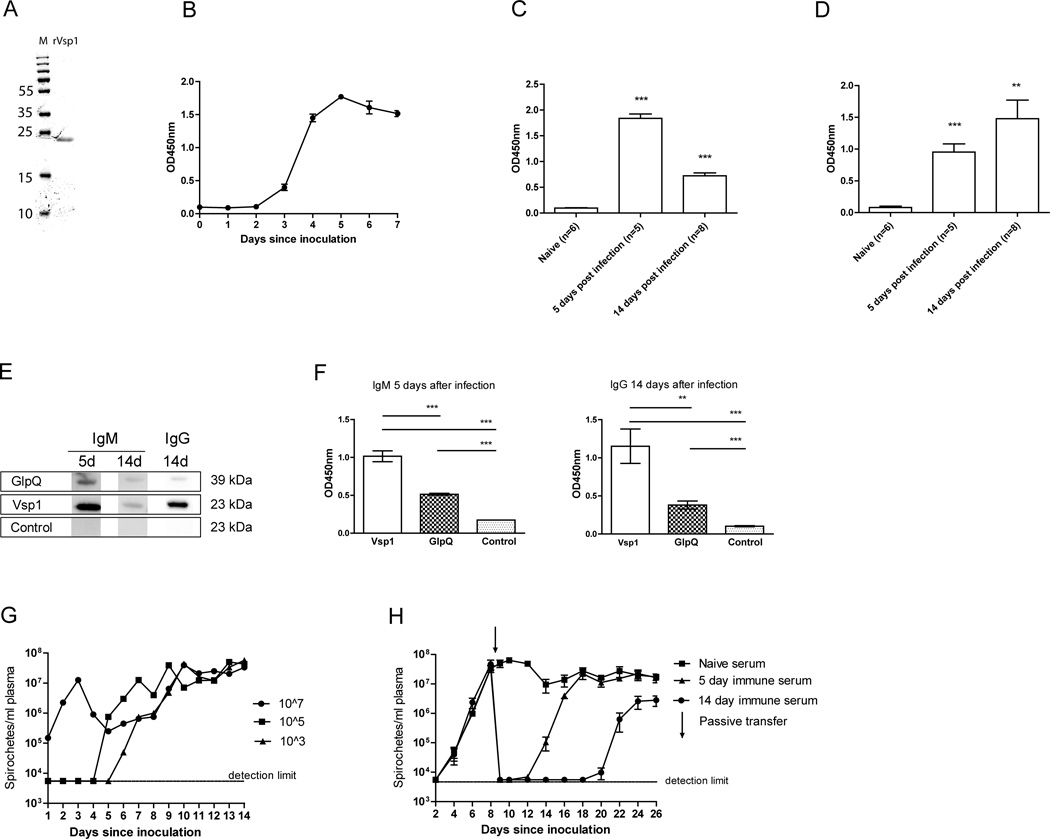
To verify our hypothesis that Vsp1 was one of the immunodominant B. miyamotoi proteins recognized by B. miyamotoi-infected mouse sera, we generated a recombinant HIS-tagged B. miyamotoi LB-2001 Vsp1 (rVsp1), without its predicted 24 amino acid signal sequence to enhance solubility (Fig. 3A). ELISAs using rVsp1 as an antigen were performed on sera from B. miyamotoi LB-2001 infected mice. Indeed, a rise in anti-Vsp1 IgM was observed in infected mice from day 3, with a maximum level at day 5 post-infection (Fig. 3B). Anti-Vsp1 IgM levels decreased at day 14 after infection (Fig. 3C), at which time anti-Vsp1 IgG levels were >3 SD higher than those of naive control mice (Fig. 3D). A western blot using pooled sera from infected C3H/HeN mice further confirmed the presence of IgM and IgG reacting with rVsp1 (Fig. 3E). Of note, C3H/HeN mice developed more robust antibody responses against Vsp1 compared to GlpQ, which is currently used as a seromarker for human infection (Fig. 3F).
FIGURE 3. A rapid Vsp-1 specific IgM response is induced upon B. miyamotoi infection.
(A) Recombinant Vsp1 loaded onto a 4–20% SDS-page gel. M: Protein marker (size in kDa on the left). (B–D) ELISAs were coated with rVSP1 and incubated with previously infected mouse plasma, detecting IgM (B and C) and IgG (D). Bars represent mean ± S.E.M. (E and F) Western blot and ELISA detecting IgM and IgG against GlpQ, Vsp1 or a negative control protein (tHRF) in mouse sera 5 or 14 days after infection. For western blot, pooled sera (n=4) were used. For ELISA, individual mouse sera (5 days after infection: n=5. 14 days after infection: n=8) were used. (G) Three groups of three SCID mice were inoculated i.p. with either 103, 105 or 107 B. miyamotoi LB-2001 spirochetes, and plasma spirochete concentrations were measured by darkfield microscopy. (H) Dark-field microscopy in plasma of B. miyamotoi-infected SCID mice (n=4 per group) receiving an i.p. passive transfer of pooled sera at t=8 days after infection. Mice received 250µl naive, 5- or 14 day immune C3H/HeN mouse sera. ** p<0.01, *** p<0.001. Error bars illustrate mean ± S.E.M., negatives were given the detection limit.
Effects of passive immunization on B. miyamotoi spirochetemia in SCID mice
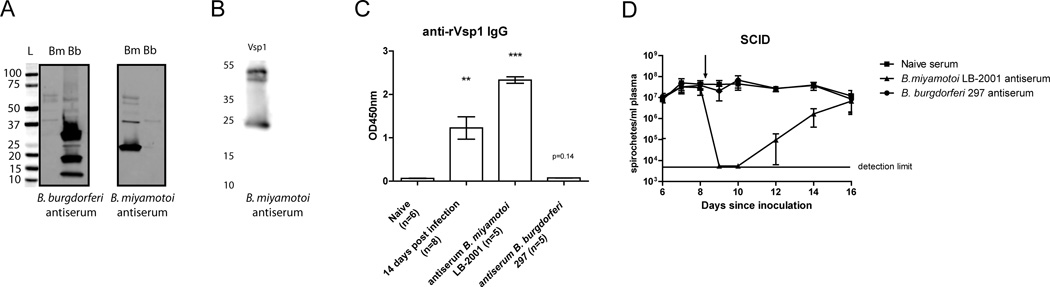
We next set out to investigate whether antibodies present in serum 5 and 14 days post-infection, which included anti- Vsp1 IgM and IgG antibodies, were sufficient to clear B. miyamotoi infection. To this end we used SCID mice that develop persistent spirochetemia upon infection with B. miyamotoi (13, 45). We therefore performed a dose-finding experiment in which we i.p. inoculated SCID mice with 103, 105 or 107 B. miyamotoi LB-2001 spirochetes. As expected, spirochetes were detected more rapidly using the highest inoculum, but all mice became spirochetemic within 6 days after inoculation and the levels plateaued at concentrations ranging between 107 and 108 spirochetes/ml (Fig. 3G). We subsequently infected groups of four SCID mice with 105 B. miyamotoi spirochetes i.p. and after 8 days, 250µl of pooled serum from previously infected C3H/HeN mice was injected i.p. to assess its potential to clear spirochetemia. Tail bleeds were performed to check for B. miyamotoi concentrations in SCID mouse plasma. While passive transfer of naive mouse sera did not show any effect on spirochetemia, 5- day and 14-day immune sera were both successful in temporarily clearing B. miyamotoi (Fig 3H). Interestingly, both sera were unable to permanently clear B. miyamotoi, and relapses were observed around day 14 and day 20–22 in mice treated with 5-day and 14-day immune sera, respectively. Finally, we immunized mice with Vsp1-expressing B. miyamotoi lysates, yielding a strong anti-Vsp1 IgG response (Fig. 4A–C). Although the anti-Vsp1 IgG levels were higher than in serum of mice experimentally infected with B. miyamotoi LB-2001 14 days post-infection, passive transfer of immunized mouse sera to infected SCID mice did not result in more efficient clearance of spirochetemia (Fig. 4D). These data demonstrate that antibodies against B. miyamotoi are involved in (partial) clearance in vivo. The fact that Vsp1 was the most dominant antigenic protein during natural infection and after immunization with B. miyamotoi lysate suggests a role for Vsp1-specific antibodies.
FIGURE 4. Serum from mice immunized against Vsp1 serotype B. miyamotoi temporarily clears B. miyamotoi spirochetemia in SCID mice.
(A) western blot of strips containing B. miyamotoi (Bm) and B. burgdorferi 297 (Bb) lysate incubated with pooled antiserum from mice immunized with either B. burgdorferi 297 or B. miyamotoi LB-2001 lysate. (B, C) Borrelia miyamotoi antiserum reacted with recombinant Vsp1 as shown by western blot and ELIS, respectively. (D) Dark-field microscopy in plasma of B. miyamotoi-infected SCID mice receiving passive transfer of pooled B. miyamotoi antiserum, B. burgdorferi antiserum or naïve serum 8 days after initial infection. ** p<0.01, *** p<0.001. Error bars illustrate mean ± S.E.M.
Vsp1 antibody-mediated killing
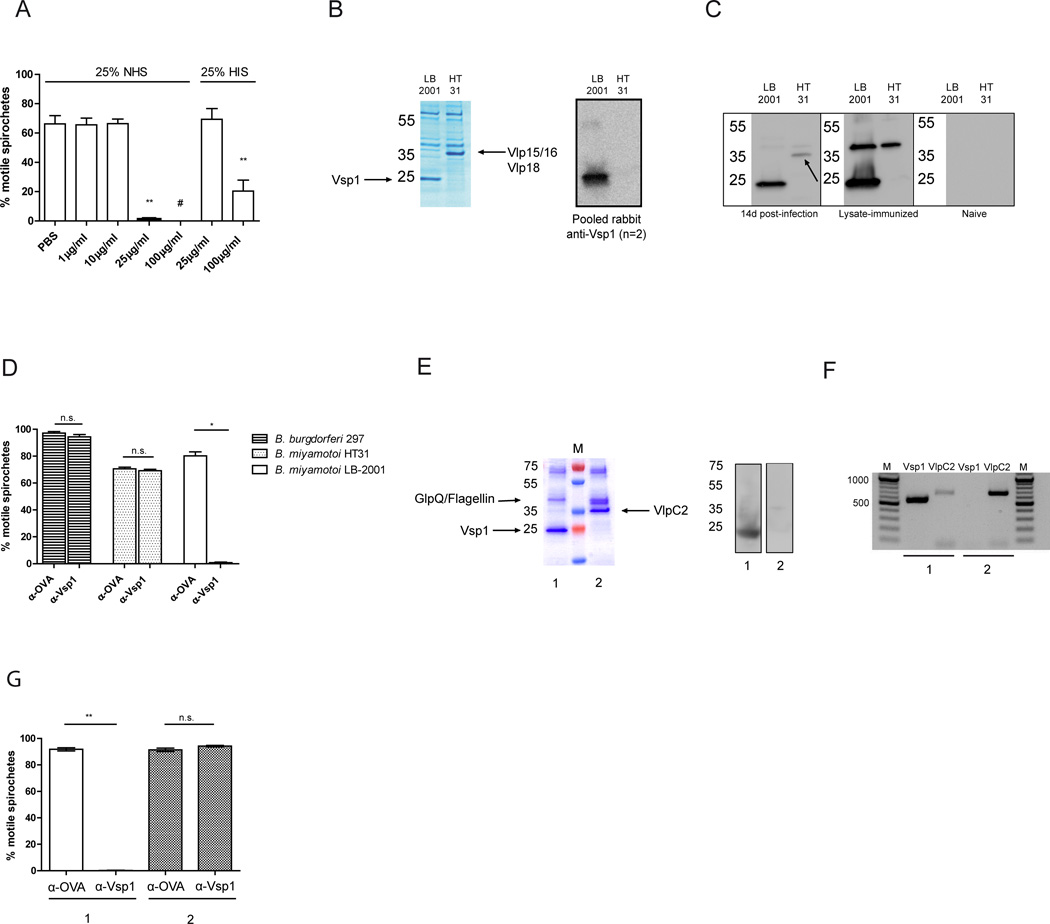
To confirm that Vsp1 antibodies are involved in the elimination of B. miyamotoi, we generated anti-Vsp1 IgG and performed killing assays on cultured B. miyamotoi spirochetes. We generated polyclonal anti-Vsp1 IgG through immunization of two rabbits with rVsp1. Next, to confirm the stability of Vsp1 expression in vitro, we cultured B. miyamotoi for 50 weeks using weekly passages, confirming stable expression after 50 passages (data not shown). Subsequently, we performed killing assays using increasing concentrations of pooled purified anti-Vsp1 IgG added to 5 replicate wells containing 105 B. miyamotoi spirochetes in the presence of 25% human serum (NHS), and determined viability after one hour by assessing the percentage of motile spirochetes during dark-field examination of 100 spirochetes per well. This resulted in a near-complete killing from 25 µg/ml antibody (Fig. 5A) with extensive bleb formation. Interestingly, when heat-inactivated human serum (HIS) was used, thus eliminating complement activity, no bactericidal effect was observed using 25µg/ml anti-Vsp1 IgG, indicating a role for complement in spirochete killing. However, killing was observed even in HIS when a higher antibody dose (100µg/ml) was administered, suggesting an additional complement-independent mechanism of antibody-mediated killing (Fig. 5A). Next, we examined whether Vsp1 is also expressed by a population of B. miyamotoi HT31, a tick isolate derived from Japan. Coomassie staining on spirochete lysates revealed that instead of a dominant ~23 kDa band, a ~37 kDa protein was expressed in B. miyamotoi HT31. Mass-spectrometric analysis of the ~37 kDa protein band revealed the presence of two Vlp proteins corresponding to the Vlp15/16 protein and Vlp18 protein (WP_025444482.1 and WP_025444235.1, respectively)(Fig. 5B). This finding was confirmed by western blot, which showed absence of anti-Vsp1 IgG binding to B. miyamotoi HT31 lysate (Fig. 5B). Additional western blots using B. miyamotoi LB-2001 infected or immunized mouse sera further confirmed the absence of expression of a Vsp protein in this isolate (Fig. 5C). Interestingly, in pooled sera from four mice infected with B. miyamotoi LB-2001 for 14 days, minor reactivity was observed with the B. miyamotoi HT31 band containing Vlp15/16 and Vlp18. However, mice immunized with LB-2001 lysate expressing Vsp1 did not produce antibodies reactive with the suspected B. miyamotoi HT31 Vlp protein (Fig. 5C). This suggests that during infection with B. miyamotoi LB-2001, a Vlp protein had been expressed at some point during the infection. 100 µg/ml anti-Vsp1 IgG did not induce killing of B. miyamotoi HT31 nor did it have any effect on B. burgdorferi viability, which further demonstrates that Vsp1 antibodies specifically kill Vsp1-expressing spirochetes (Fig. 5D). However, anti-Vsp1 antibody challenge of Vsp1-expressing B. miyamotoi LB-2001 spirochetes consistently resulted in at least 99% of counted spirochetes to be immotile. These data collectively suggest that spirochetes that express Vsp1 are affected by anti-Vsp1 IgG antibodies.
FIGURE 5. Vsp1 antibodies neutralize Vsp1-expressing B. miyamotoi spirochetes, and select for spirochetes expressing a Vlp.
(A) B. miyamotoi LB-2001 spirochetes were incubated with different concentrations of anti-Vsp1 IgG and 25% normal human serum (NHS) or 25% heat-inactivated serum (HIS). Five wells per condition were incubated at 37°C and 100 spirochetes per well were assessed by dark-field microscopy. A representative of three independent experiments is shown. (B) Coomassie blue staining of B. miyamotoi LB-2001 (expressing Vsp1), and of isolate HT31, expressing a ~37 kDa protein band which by mass-spectrometric analysis was revealed to contain Vlp15/16 and Vlp18 (left panel). Western blot analysis of Vsp1 expression in LB-2001 and HT31 lysates (right panel). (C) Western blot analysis of LB-2001 and HT31 lysates using pools of sera from 4 mice infected for 14 days with B. miyamotoi LB-2001 (left strip), mice actively immunized with B. miyamotoi LB-2001 lysate (middle strip), or naive mice (right strip). A band containing Vlp15/16 and Vlp18 in B. miyamotoi HT31 lysate is indicated by an arrow. (D) Killing assay performed using 100µg/ml anti-Vsp1 IgG (α-Vsp1) or a control antibody (α-OVA) and 25% NHS, on B. burgdorferi 297, B. miyamotoi HT31 and B. miyamotoi LB-2001. The graph is a representative of three independent experiments. (E) Coomassie blue staining of B. miyamotoi lysate 1, derived from B. miyamotoi LB-2001 previously challenged with α-OVA, and lysate 2 derived from spirochetes surviving a challenge with α-Vsp1 (E, left panel). M: Protein marker. Vsp1 expression in lysate 1 and 2 as determined by western blot using Vsp1 antiserum (E, right panel). (F) PCR on DNA extracted from isolates 1 and 2 (α-OVA and α-Vsp1 challenged, respectively) performed using an expression site promoter-specific forward primer and reverse primers for Vsp1 or VlpC2. M: Marker indicating number of base pairs (bp), with 100 bp increments. (G) Second challenge with 100µg/ml α-Vsp1 or α-OVA + 25% NHS performed on representative spirochete cultures 1 and 2 (which dominantly express Vsp1 and VlpC2, respectively). * p<0.05, ** p<0.01. # Chi squared test, p=0.002. Error bars illustrate mean ± S.E.M.
Challenge of infected mice with anti-Vsp1 antibodies selects for a different VMP
We next determined whether anti-Vsp1 induced challenge would lead to a different VMP being expressed in the surviving spirochetes, similar to the relapse mechanism observed in other TBRF species. To investigate this, we incubated B. miyamotoi LB-2001 suspensions for one hour with either anti-Vsp1 or a control antibody (anti-OVA, directed against ovalbumin) and then inoculated the suspensions into MKP-F medium to culture any surviving spirochetes. Interestingly, viable spirochetes were cultured from all five replicate wells after one week, however with lower concentrations in the samples previously exposed to anti-Vsp1 IgG compared to those incubated with control antibody (concentration mean ±SE: 2.4 ±0.4 ×105/ml versus 2.5 ±0.4 ×106/ml; p=0.0006). New lysates were made from a P3 passage of these residual spirochetes and two representative lysates are depicted, revealing survival of spirochetes expressing a ~35 kDa band rather than Vsp1 (Fig. 5E, left panel). A western blot confirmed that Vsp1 was only expressed in lysate 1, and not in the anti-Vsp1 exposed lysate 2 (Fig. 5E, right panel). Three peptides identified in this band by mass-spectrometry matched with protein “Vlp5” in strain FR64b of B. miyamotoi from Japan (WP_025444408.1). In order to obtain the B. miyamotoi LB-2001 homologous sequence, we performed a PCR on the isolate that was previously treated with anti-Vsp1 antibodies, using a forward primer based on the “-35” promoter element and a reverse primer based on the 3’ end of the gene coding for Vlp5. This approach yielded a sequence coding for 342 amino acids of the dominant VMP in the expression site of the anti-Vsp1 subjected isolate. The partial gene was annotated in Genbank (KU199715; www.ncbi.nlm.nih.gov/genbank), and shared 99% nucleotide identity with the silent B. miyamotoi VlpC2 gene on plasmid lpE (Genbank accession KU041636). All four peptides derived from the mass spectrometry that were unmatched to other proteins matched with this sequence (Supplemental Fig. 1B). Subsequently, we performed PCR on the isolates subjected to control antibody (isolate 1, expressing Vsp1) or anti-Vsp1 antibodies (isolate 2, expressing VlpC2), amplifying only the Vsp1 or VlpC2 genes located in the expression site. As expected, Vsp1 was amplified from the expression site in the population expressing Vsp1, while from the “relapsed” population the Vsp1 gene was not amplified downstream of the promoter (Fig. 5F). VlpC2 was present downstream of the expression site promoter in the “relapsed” isolate. Interestingly, from the Vsp1 expressing population we also amplified the VlpC2 gene in the expression site, albeit to a lesser extent. Isolates 1 and 2, expressing Vsp1 and VlpC2, respectively, were (re)exposed to anti-Vsp1 IgG or control antibody + 25% NHS for one hour. As expected, the B. miyamotoi LB-2001 isolate previously subjected to control antibody (thus still expressing Vsp1) showed a reduction in viability when exposed to anti-Vsp1. However, the B. miyamotoi LB-2001 isolate that expressed VlpC2 showed no reduction in viability upon rechallenge with anti-Vsp1 IgG antibodies (Fig. 5G). Taken together, these findings provide immunologic evidence that upon a borreliacidal humoral immune response to B. miyamotoi LB-2001 Vsp1, spirochetes that express Vsp1 are eliminated while surviving spirochetes that express a Vlp are unaffected, enabling them to expand. Thus, antibody-mediated selection induced a serotype switch from the dominant spirochete population to a minority serotype.
Antibody responses against VMPs in HTBRF patients
Although all mice subjected to a Vsp1-expressing B. miyamotoi isolate developed high Vsp1 antibody levels, we suspected that it might not be a sensitive seromarker in humans by itself because humans might be infected by a wide range of B. miyamotoi serotypes, some of which could be expressing Vlps, or Vsps with low or absent cross-reactivity to Vsp1. Therefore, we assessed select Vsp and Vlp reactivity of sera from Russian HTBRF patients in whom infection had been confirmed by sequence confirmation of B. miyamotoi PCR positive blood samples. The patient population from which we were able to obtain a selection of serum samples was defined by an established case definition for B. miyamotoi infection (3) and has been previously described (37). In short, the first HTBRF patient sera that were PCR-positive for B. miyamotoi during admission were collected, as well as early convalescent sera approximately one week thereafter. In addition, we also evaluated sera from Russian patients with erythema migrans (EM; diagnosed by a physician) and healthy blood donors from the same region as controls.
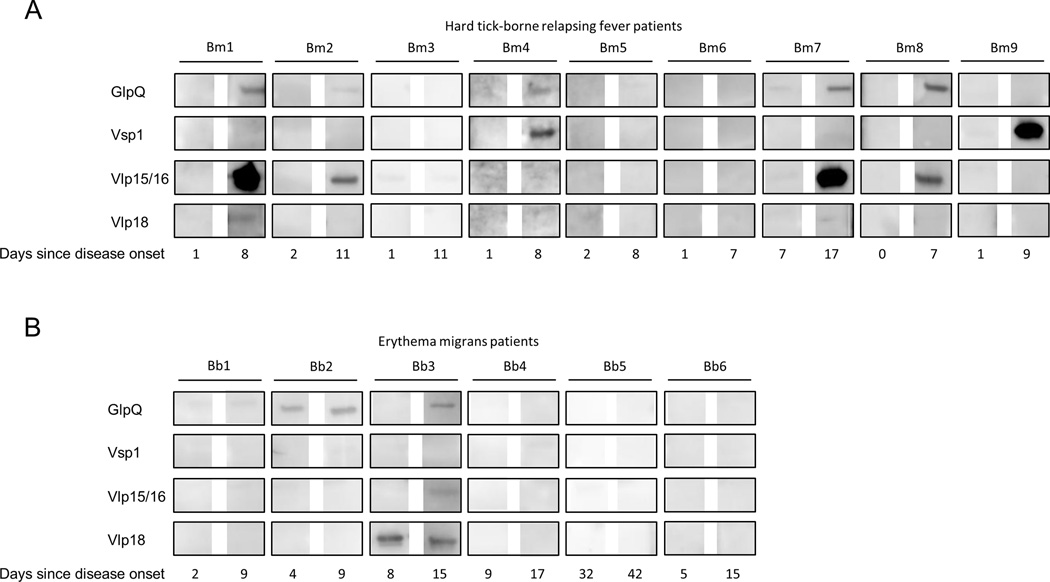
Of nine HTBRF patients, all were PCR positive at the day of admission except patient Bm2, which was positive 2 days after admission. The average time between a reported tick bite and onset of disease was 16.8 days (range: 9–29) while the average time between disease onset and admission was 1.6 days (range: 1–7). Anti-GlpQ IgM seroconversion was observed in five out of nine HTBRF patients around a week after B. miyamotoi was first detected by PCR in blood (Fig. 6A). Two out of nine patients (Bm4, Bm9) demonstrated a robust Vsp1-specific IgM seroconversion, one of which (Bm9) tested negative using GlpQ antibodies. Another four HTBRF patients (Bm1, Bm2, Bm7, Bm8) were demonstrated to have robust IgM seroconversion for Vlp15/16, all of whom were also positive for GlpQ, while a Vlp18 seroconversion occurred in Bm1 (Fig. 6A). Thus six out of nine HTBRF patients showed a seroconversion to these B. miyamotoi VMPs, while five patients showed a seroconversion to GlpQ. Of EM patients, Bb3 demonstrated a seroconversion for GlpQ and was positive for Vlp18 IgM at admission (Fig. 6B). In addition to an EM up to 20 cm diameter, this patient described chills and a headache 13 days after a tick bite followed by a fever (39°C axillary temperature), and displayed elevated ALT (124 IU/L) and AST (94 IU/L) on admission, suggestive of a PCR-negative B. miyamotoi co-infection. None of 12 Russian blood donors were reactive to GlpQ, Vsp1, Vlp15/16 or Vlp18 on Western blot (data not shown).
FIGURE 6. IgM seroconversions to Vsp1 and Vlp15/16 in acute HTBRF patients.
(A) Western blots on recombinant proteins GlpQ, Vsp1, Vlp15/16 and Vlp18. Strips were incubated in sera from nine Russian patients (Bm1 to Bm9) collected at the day they were first found B. miyamotoi PCR positive in blood, and approximately one week thereafter. IgM was detected and all blots were developed simultaneously. The following blots were considered positive: GlpQ seroconversion: Bm1, Bm2, Bm4, Bm7, Bm8; Vsp1 seroconversion: Bm4 and Bm9; Vlp15/16 seroconversion: Bm1, Bm2, Bm7, Bm8. Vlp18 seroconversion: Bm1.
(B) Sera from Russian patients admitted for suspected tick-borne disease and diagnosed by a physician with erythema migrans (Bb1 to Bb6) were tested for IgM against GlpQ, Vsp1, Vlp15/16 and Vlp18. The following blots were considered positive: GlpQ seroconversion in patient Bb3, GlpQ in acute and convalescent serum of Bb2. Vlp18 in acute and convalescent serum of patient Bb3.
Discussion
We report here the identification of Vsp1 as an immunodominant antigen of B. miyamotoi LB-2001 in mice. The antibody response was efficient in clearing B. miyamotoi from the bloodstream in most C3H/HeN mice. Passive transfer of sera from LB-2001 infected C3H/HeN mice to B. miyamotoi-infected SCID mice, which normally exhibit sustained spirochetemia, induced a transient clearance of infection followed by a relapse. In vitro we showed that killing of B. miyamotoi LB-2001 was anti-Vsp1 mediated and largely complement-dependent. Furthermore, we demonstrated that anti-Vsp1 challenge selects for a minority of spirochetes that express VMP (VlpC2) instead of Vsp1, which become resistant to anti-Vsp1 mediated killing. Although B. miyamotoi and B. burgdorferi s.l. are present in the same tick species, the resemblance of B. miyamotoi to other relapsing fever spirochetes is reflected by its presence in the blood and the ability to express different immunogenic VMPs to evade humoral immunity. Together, our data not only provide an experimental model to study B. miyamotoi infection in mice, but also reveal an important role for antibodies in clearance of B. miyamotoi. Finally, we show that VMPs induce robust antibody responses in a subset of HTBRF patients. We postulate that by combining different VMPs a serologic test could be developed to diagnose early infection in humans caused by various B. miyamotoi serotypes.
Spirochetemia patterns and concentrations found in our study were comparable to a study in DBA/2, C57BL/6 and CB17 SCID mice receiving B. miyamotoi-infected donor mouse blood through intravenous injection, with a low-level spirochetemia after 2 days followed by undetectable spirochetemia, and a persistent spirochetemia in SCID mice (45). While B. hermsii is similarly known to cause a continuous spirochetemia in SCID mice, it usually causes a more consistent pattern of relapse in wild-type mice than we observed for B. miyamotoi (31, 46–49). In that respect, B. miyamotoi is reminiscent of a type of murine relapsing fever that exhibits a similarly short-lived spirochetemia with a limited capacity for relapse (20). It is possible that other (clinical) B. miyamotoi isolates or serotypes will behave differently in future studies. On the other hand, B. miyamotoi might be less virulent than B. hermsii or less capable of relapsing efficiently. In line with that hypothesis, in humans a clear relapsing fever pattern has only been described in a small number of HTBRF patients. Alternatively, early antibiotic therapy in HTBRF patients might also account for the lack of relapsing disease in humans (3, 7, 37). In two severely immunocompromised and B-cell depleted patients, B. miyamotoi was able to cause a more chronic infection of the central nervous system, which has thus far not been demonstrated in immunocompetent patients (8, 9).
Our results of the passive transfer of previously infected wild-type mouse sera to infected SCID mice are comparable to those found for B. hermsii. Similar to what we have observed, a longer time to relapse was previously observed after transfer of late immune sera compared to transferring early immune sera (47). We speculate that this phenomenon is caused by the gradual emergence of antibodies against multiple VMPs that occurs in mice during infection. In B. hermsii, around 60 different silent vsp/vlp alleles of are described. A switch between alleles causes a different Vmp to be expressed after spirochetemic relapse, thus evading a neutralizing IgM response against the previously expressed Vmp (20–22). Further investigation is needed to identify all Vmps in B. miyamotoi, and infection with clonal isolates should provide the opportunity to demonstrate active recombination. In humans, we found antibodies against B. miyamotoi Vsp1, Vlp15/16 or Vlp18 in most of the HTBRF patients that we were able to investigate, although the contribution of cross-reactivity between yet unknown VMP antibodies remains to be investigated. Further research should elucidate how often these VMPs are recognized in larger HTBRF patient cohorts, which other VMPs are commonly expressed during human infection, cross-reactivity in Lyme borreliosis patients, and whether a combination of VMPs could increase sensitivity of HTBRF serological testing. We identified one patient that presented with an EM as well as symptoms and serology consistent with HTBRF. Although data on the incidence of co-infections is limited, studies in the U.S.A. have shown that 14% of HTBRF patients were co-infected with B. burgdorferi, while 9.8% of acute Lyme borreliosis patients were positive for GlpQ antibodies (7, 14). Thus, despite a negative PCR for B. miyamotoi, this patient could indeed have been co-infected with B. burgdorferi s.l. and B. miyamotoi. GlpQ antibodies were present in all mice and most patients in this study, however antibody responses against VMPs were consistently more robust, using our methods. Since Vsp1 antibody levels were higher than for GlpQ in mice, and were produced rapidly after infection, a VMP-based serological assay covering the most common VMP serotypes could improve serologic diagnosis of B. miyamotoi infections.
Previous studies in B. miyamotoi have shown both isolates LB-2001 and HT31 to be resistant to direct complement-mediated killing in vitro, similar to B. hermsii (12, 50). In this study, using polyclonal rabbit IgG against Vsp1, we were able to demonstrate that antibody-mediated killing was mostly complement-dependent at moderate antibody concentrations. Although complement was previously demonstrated to significantly enhance antibody-mediated killing of B. hermsii in vitro (49), several studies have demonstrated that complement is not essential for antibody-mediated killing and clearance of relapsing fever spirochetes, and complement-and phagocyte independent bactericidal antibodies have also been described for B. burgdorferi, causing outer membrane damage via a yet to be unraveled process (20, 51–55). Further studies using monoclonal IgM against VMPs should expand our understanding of this phenomenon in B. miyamotoi. IgM appeared to play a major role in B. miyamotoi clearance in wild-type mice, as its rise coincided with B. miyamotoi clearance, and passive transfer of 5-day immune sera to SCID mice cleared spirochetemia. In other relapsing fever spirochetes, T-cell independent IgM has a major role in early clearance, facilitated by B1b cells and splenic marginal zone B cells (21, 46, 48, 56). IgM specific for Vlp7 has been demonstrated to directly neutralize Vlp7-expressing B. hermsii in vitro and in vivo upon passive immunization. However, active vaccination of mice with recombinant Vlp7 did not result in protection (21). In our experiments, the humoral immune response elicited by active immunization of mice with native Vsp1 (i.e. Vsp1-expressing B. miyamotoi lysate) was able to temporarily clear infection upon passive transfer to infected SCID mice. Although we did not actively immunize mice with rVsp1 to assess protection, we did observe direct killing in vitro by antibodies derived from rabbits actively immunized with rVsp1, and further studies should aim to identify the potential of recombinant B. miyamotoi VMPs in inducing protective immunity.
To conclude, although we are only just beginning to understand the biology of B. miyamotoi and the (immuno)pathogenesis of HTBRF, we here show that B. miyamotoi is able to express various variable major proteins (VMPs) to evade host humoral immune responses. The findings presented in this study provide the basis for future research on additional VMPs in B. miyamotoi and the scientific impetus to explore the clinical utility of a combined VMP-based serological test for early detection of HTBRF.
Supplementary Material
Acknowledgments
We would like to thank Alan Barbour for his review and feedback on the manuscript, and we thank Cees van ‘t Veer, Anneke Oei, Bob de Wever, Jasmin Ersöz, Nadezhda Kolyasnikova, Olga Platonova, and Anton Titkov for their input and technical assistance.
Grant support: JWH is a recipient of a VENI stipend (91611065) from the Netherlands Organization for Health Research and Development (ZonMw). EF is an investigator of the Howard Hughes Medical Institute. This study was supported by a grant of the Russian Scientific Foundation (project number 15-15-00072) for AEP, DSS, and LSK. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations used in this article
- EM
erythema migrans
- GlpQ
glycerophosphodiester phosphodiesterase
- HIS
Heat-inactivated human serum
- HTBRF
hard tick-borne relapsing fever
- NHS
normal human serum
- Vlp
variable large protein
- VMP
variable major protein
- Vsp
variable small protein
- TBRF
tick-borne relapsing fever
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Wagemakers A, Staarink PJ, Sprong H, Hovius JW. Borrelia miyamotoi: a widespread tick-borne relapsing fever spirochete. Trends Parasitol. 2015;31:260–269. doi: 10.1016/j.pt.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 2.Krause PJ, Fish D, Narasimhan S, Barbour AG. Borrelia miyamotoi infection in nature and in humans. Clin Microbiol Infect. 2015;21:631–639. doi: 10.1016/j.cmi.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Platonov AE, Karan LS, Kolyasnikova NM, Makhneva NA, Toporkova MG, Maleev VV, Fish D, Krause PJ. Humans infected with relapsing fever spirochete Borrelia miyamotoi, Russia. Emerg Infect Dis. 2011;17:1816–1823. doi: 10.3201/eid1710.101474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krause PJ, Narasimhan S, Wormser GP, Rollend L, Fikrig E, Lepore T, Barbour A, Fish D. Human Borrelia miyamotoi infection in the United States. N Engl J Med. 2013;368:291–293. doi: 10.1056/NEJMc1215469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chowdri HR, Gugliotta JL, Berardi VP, Goethert HK, Molloy PJ, Sterling SL, Telford SR. Borrelia miyamotoi infection presenting as human granulocytic anaplasmosis: a case report. Ann Intern Med. 2013;159:21–27. doi: 10.7326/0003-4819-159-1-201307020-00005. [DOI] [PubMed] [Google Scholar]
- 6.Sato K, Takano A, Konnai S, Nakao M, Ito T, Koyama K, Kaneko M, Ohnishi M, Kawabata H. Human infections with Borrelia miyamotoi, Japan. Emerg Infect Dis. 2014;20:1391–1393. doi: 10.3201/eid2008.131761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Molloy PJ, Telford SR, Iii, Chowdri HR, Lepore TJ, Gugliotta JL, Weeks KE, Hewins ME, Goethert HK, Berardi VP. Borrelia miyamotoi Disease in the Northeastern United States: A Case Series. Ann Intern Med. 2015;163:91–98. doi: 10.7326/M15-0333. [DOI] [PubMed] [Google Scholar]
- 8.Gugliotta JL, Goethert HK, Berardi VP, Telford SR., 3rd Meningoencephalitis from Borrelia miyamotoi in an immunocompromised patient. N Engl J Med. 2013;368:240–245. doi: 10.1056/NEJMoa1209039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hovius JW, de Wever B, Sohne M, Brouwer MC, Coumou J, Wagemakers A, Oei A, Knol H, Narasimhan S, Hodiamont CJ, Jahfari S, Pals ST, Horlings HM, Fikrig E, Sprong H, van Oers MH. A case of meningoencephalitis by the relapsing fever spirochaete Borrelia miyamotoi in Europe. Lancet. 2013;382:658. doi: 10.1016/S0140-6736(13)61644-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krause PJ, Barbour AG. Borrelia miyamotoi: The Newest Infection Brought to Us by Deer Ticks. Ann Intern Med. 2015;163:141–142. doi: 10.7326/M15-1219. [DOI] [PubMed] [Google Scholar]
- 11.Margos G, Stockmeier S, Hizo-Teufel C, Hepner S, Fish D, Dautel H, Sing A, Dzaferovic E, Rieger M, Jungnick S, Binder K, Straubinger RK, Fingerle V. Long-term in vitro cultivation of Borrelia miyamotoi. Ticks Tick Borne Dis. 2015;6:181–184. doi: 10.1016/j.ttbdis.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Wagemakers A, Oei A, Fikrig MM, Miellet WR, Hovius JW. The relapsing fever spirochete Borrelia miyamotoi is cultivable in a modified Kelly-Pettenkofer medium, and is resistant to human complement. Parasit Vectors. 2014;7:418. doi: 10.1186/1756-3305-7-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hue F, Ghalyanchi Langeroudi A, Barbour AG. Chromosome Sequence of Borrelia miyamotoi, an Uncultivable Tick-Borne Agent of Human Infection. Genome Announc. 2013;1 doi: 10.1128/genomeA.00713-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krause PJ, Narasimhan S, Wormser GP, Barbour AG, Platonov AE, Brancato J, Lepore T, Dardick K, Mamula M, Rollend L, Steeves TK, Diuk-Wasser M, Usmani-Brown S, Williamson P, Sarksyan DS, Fikrig E, Fish D G. Tick Borne Diseases. Borrelia miyamotoi sensu lato Seroreactivity and Seroprevalence in the Northeastern United States. Emerg Infect Dis. 2014;20:1183–1190. doi: 10.3201/eid2007.131587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwan TG, Schrumpf ME, Hinnebusch BJ, Anderson DE, Jr, Konkel ME. GlpQ: an antigen for serological discrimination between relapsing fever and Lyme borreliosis. J Clin Microbiol. 1996;34:2483–2492. doi: 10.1128/jcm.34.10.2483-2492.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lopez JE, Porcella SF, Schrumpf ME, Raffel SJ, Hammer CH, Zhao M, Robinson MA, Schwan TG. Identification of conserved antigens for early serodiagnosis of relapsing fever Borrelia. Microbiology. 2009;155:2641–2651. doi: 10.1099/mic.0.029918-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jahfari S, Herremans T, Platonov AE, Kuiper H, Karan LS, Vasilieva O, Koopmans MP, Hovius JW, Sprong H. High seroprevalence of Borrelia miyamotoi antibodies in forestry workers and individuals suspected of human granulocytic anaplasmosis in the Netherlands. New Microbes New Infect. 2014;2:144–149. doi: 10.1002/nmi2.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamase A, Takahashi Y, Nohgi K, Fukunaga M. Homology of variable major protein genes between Borrelia hermsii and Borrelia miyamotoi. FEMS Microbiol. Lett. 1996;140:131–137. doi: 10.1016/0378-1097(96)00168-1. [DOI] [PubMed] [Google Scholar]
- 19.Barbour AG. Multiple and Diverse vsp and vlp Sequences in Borrelia miyamotoi, a Hard Tick-Borne Zoonotic Pathogen. PloS One. 2016;11:e0146283. doi: 10.1371/journal.pone.0146283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Connolly SE, Benach JL. Cutting edge: the spirochetemia of murine relapsing fever is cleared by complement-independent bactericidal antibodies. J Immunol. 2001;167:3029–3032. doi: 10.4049/jimmunol.167.6.3029. [DOI] [PubMed] [Google Scholar]
- 21.Barbour AG, Bundoc V. In vitro and in vivo neutralization of the relapsing fever agent Borrelia hermsii with serotype-specific immunoglobulin M antibodies. Infect Immun. 2001;69:1009–1015. doi: 10.1128/IAI.69.2.1009-1015.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barbour AG, Dai Q, Restrepo BI, Stoenner HG, Frank SA. Pathogen escape from host immunity by a genome program for antigenic variation. Proc Natl Acad Sci U S A. 2006;103:18290–18295. doi: 10.1073/pnas.0605302103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Plasterk RH, Simon MI, Barbour AG. Transposition of structural genes to an expression sequence on a linear plasmid causes antigenic variation in the bacterium Borrelia hermsii. Nature. 1985;318:257–263. doi: 10.1038/318257a0. [DOI] [PubMed] [Google Scholar]
- 24.Meier JT, Simon MI, Barbour AG. Antigenic variation is associated with DNA rearrangements in a relapsing fever Borrelia. Cell. 1985;41:403–409. doi: 10.1016/s0092-8674(85)80013-1. [DOI] [PubMed] [Google Scholar]
- 25.Dai Q, Restrepo BI, Porcella SF, Raffel SJ, Schwan TG, Barbour AG. Antigenic variation by Borrelia hermsii occurs through recombination between extragenic repetitive elements on linear plasmids. Mol Microbiol. 2006;60:1329–1343. doi: 10.1111/j.1365-2958.2006.05177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hinnebusch BJ, Barbour AG, Restrepo BI, Schwan TG. Population structure of the relapsing fever spirochete Borrelia hermsii as indicated by polymorphism of two multigene families that encode immunogenic outer surface lipoproteins. Infect Immun. 1998;66:432–440. doi: 10.1128/iai.66.2.432-440.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mehra R, Londono D, Sondey M, Lawson C, Cadavid D. Structure-function investigation of vsp serotypes of the spirochete Borrelia hermsii. PloS One. 2009;4:e7597. doi: 10.1371/journal.pone.0007597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Restrepo BI, Kitten T, Carter CJ, Infante D, Barbour AG. Subtelomeric expression regions of Borrelia hermsii linear plasmids are highly polymorphic. Mol Microbiol. 1992;6:3299–3311. doi: 10.1111/j.1365-2958.1992.tb02198.x. [DOI] [PubMed] [Google Scholar]
- 29.Cadavid D, Pennington PM, Kerentseva TA, Bergstrom S, Barbour AG. Immunologic and genetic analyses of VmpA of a neurotropic strain of Borrelia turicatae. Infect Immun. 1997;65:3352–3360. doi: 10.1128/iai.65.8.3352-3360.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stoenner HG, Dodd T, Larsen C. Antigenic variation of Borrelia hermsii. J Exp Med. 1982;156:1297–1311. doi: 10.1084/jem.156.5.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raffel SJ, Battisti JM, Fischer RJ, Schwan TG. Inactivation of genes for antigenic variation in the relapsing fever spirochete Borrelia hermsii reduces infectivity in mice and transmission by ticks. PLoS Pathog. 2014;10:e1004056. doi: 10.1371/journal.ppat.1004056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lawson CL, Yung BH, Barbour AG, Zuckert WR. Crystal structure of neurotropism-associated variable surface protein 1 (Vsp1) of Borrelia turicatae. J Bacteriol. 2006;188:4522–4530. doi: 10.1128/JB.00028-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cadavid D, Pachner AR, Estanislao L, Patalapati R, Barbour AG. Isogenic serotypes of Borrelia turicatae show different localization in the brain and skin of mice. Infect Immun. 2001;69:3389–3397. doi: 10.1128/IAI.69.5.3389-3397.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cadavid D, Thomas DD, Crawley R, Barbour AG. Variability of a bacterial surface protein and disease expression in a possible mouse model of systemic Lyme borreliosis. J Exp Med. 1994;179:631–642. doi: 10.1084/jem.179.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pennington PM, Allred CD, West CS, Alvarez R, Barbour AG. Arthritis severity and spirochete burden are determined by serotype in the Borrelia turicatae-mouse model of Lyme disease. Infect Immun. 1997;65:285–292. doi: 10.1128/iai.65.1.285-292.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Setubal JC, Reis M, Matsunaga J, Haake DA. Lipoprotein computational prediction in spirochaetal genomes. Microbiology. 2006;152:113–121. doi: 10.1099/mic.0.28317-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sarksyan DS, Platonov AE, Karan LS, Malinin IE, Khalitova LI, Shakhov VI, Dudarev MV, Malinin OV, Maleev VV. Clinical presentation of "new" tick-borne borreliosis caused by Borrelia miyamotoi. Ter Arkh. 2012;84:34–41. [PubMed] [Google Scholar]
- 38.Larsson C, Bergstrom S. A novel and simple method for laboratory diagnosis of relapsing Fever borreliosis. Open Microbiol J. 2008;2:10–12. doi: 10.2174/1874285800802010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schuijt TJ, Coumou J, Narasimhan S, Dai J, Deponte K, Wouters D, Brouwer M, Oei A, Roelofs JJ, van Dam AP, van der Poll T, Van't Veer C, Hovius JW, Fikrig E. A tick mannose-binding lectin inhibitor interferes with the vertebrate complement cascade to enhance transmission of the lyme disease agent. Cell Host Microbe. 2011;10:136–146. doi: 10.1016/j.chom.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kenedy MR, Vuppala SR, Siegel C, Kraiczy P, Akins DR. CspA-mediated binding of human factor H inhibits complement deposition and confers serum resistance in Borrelia burgdorferi. Infect Immun. 2009;77:2773–2782. doi: 10.1128/IAI.00318-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Burgel ND, Kraiczy P, Schuijt TJ, Zipfel PF, van Dam AP. Identification and functional characterisation of Complement Regulator Acquiring Surface Protein-1 of serum resistant Borrelia garinii OspA serotype 4. BMC Microbiol. 2010;10:43. doi: 10.1186/1471-2180-10-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Dam AP, Oei A, Jaspars R, Fijen C, Wilske B, Spanjaard L, Dankert J. Complement-mediated serum sensitivity among spirochetes that cause Lyme disease. Infect Immun. 1997;65:1228–1236. doi: 10.1128/iai.65.4.1228-1236.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Narasimhan S, Coumou J, Schuijt TJ, Boder E, Hovius JW, Fikrig E. A tick gut protein with fibronectin III domains aids Borrelia burgdorferi congregation to the gut during transmission. PLoS Pathog. 2014;10:e1004278. doi: 10.1371/journal.ppat.1004278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kelley LA, Mezulis S, Yates CM, Wass MN, Sternberg MJ. The Phyre2 web portal for protein modeling, prediction and analysis. Nat Protoc. 2015;10:845–858. doi: 10.1038/nprot.2015.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krause PJ, Hendrickson JE, Steeves TK, Fish D. Blood transfusion transmission of the tick-borne relapsing fever spirochete Borrelia miyamotoi in mice. Transfusion. 2015;55:593–597. doi: 10.1111/trf.12879. [DOI] [PubMed] [Google Scholar]
- 46.Alugupalli KR, Gerstein RM, Chen J, Szomolanyi-Tsuda E, Woodland RT, Leong JM. The resolution of relapsing fever borreliosis requires IgM and is concurrent with expansion of B1b lymphocytes. J Immunol. 2003;170:3819–3827. doi: 10.4049/jimmunol.170.7.3819. [DOI] [PubMed] [Google Scholar]
- 47.Bolz DD, Sundsbak RS, Ma Y, Akira S, Weis JH, Schwan TG, Weis JJ. Dual role of MyD88 in rapid clearance of relapsing fever Borrelia spp. Infect Immun. 2006;74:6750–6760. doi: 10.1128/IAI.01160-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vuyyuru R, Liu H, Manser T, Alugupalli KR. Characteristics of Borrelia hermsii infection in human hematopoietic stem cell-engrafted mice mirror those of human relapsing fever. Proc Natl Acad Sci U S A. 2011;108:20707–20712. doi: 10.1073/pnas.1108776109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Woodman ME, Cooley AE, Avdiushko R, Bowman A, Botto M, Wooten RM, van Rooijen N, Cohen DA, Stevenson B. Roles for phagocytic cells and complement in controlling relapsing fever infection. J Leukoc Biol. 2009;86:727–736. doi: 10.1189/jlb.0309169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Teegler A, Herzberger P, Margos G, Fingerle V, Kraiczy P. The relapsing fever spirochete Borrelia miyamotoi resists complement-mediated killing by human serum. Ticks Tick Borne Dis. 2014 doi: 10.1016/j.ttbdis.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 51.LaRocca TJ, Katona LI, Thanassi DG, Benach JL. Bactericidal action of a complement-independent antibody against relapsing fever Borrelia resides in its variable region. J Immunol. 2008;180:6222–6228. doi: 10.4049/jimmunol.180.9.6222. [DOI] [PubMed] [Google Scholar]
- 52.Newman K, Jr, Johnson RC. In vivo evidence that an intact lytic complement pathway is not essential for successful removal of circulating Borrelia turicatae from mouse blood. Infect Immun. 1981;31:465–469. doi: 10.1128/iai.31.1.465-469.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Connolly SE, Thanassi DG, Benach JL. Generation of a complement-independent bactericidal IgM against a relapsing fever Borrelia. J Immunol. 2004;172:1191–1197. doi: 10.4049/jimmunol.172.2.1191. [DOI] [PubMed] [Google Scholar]
- 54.Coleman JL, Rogers RC, Benach JL. Selection of an escape variant of Borrelia burgdorferi by use of bactericidal monoclonal antibodies to OspB. Infect Immun. 1992;60:3098–3104. doi: 10.1128/iai.60.8.3098-3104.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sadziene A, Jonsson M, Bergstrom S, Bright RK, Kennedy RC, Barbour AG. A bactericidal antibody to Borrelia burgdorferi is directed against a variable region of the OspB protein. Infect Immun. 1994;62:2037–2045. doi: 10.1128/iai.62.5.2037-2045.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Belperron AA, Dailey CM, Bockenstedt LK. Infection-induced marginal zone B cell production of Borrelia hermsii-specific antibody is impaired in the absence of CD1d. J Immunol. 2005;174:5681–5686. doi: 10.4049/jimmunol.174.9.5681. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.