Abstract
Mounting evidence shows that hyperhomocysteinemia is a risk factor for cognitive decline. This study enrolled subjects with normal serum levels of B12 and folate and performed thorough neuropsychological assessments to illuminate the independent role of homocysteine on cognitive functions.
Participants between ages 50 and 85 were enrolled with Modified Hachinski ischemic score of <4, adequate visual and auditory acuity to allow neuropsychological testing, and good general health. Subjects with cognitive impairment resulting from secondary causes were excluded. Each of the participants completed evaluations of general intellectual function, including the Mini-Mental State Examination, Cognitive Abilities Screening Instrument, Clinical Dementia Rating, and a battery of neuropsychological assessments.
This study enrolled 225 subjects (90 subjects younger than 65 years and 135 subjects aged 65 years or older). The sex proportion was similar between the 2 age groups. Years of education were significantly fewer in the elderly (7.49 ± 5.40 years) than in the young (9.76 ± 4.39 years, P = 0.001). There was no significant difference in body mass index or levels of vitamin B12 and folate between the 2 age groups. Homocysteine levels were significantly higher in the elderly group compared to the younger group (10.8 ± 2.7 vs. 9.5 ± 2.5 μmol/L, respectively, P = 0.0006). After adjusting for age, sex, and education, only the Digit Symbol Substitution (DSS) score was significantly lower in subjects with hyperhomocysteinemia (homocysteine >12 μmol/L) than those with homocysteine ≤12 μmol/L in the elderly group (DSS score: 7.1 ± 2.7 and 9.0 ± 3.0, respectively, beta = −1.6, 95% confidence interval [CI] = −2.8∼−0.5, P = 0.001) and borderline significance was noted in the combined age group (beta = −1.1, 95% CI = −2.1∼−0.1, P = 0.04). We did not find an association between hyperhomocysteinemia and other neuropsychological assessments.
This is the first study to demonstrate a significant association between hyperhomocysteinemia (>12 μmol/L) and low DSS score, suggesting that DSS score may be an independent marker of cognitive impairment in response to hyperhomocysteinemia, especially in the elderly. Further replication studies with larger cohorts are needed to confirm our results.
Keywords: cobalamide, cognitive impairment, folate, homocysteine, vitamin B12
1. Introduction
Epidemiological reports consistently demonstrate an association between elevated plasma homocysteine and increased risk for Alzheimer disease (AD).[1,2] Hyperhomocysteinemia was shown to be an independent risk factor for AD in the Framingham study, in which homocysteine concentration over 14 μmol/L doubled the risk for AD.[2] Although the Framingham study did not find a relationship between plasma concentrations of folate, vitamin B6, or B12 and AD risk, a Swedish population-based longitudinal study showed that nondemented people with very low vitamin B12 (150 pmol/L and lower) and folate concentrations (10 nmol/L and lower) had twice higher AD risk compared to those with normal levels.[3] However, the association between high homocysteine concentrations and AD is largely confounded by age[4,5] and conflicting evidence showed no association between homocysteine and cognitive decline in people age 55 and older in the Rotterdam study[6] and in the other reports.[7] In addition, treatments for lower homocysteine concentration failed to demonstrate global benefit in clinical trials, with supplementation of vitamin B12 reducing risk only in subsets of the populations studied.[8–10]
These clinical data suggest that homocysteine, folate, and vitamin B12 have a more complicated relationship with AD risk than just serving as simple biomarkers.
Homocysteine is a precursor of methionine and cysteine. Folate and vitamin B12 are essential for the conversion of homocysteine to methionine. Methylcobalamin in the cytosol and adenosylcobalamin in the mitochondria are 2 major forms of vitamin B12.[11] Methylcobalamin functions as an essential coenzyme to methionine synthase, which catalyzes the remethylation of homocysteine to methionine.[11] Being the most important coenzyme in methyl group transfer, methylcobalamin facilitates the methylation of homocysteine by methyltetrahydrofolate (MTF) into methionine and tetrahydrofolate (THF). THF is necessary for normal DNA synthesis in all cells, including blood cell precursors and myelin-producing oligodendrocytes. A deficiency of vitamin B12 leads to accumulation of homocysteine. Increase in serum homocysteine level therefore indicates a possible deficiency of vitamin B12. In contrast, however, homocysteine is not a specific B12 deficiency marker as deficiencies of vitamin B6 and folate can also raise homocysteine levels. This metabolic pathway is not only involved in the regulation of B vitamins, homocysteine, and methionine, but also in the methylation of proteins, histones, DNA, and RNA. Furthermore, adenosylcobalamin, the other form of vitamin B12, is formed in the mitochondria and is an important cofactor in rearrangement reactions. Vitamin B12 is also a component of fatty acid, carbohydrate, and nucleic acid metabolism.[11] In addition to vitamin B12, folates also participate in various enzymatic reactions mostly involving transmethylations and synthesis of methionine, glycine, and thymidine. Both B12 and folates are necessary in the regulation of homocysteine levels and DNA/RNA metabolism.
Hyperhomocysteinemia and deficiency in folate or vitamin B12 are not uncommon. In our registry of dementia clinics of Chang Gung Memorial Hospital (CGMH), 148 of 895 (14.8%) of consecutive patients had a history or current status of hyperhomocysteinemia or deficiency of folate or vitamin B12 (unpublished). Given that homocysteine, folate, and vitamin B12 are involved in AD risk, this study enrolled subjects with normal concentrations of B12 and folate and performed thorough neuropsychological assessments to illuminate the independent role of homocysteine on cognitive function.
2. Methods
2.1. Subjects
Subjects were recruited from the CGMH. Patients with a previous clinical history of neurological, psychiatric, somatic, stroke, or toxic causes for dementia were excluded. The inclusion criteria included age between 50 and 85 years, Modified Hachinski ischemic score of <4, adequate visual and auditory acuity with or without aid to allow neuropsychological testing, and good general health with no additional diseases expected to interfere with the study. Exclusion criteria included cognitive impairment resulting from cerebral trauma, subdural hematoma, or hypoxic cerebral damage regardless of etiology, vitamin deficiency states, including folate, vitamin B12, and other B complex deficiencies, such as thiamine deficiency in Korsakoff's syndrome, central nervous system infection, brain neoplasm, significant endocrine or metabolic disease, including thyroid or pituitary disease, Cushing syndrome, severe renal failure, or mental retardation, and vascular dementia fulfilling the Diagnostic and Statistical Manual of Mental Disorders IV (DSM-IV) criteria[12] for vascular dementia and the requirements of the National Institute of Neurological Disorders and Stroke-Association Internationale pour la Recherche et l’Enseignement en Neurosciences (NINDS-AIREN) criteria[13] for probable vascular dementia. The dementia group was defined by the following criteria: Clinical Dementia Rating (CDR) = 0.5, 1.0, or 2.0 and for those with CDR 0.5, the diagnosis of incipient AD depended on the combined consensus of the investigators at the clinical level, activities of daily living (ADL), and neuropsychological impairment[14]; probable AD defined by NINCDS/ADRDA criteria[15]; information from caregiver/informant accompanying patient to all scheduled visits; and Cornell Scale for Depression in Dementia (CSDD) score <8. This study was approved by the institutional review board of CGMH. All subjects gave informed consent for the study.
2.2. Clinical information
Anthropometric data and 12-hour fasting blood were collected from all participants. Education level was documented in years. Body mass index (BMI) was calculated by weight in kilograms divided by squared height in meters.
2.3. Neuropsychological tests and assessment
Each subject completed evaluations of general intellectual function, including the Mini-Mental State Examination (MMSE)[16] and Cognitive Abilities Screening Instrument (CASI), Chinese Version.[17] DR sum of box (SOB)[18] was also recorded. A battery of neuropsychological assessments included Memory— the Taiwanese version of the Wechsler Memory Scale, Third Edition (mainly logical memory and visual reproduction subtests)[19] and the Word Sequence Learning Test[20]; Language—semantic association of verbal fluency[21]; visuospatial function—3-Dimensional Block Construction Models (3-D BCM)[22]; attention—Digit Span and Digit Symbol Substitution subtests (DSS) of the Taiwanese version of the Wechsler Adult Intelligence Scale, Third Edition[23]; and executive function—modified Wisconsin card sorting test (WCST).[24]
2.4. Statistical analysis and power estimation
The Pearson χ2 test or t test was utilized to compare demographic data between controls and cases. Correlation coefficients between serum homocysteine levels and cognitive assessments were evaluated by Spearman rank correlation coefficient. Two-tailed P values were derived from the χ2 test or Fisher exact test. Association analyses were performed first stratified by sexes and then stratified by the age of 65 years. The elderly group was defined as subjects ≥65 years. Associations of dementia and cognitive assessments with homocysteine were examined by logistic regression and general linear models where appropriate. Confounding factors were adjusted in the models, including age, sex, hypertension, diabetes mellitus, smoking, total cholesterol level, and education.
Based on the present setting, when the significance level was set at 0.01 as Bonferroni correction, at least a sample size of 50 was required to obtain a power >0.8 to detect the difference of MMSE between the normal and hyperhomocystenemia groups. Analyses were performed using SAS software version 9.1.3 (SAS Institute, Cary, NC).
3. Results
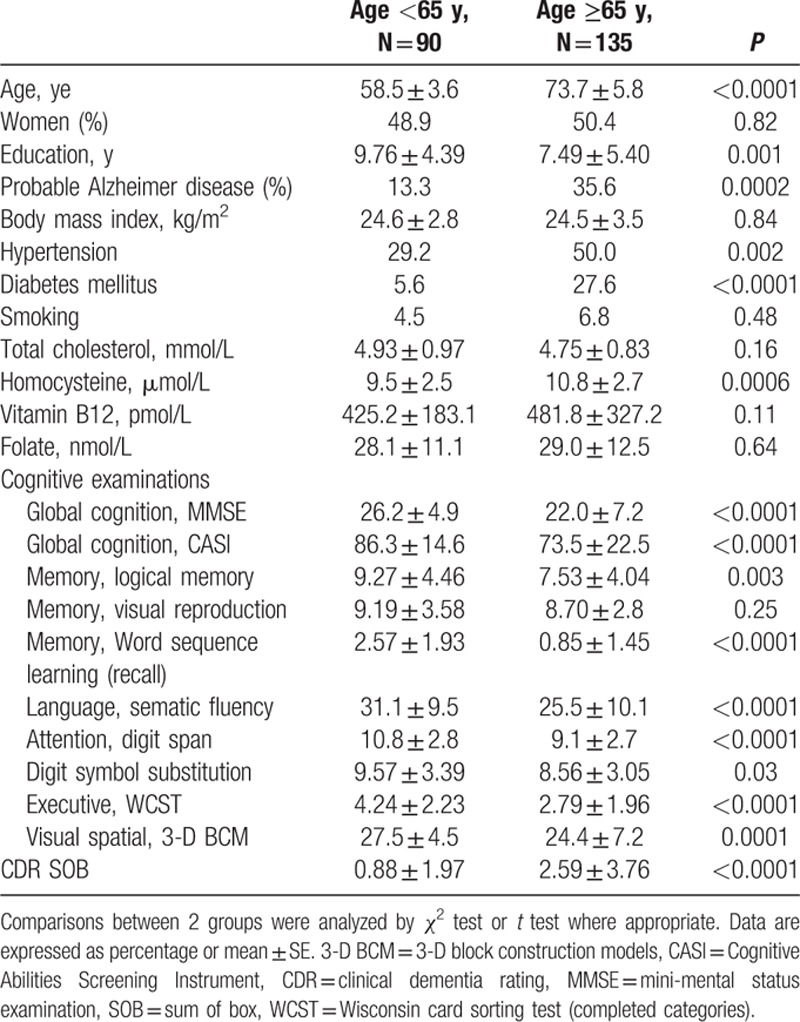
This study enrolled 225 subjects, including 90 persons who were younger than 65 years (range 50–64 years) and 135 subjects aged 65 years or older (range 65–85 years). The demographic data are shown in Table 1. The gender proportion was similar between the 2 age groups. Years of education were significantly fewer in the elderly (7.49 ± 5.40 years) than in the young (9.76 ± 4.39 years, P = .0001). The proportion of probable AD was higher in the elderly than in the young group (35.6% and 13.3%, respectively). The proportion of hypertension and diabetes was higher in the elderly (50% and 27.6%) compared to those in the young group (29.2% and 5.6%). There was no significant difference in BMI, smoking, or levels of total cholesterol, vitamin B12, and folate between the 2 age groups. However, homocysteine levels were significantly higher in the elderly group than in the younger group (10.8 ± 2.7 vs. 9.5 ± 2.5 μmol/L, respectively, P = 0.0006). As expected, subjects younger than 65 years tended to have better general cognitive function as well as executive function (Table 1). Specifically, compared to the older subjects, younger subjects had significantly higher scores in the MMSE, CASI, logic memory scale, word sequence learning, sematic fluency, digit span, WCST (completed categories), 3-D BCM, and function status (CDR-SOB). There was a borderline difference in DSS score (P = 0.03) and no difference in visual production (P = 0.25) between the 2 age groups.
Table 1.
Characteristics of the subjects at baseline.
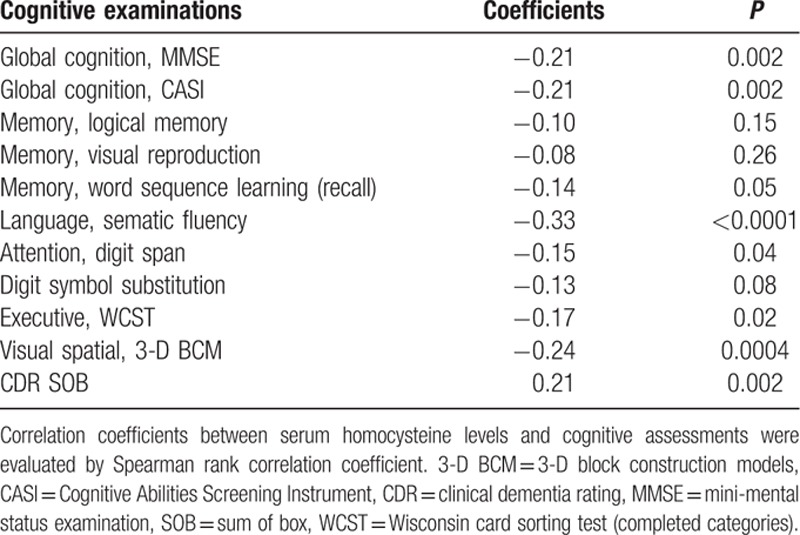
Correlation coefficients between serum homocysteine levels and cognitive assessments are shown in Table 2. We found a weak negative linear relationship (coefficients <−0.30) between homocysteine levels and semantic fluency of language. There were borderline negative linear relationships (coefficients −0.30 to −0.20) between homocysteine levels and global assessments (MMSE and CASI) and visual spatial function. The positive linear relationship with CDR-SOB was also borderline (coefficients = 0.21).
Table 2.
Correlation coefficients between serum homocysteine levels and cognitive assessments.
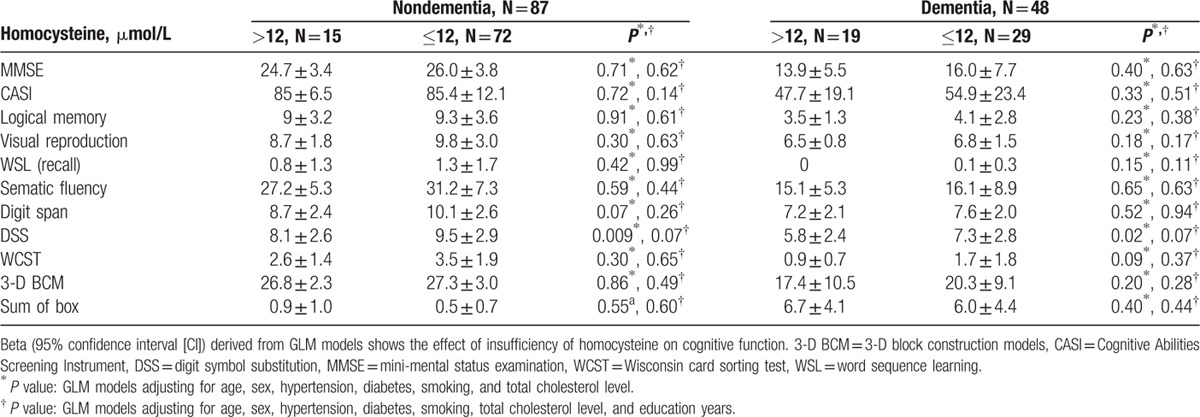
Associations between cognitive assessment and homocysteine are shown in Table 3. Analyses were performed first stratified by age group and then combined. Education level and age had a significant effect on cognitive function (P < 0.0001, data not shown in Tables). When adjusted for age, sex, and education, we found that only DSS score was significantly lower in subjects with hyperhomocysteinemia (homocysteine >12 μmol/L) than in those with homocysteine ≤12 μmol/L in the elderly group (DSS score: 7.1 ± 2.7 and 9.0 ± 3.0, respectively, beta = −1.9, 95% confidence interval [CI] = −3.1∼−0.6, P = 0.003) and borderline significance was noted in the combined age group (beta = −1.1, 95% CI = −2.1∼−0.04, P = 0.04). Except for DSS score, we did not find any other association between hyperhomocysteinemia and dementia as well as other neuropsychological assessments. Additional analyses stratified by dementia were performed to clarify whether the observed associations in the elderly group were entirely driven by dementia patients (Table 4). The significance of association between homocysteine levels and DSS in the dementia and nondementia groups were both borderline with similar magnitude and direction (P = 0.07), suggesting that hyperhomocysteinemia is associated with low DSS score in the elderly regardless of dementia diagnosis.
Table 3.
Differences in cognitive evaluation as a function of hyperhomocysteine (>12 μmol/L) in 2 age groups.
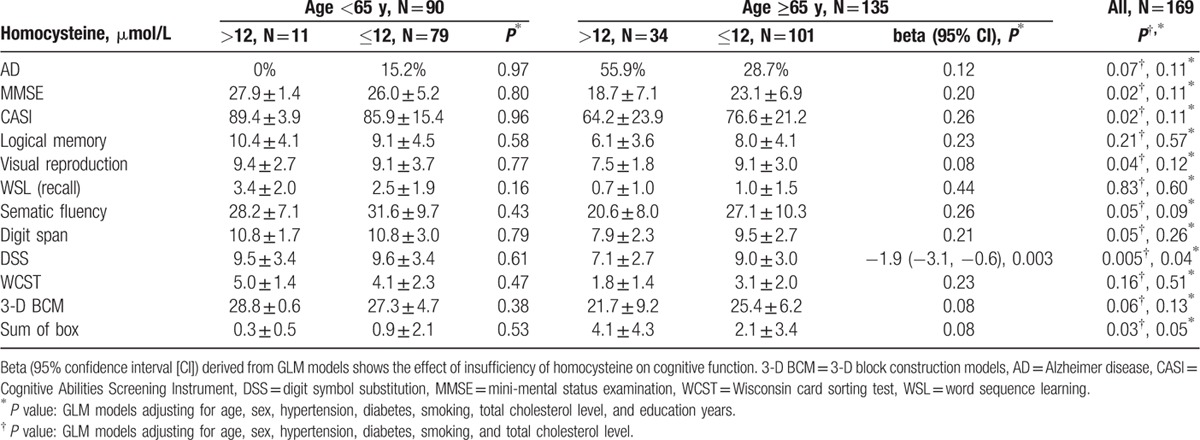
Table 4.
Differences in cognitive evaluation as a function of hyperhomocysteine (>12 μmol/L) in the elderly group (age ≥65 years).
4. Discussion
This is the first study that demonstrated a correlation between hyperhomocystinemia (>12 μmol/L) with significant decline of DSS score in participants aged 65 years or older who had normal concentrations of vitamin B12 and folate. Although within the normal limit, homocysteine levels were significantly higher in the elderly compared to the young group (10.8 ± 2.7 vs. 9.5 ± 2.5 μmol/L, respectively, P = 0.0006), suggesting a potential role of homocysteine in aging and related pathophysiology. This study also indicates that homocysteine level and its effect on DSS score are age-dependent. DSS score may be an independent marker of cognitive impairment in response to hyperhomocysteinemia in the elderly regardless of dementia diagnosis. In addition to age, education level significantly confounded the associations between homocysteine and almost all of the neuropsychological assessments. Because this study enrolled subjects with normal vitamin B12 and folate levels, one could expect that the effect size of homocysteine alone on cognition should be small given the a priori knowledge of metabolic intercorrelation between B12 and homocysteine. Further replication studies with larger cohorts are needed to confirm our results.
The DSS score is a measure of executive function, working memory, processing speed, and visuospatial attention.[18,22,25] The Cardiovascular Health Study[25] demonstrated that in the elderly, DSS score was significantly associated with gait speed, and low DSS score was an independent risk factor for mortality and developing disability in well-functioning older adults (data from 3156 participants with mean age 70.4 years who were free from stroke and physical disability). It has been suggested that lower DSS score reflects early focal changes affecting the brain integrity of specific executive function-related networks. Future neuroimaging and electrophysiological studies are needed to illuminate the relationship between the localization of damage in executive control function networks and hyperhomocysteinemia.
Increased plasma homocysteine concentration is related to atherosclerosis, stroke, and AD.[1,2,26] Hyperhomocysteinemia is related to brain aging through the pathological processes of cerebral macro- and microangiopathy,[26,27] nitric oxide activity, oxidative stress, and endothelial dysfunction.[26] Homocysteine could lead to disturbed methylation and redox potentials, promoting calcium influx, amyloid and tau protein accumulation, and neuronal death.[28] A positive association between homocysteine and Aβ1–40 levels has been shown in aging and neurodegenerative disease.[29] In addition, homocysteine can induce accumulation and deposition of intracellular and extracellular Aβ and increase neuronal vulnerability to Aβ damage.[28,30] Although hyperhomocysteinemia is a recognized risk factor for cognitive decline in older adults (an approximate 20% reduction in risk of dementia from treatment with folic acid and B12 was estimated under assumptions in a meta-analysis),[31] treatments to lower homocysteine concentration failed to demonstrate global benefits in clinical trials, with supplementation of vitamin B12 reducing risk only in subsets of the populations studied.[8–10] There is no consistent evidence showing that folic acid and vitamin B12 have a beneficial effect on cognitive function in healthy or cognitively impaired older people.[32,33] Further clinical trials that utilize different cognitive modalities and stratified heterogeneity of recruited participants are needed for this important issue. Still, vitamin supplementation could be recognized as a beneficial intervention to hyperhomocysteinemia with DSS score improvements.
It is worth mentioning, however, that there are limitations in our study. First, this cross-sectional study is not able to address the disparity between the 2 age groups regarding the associations of hyperhomocysteinemia with low DSS score. Second, the biological relevancies of brain integrity and hyperhomocysteinemia are not clear and remain to be investigated. Third, the significance of the discovered associations might not survive a more rigorous statistic correction of multiple testing. The sample size of subjects younger than 65 years is small. Further cohort studies with adequate sample size are needed to confirm our results before the DSS score can be viewed as an independent marker of cognitive impairment in hyperhomocysteinemic subjects.
In summary, this is the first study that demonstrated a significant association of hyperhomocysteinemia with low DSS score, suggesting that DSS score may be an independent marker of cognitive impairment in response to hyperhomocysteinemia in the elderly. Further neuroimaging and longitudinal studies are needed to clarify the pathophysiology underlying the association between this biochemical pathway and brain integrity to provide additional prevention and treatment targets for dementia.
Acknowledgements
The authors wish to thank the staff at the Department of Neurology, Chang Gung Memorial Hospital Linkou Medical Center and especially the participants in this study for their valuable contributions.
Footnotes
Abbreviations: 3-D BCM = three-dimensional block construction, AD = Alzheimer disease, ADL = activities of daily living, B12 = vitamin B12, BMI = body mass index, CASI = Cognitive Abilities Screening Instrument, CDR = clinical dementia rating, CGMH = Chang Gung Memorial Hospital, CHS = Cardiovascular Health Study, DNA = deoxyribonucleic acid, DSM-IV = Diagnostic and Statistical Manual of Mental Disorders IV, DSS = Digit Symbol Substitution subtests, MMSE = Mini-Mental State Examination, MTF = methyltetrahydrofolate, NINDS-AIREN = National Institute of Neurological Disorders and Stroke–Association Internationale pour la Recherche et l’Enseignement en Neurosciences, RNA = ribonucleic acid, SOB = sum of box, THF = tetrahydrofolate, WCST = Modified Wisconsin Card Sorting Test, WSL = word sequence learning test.
This study was supported by CGMH, Taiwan (CMRPG37136, CMRPG37172, CMRPG3E1501, and CMRPG3C174). The financial supporters had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors report no conflicts of interest.
References
- 1.Seshadri S, Beiser A, Selhub J, et al. Plasma homocysteine as a risk factor for dementia and Alzheimer's disease. N Engl J Med 2002; 346:476–483. [DOI] [PubMed] [Google Scholar]
- 2.Seshadri S. Elevated plasma homocysteine levels: risk factor or risk marker for the development of dementia and Alzheimer's disease? J Alzheimers Dis 2006; 9:393–398. [DOI] [PubMed] [Google Scholar]
- 3.Wang HX, Wahlin A, Basun H, et al. Vitamin B(12) and folate in relation to the development of Alzheimer's disease. Neurology 2001; 56:1188–1194. [DOI] [PubMed] [Google Scholar]
- 4.Luchsinger JA, Tang MX, Shea S, et al. Plasma homocysteine levels and risk of Alzheimer disease. Neurology 2004; 62:1972–1976. [DOI] [PubMed] [Google Scholar]
- 5.Elias MF, Sullivan LM, D’Agostino RB, et al. Homocysteine and cognitive performance in the Framingham offspring study: age is important. Am J Epidemiol 2005; 162:644–653. [DOI] [PubMed] [Google Scholar]
- 6.Kalmijn S, Launer LJ, Lindemans J, et al. Total homocysteine and cognitive decline in a community-based sample of elderly subjects: the Rotterdam Study. Am J Epidemiol 1999; 150:283–289. [DOI] [PubMed] [Google Scholar]
- 7.Luchsinger JA, Mayeux R. Dietary factors and Alzheimer's disease. Lancet Neurol 2004; 3:579–587. [DOI] [PubMed] [Google Scholar]
- 8.Lippi G, Plebani M. Hyperhomocysteinemia in health and disease: where we are now, and where do we go from here? Clin Chem Lab Med 2012; 50:2075–2080. [DOI] [PubMed] [Google Scholar]
- 9.Spence JD, Stampfer MJ. Understanding the complexity of homocysteine lowering with vitamins: the potential role of subgroup analyses. JAMA 2011; 306:2610–2611. [DOI] [PubMed] [Google Scholar]
- 10.Smith AD, Smith SM, de Jager CA, et al. Homocysteine-lowering by B vitamins slows the rate of accelerated brain atrophy in mild cognitive impairment: a randomized controlled trial. PLoS One 2010; 5:e12244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grober U, Kisters K, Schmidt J. Neuroenhancement with vitamin B12-underestimated neurological significance. Nutrients 2013; 5:5031–5045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wetterling T, Kanitz RD, Borgis KJ. Comparison of different diagnostic criteria for vascular dementia (ADDTC, DSM-IV, ICD-10, NINDS-AIREN). Stroke 1996; 27:30–36. [DOI] [PubMed] [Google Scholar]
- 13.Erkinjuntti T. Clinical criteria for vascular dementia: the NINDS-AIREN criteria. Dementia 1994; 5:189–192. [DOI] [PubMed] [Google Scholar]
- 14.Morris JC, Storandt M, Miller JP, et al. Mild cognitive impairment represents early-stage Alzheimer disease. Arch Neurol 2001; 58:397–405. [DOI] [PubMed] [Google Scholar]
- 15.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984; 34:939–944. [DOI] [PubMed] [Google Scholar]
- 16.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189–198. [DOI] [PubMed] [Google Scholar]
- 17.Lin KN, Wang PN, Liu HC, et al. Cognitive Abilities Screening Instrument, Chinese Version 2.0 (CASI C-2.0): administration and clinical application. Acta Neurol Taiwan 2012; 21:180–189. [PubMed] [Google Scholar]
- 18.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993; 43:2412–2414. [DOI] [PubMed] [Google Scholar]
- 19.Hua MS, Chang BS, Lin KN, et al. Wechsler Memory Scale. 3rd edTaipei: Chinese Behavioral Science Corp; 2005. [Google Scholar]
- 20.Hamsher Kd, Roberts RJ. Word Sequence Learning Test. Milwaukee: Department of Neurology, University of Wisconsin Medical School; 1983. [Google Scholar]
- 21.Hua MS, Chang S H, Chen S T. Factor structure and age effects with an aphasia test battery in normal Taiwanese adults. Neuropsychology 1997; 11:156–162. [DOI] [PubMed] [Google Scholar]
- 22.Benton AL, Sivan AB, Hamsher Kd, et al. Contributions to neuropsychological assessment: a clinical manual. 2nd edNew York: Oxford Univ. Press; 1994. [Google Scholar]
- 23.Chen JH, Chen HY. Wechsler Adult Intelligence Scale [Manual]. 3rd ed.[In Chinese] Taipei: Chinese Behavioral Science Corp; 2002. [Google Scholar]
- 24.Nelson HE. A modified card sorting test sensitive to frontal lobe defects. Cortex; a journal devoted to the study of the nervous system and behavior 1976; 12:313–324. [DOI] [PubMed] [Google Scholar]
- 25.Rosano C, Newman AB, Katz R, et al. Association between lower digit symbol substitution test score and slower gait and greater risk of mortality and of developing incident disability in well-functioning older adults. J Am Geriatr Soc 2008; 56:1618–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kamat PK, Vacek JC, Kalani A, et al. Homocysteine induced cerebrovascular dysfunction: a link to Alzheimer's disease etiology. Open Neurol J 2015; 9:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fassbender K, Mielke O, Bertsch T, et al. Homocysteine in cerebral macroangiography and microangiopathy. Lancet 1999; 353:1586–1587. [DOI] [PubMed] [Google Scholar]
- 28.Obeid R, Herrmann W. Mechanisms of homocysteine neurotoxicity in neurodegenerative diseases with special reference to dementia. FEBS Lett 2006; 580:2994–3005. [DOI] [PubMed] [Google Scholar]
- 29.Irizarry MC, Gurol ME, Raju S, et al. Association of homocysteine with plasma amyloid beta protein in aging and neurodegenerative disease. Neurology 2005; 65:1402–1408. [DOI] [PubMed] [Google Scholar]
- 30.Hasegawa T, Ukai W, Jo DG, et al. Homocysteic acid induces intraneuronal accumulation of neurotoxic Abeta42: implications for the pathogenesis of Alzheimer's disease. J Neurosci Res 2005; 80:869–876. [DOI] [PubMed] [Google Scholar]
- 31.Wald DS, Kasturiratne A, Simmonds M. Serum homocysteine and dementia: meta-analysis of eight cohort studies including 8669 participants. Alzheimers Dement 2011; 7:412–417. [DOI] [PubMed] [Google Scholar]
- 32.Malouf R, Grimley Evans J. Folic acid with or without vitamin B12 for the prevention and treatment of healthy elderly and demented people. Cochrane Database Syst Rev 2008; 4:CD004514. [DOI] [PubMed] [Google Scholar]
- 33.McCaddon A, Miller JW. Assessing the association between homocysteine and cognition: reflections on Bradford Hill, meta-analyses, and causality. Nutr Rev 2015; 73:723–735. [DOI] [PubMed] [Google Scholar]


