Abstract
It is well known in clinical and animal studies that women and men have different disease risk as well as different disease physiology. Women of reproductive age are protected from metabolic and cardiovascular disease compared with postmenopausal women and men. Most murine studies are skewed toward the use of male mice to study obesity-induced metabolic dysfunction because of similar protection in female mice. We have investigated dietary obesity in a mouse model and have directly compared inflammatory responses in males and females. In this review we will summarize what is known about sex differences in diet-induced inflammation and will summarize our data on this topic. It is clear that sex differences in high-fat diet-induced inflammatory activation are due to cell intrinsic differences in hematopoietic responses to obesogenic cues, but further research is needed to understand what leads to sexually dimorphic responses.
Keywords: diabetes, high-fat diet, myelopoisis, obesity, sexual dimorphism
global obesity rates have risen drastically in the past several decades with around one in every three individuals currently categorized as obese (34). The overall incidence of obesity-related diseases such as diabetes and cardiovascular disease (CVD) also continues to rise as a result (3a). Obesity manifests as the result of an imbalance of caloric intake and energy expenditure. A major contributing factor to the increase in obesity rates is an increase in the consumption of calorie-dense foods rich in saturated fatty acids (4). With increased consumption of high-fat foods, individuals accrue body fat and thus have an elevated risk of developing obesity-related diseases. In this brief review we will emphasize the effects of diet-induced obesity, focusing primarily on sexually dimorphic responses of high-fat diet (HFD) priming of the immune system.
An individual's response to HFD is dependent on several factors including sex, age, and ethnicity. What has become increasingly striking is that there is a clear sexual dimorphism in obesity and diabetes rates. While obesity rates are higher in women (34), men have higher rates of cardiovascular disease (CVD) and Type 2 diabetes (30, 36), suggesting that females are protected from the adverse effects of obesity (30). This is of particular importance because when investigating diabetes and CVD, many preclinical studies have been performed in males alone, leaving gaps in our knowledge of sexually dimorphic responses to obesity (45). Therefore, guidelines and therapies are being created based on investigations in males but are being implemented in men and women (13).
It is important to investigate males and females to understand the contributing factors to these sex-specific differences. Previous studies have focused on altered hormone environments, anatomical fat distribution (17, 19), and energy expenditure differences. Women have been found to have 10% higher total body fat content compared with males of the same body mass index (BMI) (15). This dimorphism is especially profound in poor socioeconomic conditions, whereas richer environments show a smaller variance in adiposity between the sexes (11). This suggests that estrogen largely influences fat accumulation regardless of socioeconomic status (11). Additionally, when adiposity matched, females display a greater volume of subcutaneous fat than males, whereas males have a greater volume of intra-abdominal or visceral fat (5, 9). Women are also known to have higher energy expenditure rates (47, 49). Limited data exist explaining this, but they indicate increases in brown adipose tissue (BAT) in females (17), and recent studies demonstrate the role of estrogen in sex differences in muscle metabolism (40). It has also been found that estradiol may influence the brain to decrease food intake and stimulate voluntary exercise, independent of its metabolic effects (51).
The prevalence of metabolic syndrome increases with age as women face higher BMIs (1), demonstrating a loss of inherent protection. One factor that contributes to this is menopause. Clinically, during menopause there is a shift in the circulating estradiol levels and an increase in the ratio of androgens that places women at increased risk for CVD and Type 2 diabetes (36, 42). Specifically, low levels of sex hormone binding globulin (SHBG), high free androgen levels, and low estradiol levels have been implicated in the CVD risk in perimenopausal women (48). In mouse models, using ovarian failure to mimic the onset of menopause corresponds with an increase in insulin resistance (42). In this mouse model, the onset of insulin resistance was prevented following ovarian failure using estrogen replacement (17-β estradiol) therapy, thus further proving the importance of sex hormones on metabolic disease manifestation (42).
While sex hormones are a logical explanation for male and female differential responses to obesity, the clinical studies that have used estrogen replacement therapies have not been successful in preventing cardiovascular diseases (26). Thus further exploration in novel areas of investigation is required to close the gaps in our understanding of what links obesity to diseases in males and females.
Obesity is Associated With Chronic Inflammation
Inflammation may be either acute or chronic in nature, with the former having a protective role in the adipose tissue. In obesity-induced acute inflammation, leukocytes expand within the adipose tissue (55) to correct the physiological imbalance initiated by damaged adipocytes. If the stimulus is not resolved, chronic inflammation ensues, which has a more significant adverse effect on local and system tissue dysfunction (22). Though the mechanisms underlying the link between obesity and metabolic disorders are not completely understood, it is evident that they are associated with chronic low-grade inflammation (25). Obesity-induced inflammation, also known as meta-inflammation, has been implicated as a primary cause of metabolic disorders via activation of leukocytes, particularly of the myeloid lineage, in both human and murine models (20, 33). In obese individuals, several key inflammatory factors including pro-inflammatory cytokines such as IL-6, IL-1β, tumor necrosis factor-α (TNF-α), and chemokines such as MCP-1/CCR2 are elevated and associated with insulin resistance (32, 44). A major source of these systemic cytokines is the adipose tissue, specifically the visceral white adipose tissue (2). Within this depot, the adipose tissue macrophage (ATM) population is expanded under the influence of obesogenic diets, which leads to the synthesis and secretion of these pro-inflammatory cytokines (23, 24). Dampening inflammation in obese subjects has been shown to improve insulin resistance (52, 56) proving the importance of ATMs.
ATMs Recruited During Obesity Promote Insulin Resistance
Macrophages have been increasingly implicated in contributing to metabolic diseases. As key mediators of the immune system, macrophages play many roles in both innate and adaptive immunity: they recognize and carry out phagocytosis of microbes, parasites, and foreign substances; release cytokines; secrete chemoattractants to recruit other immune cells; and present foreign antigens to lymphocytes (10). Macrophages are found within nearly all tissues and have distinct populations and properties determined by their environment (53). During the normal homeostatic state there are resident tissue macrophages, representing between 10 to 15% of all cells in the lean visceral adipose tissue (53). During obesity, however, there is an increase in proinflammatory tissue macrophages, which can lead them to represent between 45 to 60% of all cells in the visceral adipose tissue (53).
There are two distinguishable types of ATMs that have been identified: recruited ATMs, which are found predominantly in obese individuals and display a classic M1 activation pattern, and resident ATMs, found predominantly in lean individuals, which have an alternative activation similar to M2 macrophages (23). Recruited ATMs are regulated through MGL1, a cell surface receptor that controls monocyte/macrophage activation and trafficking of Ly6chi monocytes to adipose tissue (54). These ATMs overexpress genes important in macrophage migration and phagocytosis and are distinguishable by the CD11c cell-surface marker not found in resident ATMs (23). The activated ATMs collect in areas known as crown-like structures (CLS) that form around dying adipocytes. Activated macrophages within these clusters release several inflammatory cytokines that have adverse effects on adipocyte function, such as decreased insulin-mediated glucose uptake and decreased adipogenesis (23).
In a high-fat environment, recruited macrophages expand within adipose tissue and accumulate in additional sites including the brain (21), liver (16), muscle, and pancreas (31), leading to systemic inflammation and disease. We have recently found evidence that fundamental changes occur within hematopoietic stem and progenitor cells (HSPCs) after HFD feeding, leading to an increase in production of macrophages. These changes in HSPCs lead to an increased production of granulocyte and macrophage progenitors and generate activated monocytes that are then recruited to become activated tissue macrophages (46).
Through this finding of hematopoietic stem cell (HSC) expansion and myeloid progenitor increases, we were able to conclude that dietary priming of hematopoietic progenitor cells leads to adipose tissue inflammation and that leukocyte production is enhanced through obesogenic signals (46). Other groups have also found that in murine models, obesity is a driver of proliferation and expansion of the bone marrow myeloid progenitors, with increasing monocytosis and neutrophilia in obese rodents compared with their lean counterparts (33). Overall, the drivers of this HSC activation in obese individuals remain unresolved.
Sexually Dimorphic Responses in High-Fat Diet
As previously stated, many of the above investigations that have been done to characterize inflammatory changes during diet-induced obesity have been performed in males. Female mice have been shown to be protected from insulin resistance and overall have dampened responses to HFDs in the laboratory. Several mechanisms have been investigated in an attempt to understand what protects females from the same metabolic impairments seen in males. The research in this area to date shows that male and female mice exhibit profound differences in anatomical adipose tissue distribution and expansion. Studies have shown that even when controlling for diet and other environmental conditions, male mice show significantly greater expansion of total body mass including subcutaneous adipose tissue (SAT), visceral adipose tissue, and liver than their female counterparts (12, 14).
To understand mechanistic differences between males and females, investigations have focused on a variety of hormone models. Among these, it has been found that estrogen receptor α (ERα) is critical for protection against tissue inflammation observed in female mice (41).
Consistent with this, ovariectomized mice have a similar increased inflammation and insulin resistance (50). More recently, androgen receptor knockout models have shown similar benefits, with female knockout mice having a greater propensity toward developing metabolic disease (8). These studies have demonstrated that sex hormones are critical for the metabolic disease protection seen in females in response to obesity. While inflammation is so tightly linked to metabolic disease, there have been very few studies looking at changes in inflammation in female obese animals. Of the studies that have assessed metainflammation, an increase in inflammatory cytokines (7) and a decrease in T regulatory cells in obese male mice have been identified (37). These studies have experienced difficulty in creating a perfect model, because when exposed to HFD for the same time interval, female mice tend to gain weight more slowly than males. Thus females typically weigh less at the time of data collection. Interestingly, even when females have an equal body weight, they have more adiposity but dampened cytokine gene expression and glucose impairment compared with males (29, 35). Although there is likely a direct link of estrogen on macrophage development (3), the effects of this in the context of diet-induced obesity have not been directly investigated.
Sexually Dimorphic Macrophage Responses to High-Fat Diet
The mechanism behind this sexually dimorphic variance remains mysterious, especially in terms of regulation of myeloid inflammation. To understand the different inflammatory responses to HFD, we looked at ATMs and HSC/bone marrow (BM) populations in male and female animals on HFD (47). Using a 60% lard-based HFD chow, both males and females were able to gain weight and adiposity, though males gained more body weight compared with females. While females gained weight, adiposity, and had adipocyte hypertrophy on HFD, they had normal glucose tolerance and lower insulin levels compared with male mice. When we next looked at adipose tissue inflammatory changes in males after 16 wk of HFD, there was a clear expansion of macrophages, specifically of the CD11c+ ATMs that formed CLS. Females also exhibited an expansion of macrophages but primarily of the CD11c− type. To understand the inflammatory environment created in the adipose tissue, we looked at the adipose tissue gene expression of inflammatory cytokines and saw that expression was reduced in females on HFD compared with males on HFD (47).
With our prior findings that obese males have expansion of HSCs and myeloid progenitors, we evaluated monocytes and hematopoietic progenitors in this model. We found that while females had normal myeloid progenitors at baseline, these cells did not expand with HFD as we saw in males. Examining the bone marrow ex vivo, we found that female BM produced less granulocyte and macrophage colonies than males after palmitic acid (saturated fatty acid) stimulation and produced lower cytokine responses to lipopolysaccharides (LPS) (47).
Given the intrinsic BM changes along with the concern for differential weight gain and energy expenditure in males versus females, we next performed a competitive BM transplant (BMT) where male and female bone marrow could be evaluated in either a male or female recipient animal. After BMT we challenged recipient animals to HFD and found that no matter the recipient sex, male BM cells responded to diet-induced obesity by producing more ATMs, specifically more CD11c+ ATMs. This suggests that there is a cell-intrinsic difference in hematopoietic responses to obesity between the sexes. It may also indicate that there is a permanent, lifelong change within the HSC progenitor population after exposure to a HFD (47).
DISCUSSION
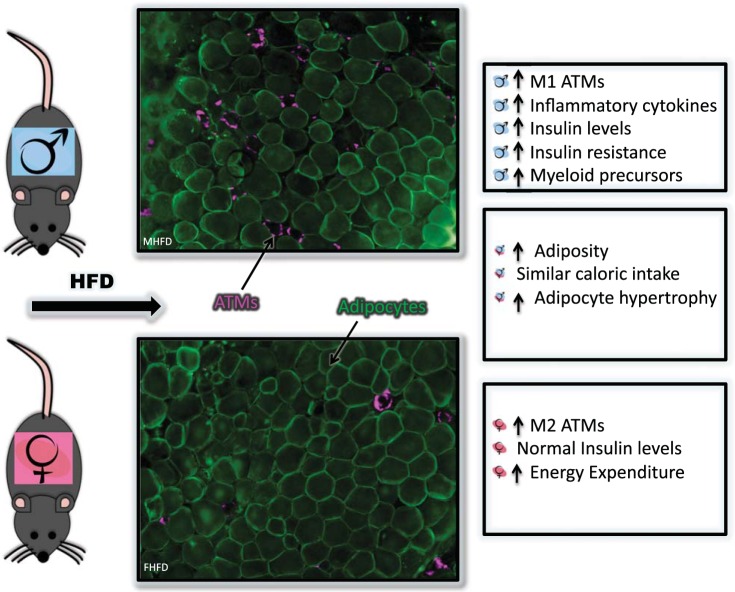
Over the last few decades there has been a greater understanding of the impact of diet-induced obesity on inflammation and insulin resistance, but there continue to be significant gaps in our knowledge on sexually dimorphic inflammatory responses. Through the use of male and female competitive BMTs we were able to conclude that there is an intrinsic sexual dimorphism in the bone marrow HSC and progenitor populations in response to diet-induced obesity, which is independent of differential weight gain and energy expenditure, though the driver of this dimorphism is still unclear (Fig. 1).
Fig. 1.
Sexually dimorphic inflammatory responses to diet-induced obesity. Immunofluorescence of gonadal white adipose tissue (staining for caveolin, green; Mac2, magenta) and summary of male responses (top), female responses (bottom) and sexually similar responses (middle) to diet-induced obesity. HFD, high-fat diet; ATM, adipose tissue macrophage.
Research in this field has identified that sex hormones, adipocyte properties, and genetics are key drivers of male and female differences in obesity, diabetes, and cardiovascular disease. Estrogen plays a systemic role, improving energy balance through neuronal signals, pancreatic β cell survival, improved lipid metabolism, and insulin sensitivity within the liver and muscle (28). It has been shown that the effects of ERα occur via its activation in adipocytes especially in males. In the context of cardiovascular disease, myeloid-specific ERα deletion actually has been shown to induce insulin resistance and atherosclerosis (39). As was mentioned previously, ovariectomized mice also show an increase in adipose tissue inflammation and insulin resistance (50) likely via alterations in MCP1 and increases in reactive oxygen species (ROS) after ovariectomy (18).
Given that hormone replacement therapy has proven ineffective at decreasing the risk of metabolic and cardiovascular disease in obese patients, hormone independent changes have become an important area of work. In our studies there was an observable, hormone-independent, dimorphism in male and female myeloid cells based on ex vivo culture studies and competitive BMT results, finding that male myeloid cells are more activated and proinflammatory. This phenomenon of sexually dimorphic inflammatory responses has been under investigated, but studies have demonstrated lower systemic LPS responses in females (27). Contrary to this, Calippe et al. (3) showed that estradiol promotes proinflammatory cytokines via ERα activation on macrophages after ovariectomy. It is still unclear what role estrogen has directly on macrophage production and macrophage polarization, especially in specific clinical scenarios such as diet-induced obesity. In addition, whereas it was not in the scope of this current review, there is some data suggesting that testosterone supplementation may increase muscle mass and decrease fat mass in males with low levels of testosterone (57). Testosterone administration in female to male transsexuals alters adipose tissue distribution, with long-term administration instigating increased visceral fat and decreased subcutaneous fat deposits (6). Additionally, ATMs have been found to express the androgen receptor, potentially contributing to the metabolic effects of androgens in males (43).
Perspectives and Significance
It has been clear for centuries that there are sex differences in body composition and responses to HFD, but it has only recently become clear that there are inflammatory differences between the sexes regarding diet-induced inflammation. The findings in our studies and others emphasize that it is not simply hormones that directly affect insulin resistance, but rather, a combination of body composition, energy expenditure, appetite, hormone effects on insulin production, and inflammatory responses to diet that create the sexually dimorphic rates of obesity-related diseases. It is necessary to continue further investigating these differences to truly have a greater understanding of what leads to the clinical paradigm of sex differences in response to obesity.
GRANTS
This review is supported on K08DK101755 NIDDK/NIH and Taubman Emerging Scholars Program.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
C.G. and K.S. interpreted results of experiments; C.G. and K.S. prepared figures; C.G., N.L., L.E., and K.S. drafted manuscript; C.G., N.L., L.E., and K.S. edited and revised manuscript; C.G. and K.S. approved final version of manuscript; K.S. conception and design of research; K.S. performed experiments; K.S. analyzed data.
REFERENCES
- 1.Arnetz L, Ekberg NR, Alvarsson M. Sex differences in type 2 diabetes: focus on disease course and outcomes. Diabetes Metab Syndr Obes 7: 409–420, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res 96: 939–949, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Calippe B, Douin-Echinard V, Delpy L, Laffargue M, Lélu K, Krust A, Pipy B, Bayard F, Arnal JF, Guéry JC, Gourdy P. 17Beta-estradiol promotes TLR4-triggered proinflammatory mediator production through direct estrogen receptor alpha signaling in macrophages in vivo. J Immunol 185: 1169–1176, 2010. [DOI] [PubMed] [Google Scholar]
- 3a.Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2014. Atlanta, GA: US Department of Health and Human Services, 2014. [Google Scholar]
- 4.Cordain L, Eaton SB, Sebastian A, Mann N, Lindeberg S, Watkins BA, O'Keefe JH, Brand-Miller J. Origins and evolution of the Western diet: health implications for the 21st century. Am J Clin Nutr 81: 341–354, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Demerath EW, Sun SS, Rogers N, Lee M, Reed D, Choh AC, Couch W, Czerwinski SA, Chumlea WC, Siervogel RM, Towne B. Anatomical patterning of visceral adipose tissue: race, sex, and age variation. Obesity (Silver Spring) 15: 2984–2993, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elbers JM, Asscheman H, Seidell JC, Megens JA, Gooren LJ. Long-term testosterone administration increases visceral fat in female to male transsexuals. J Clin Endocrinol Metab 82: 2044–2047, 1997. [DOI] [PubMed] [Google Scholar]
- 7.Estrany ME, Proenza AM, Gianotti M, Lladó I. High-fat diet feeding induces sex-dependent changes in inflammatory and insulin sensitivity profiles of rat adipose tissue. Cell Biochem Funct 31: 504–510, 2013. [DOI] [PubMed] [Google Scholar]
- 8.Fagman JB, Wilhelmson AS, Motta BM, Pirazzi C, Alexanderson C, De Gendt K, Verhoeven G, Holmäng A, Anesten F, Jansson JO, Levin M, Borén J, Ohlsson C, Krettek A, Romeo S, Tivesten Å. The androgen receptor confers protection against diet-induced atherosclerosis, obesity, and dyslipidemia in female mice. FASEB J 29: 1540–1550, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fried SK, Lee MJ, Karastergiou K. Shaping fat distribution: New insights into the molecular determinants of depot- and sex-dependent adipose biology. Obesity (Silver Spring) 23: 1345–1352, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gordon S. The role of the macrophage in immune regulation. Res Immunol 149: 685–688, 1998. [DOI] [PubMed] [Google Scholar]
- 11.Grantham JP, Henneberg M. The estrogen hypothesis of obesity. PLoS One 9: e99776, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grove KL, Fried SK, Greenberg AS, Xiao XQ, Clegg DJ. A microarray analysis of sexual dimorphism of adipose tissues in high-fat-diet-induced obese mice. Int J Obes 34: 989–1000, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holdcroft A. Gender bias in research: how does it affect evidence based medicine? J R Soc Med 100: 2–3, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hong J, Stubbins RE, Smith RR, Harvey AE, Núñez NP. Differential susceptibility to obesity between male, female and ovariectomized female mice. Nutr J 8: 11, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jackson AS, Stanforth PR, Gagnon J, Rankinen T, Leon AS, Rao DC, Skinner JS, Bouchard C, Wilmore JH. The effect of sex, age and race on estimating percentage body fat from body mass index: The Heritage Family Study. Int J Obes Relat Metab Disord 26: 789–796, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, Kasuga M. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest 116: 1494–1505, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karastergiou K, Smith SR, Greenberg AS, Fried SK. Sex differences in human adipose tissues-the biology of pear shape. Biol Sex Differ 3: 13, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim WK, Choi EK, Sul OJ, Park YK, Kim ES, Yu R, Suh JH, Choi HS. Monocyte chemoattractant protein-1 deficiency attenuates oxidative stress and protects against ovariectomy-induced chronic inflammation in mice. PLoS One 8: e72108, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krotkiewski M, Björntorp P, Sjöström L, Smith U. Impact of obesity on metabolism in men and women. Importance of regional adipose tissue distribution. J Clin Invest 72: 1150–1162, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kullo IJ, Hensrud DD, Allison TG. Comparison of numbers of circulating blood monocytes in men grouped by body mass index (<25, 25 to <30, > or = 30). Am J Cardiol 89: 1441–1443, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Lawrence CB, Brough D, Knight EM. Obese mice exhibit an altered behavioural and inflammatory response to lipopolysaccharide. Dis Model Mech 5: 649–659, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee YS, Li P, Huh JY, Hwang IJ, Lu M, Kim JI, Ham M, Talukdar S, Chen A, Lu WJ, Bandyopadhyay GK, Schwendener R, Olefsky J, Kim JB. Inflammation is necessary for long-term but not short-term high-fat diet-induced insulin resistance. Diabetes 60: 2474–2483, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 117: 175–184, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lumeng CN, DelProposto JB, Westcott DJ, Saltiel AR. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes 57: 3239–3246, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest 121: 2111–2117, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manson JE, Chlebowski RT, Stefanick ML, Aragaki AK, Rossouw JE, Prentice RL, Anderson G, Howard BV, Thomson CA, LaCroix AZ, Wactawski-Wende J, Jackson RD, Limacher M, Margolis KL, Wassertheil-Smoller S, Beresford SA, Cauley JA, Eaton CB, Gass M, Hsia J, Johnson KC, Kooperberg C, Kuller LH, Lewis CE, Liu S, Martin LW, Ockene JK, O'Sullivan MJ, Powell LH, Simon MS, Van Horn L, Vitolins MZ, Wallace RB. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women's Health Initiative randomized trials. JAMA 310: 1353–1368, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marriott I, Bost KL, Huet-Hudson YM. Sexual dimorphism in expression of receptors for bacterial lipopolysaccharides in murine macrophages: a possible mechanism for gender-based differences in endotoxic shock susceptibility. J Reprod Immunol 71: 12–27, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Mauvais-Jarvis F, Clegg DJ, Hevener AL. The role of estrogens in control of energy balance and glucose homeostasis. Endocr Rev 34: 309–338, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Medrikova D, Jilkova ZM, Bardova K, Janovska P, Rossmeisl M, Kopecky J. Sex differences during the course of diet-induced obesity in mice: adipose tissue expandability and glycemic control. Int J Obes 36: 262–272, 2012. [DOI] [PubMed] [Google Scholar]
- 30.Meyer MR, Haas E, Barton M. Gender differences of cardiovascular disease: new perspectives for estrogen receptor signaling. Hypertension 47: 1019–1026, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Morris DL. Minireview: emerging concepts in islet macrophage biology in Type 2 diabetes. Mol Endocrinol 29: 946–962, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morris DL, Singer K, Lumeng CN. Adipose tissue macrophages: phenotypic plasticity and diversity in lean and obese states. Curr Opin Clin Nutr Metab Care 14: 341–346, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagareddy PR, Kraakman M, Masters SL, Stirzaker RA, Gorman DJ, Grant RW, Dragoljevic D, Hong ES, Abdel-Latif A, Smyth SS, Choi SH, Korner J, Bornfeldt KE, Fisher EA, Dixit VD, Tall AR, Goldberg IJ, Murphy AJ. Adipose tissue macrophages promote myelopoiesis and monocytosis in obesity. Cell Metab 19: 821–835, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, Mullany EC, Biryukov S, Abbafati C, Abera SF, Abraham JP, Abu-Rmeileh NM, Achoki T, AlBuhairan FS, Alemu ZA, Alfonso R, Ali MK, Ali R, Guzman NA, Ammar W, Anwari P, Banerjee A, Barquera S, Basu S, Bennett DA, Bhutta Z, Blore J, Cabral N, Nonato IC, Chang JC, Chowdhury R, Courville KJ, Criqui MH, Cundiff DK, Dabhadkar KC, Dandona L, Davis A, Dayama A, Dharmaratne SD, Ding EL, Durrani AM, Esteghamati A, Farzadfar F, Fay DF, Feigin VL, Flaxman A, Forouzanfar MH, Goto A, Green MA, Gupta R, Hafezi-Nejad N, Hankey GJ, Harewood HC, Havmoeller R, Hay S, Hernandez L, Husseini A, Idrisov BT, Ikeda N, Islami F, Jahangir E, Jassal SK, Jee SH, Jeffreys M, Jonas JB, Kabagambe EK, Khalifa SE, Kengne AP, Khader YS, Khang YH, Kim D, Kimokoti RW, Kinge JM, Kokubo Y, Kosen S, Kwan G, Lai T, Leinsalu M, Li Y, Liang X, Liu S, Logroscino G, Lotufo PA, Lu Y, Ma J, Mainoo NK, Mensah GA, Merriman TR, Mokdad AH, Moschandreas J, Naghavi M, Naheed A, Nand D, Narayan KM, Nelson EL, Neuhouser ML, Nisar MI, Ohkubo T, Oti SO, Pedroza A, Prabhakaran D, Roy N, Sampson U, Seo H, Sepanlou SG, Shibuya K, Shiri R, Shiue I, Singh GM, Singh JA, Skirbekk V, Stapelberg NJ, Sturua L, Sykes BL, Tobias M, Tran BX, Trasande L, Toyoshima H, van de Vijver S, Vasankari TJ, Veerman JL, Velasquez-Melendez G, Vlassov VV, Vollset SE, Vos T, Wang C, Wang X, Weiderpass E, Werdecker A, Wright JL, Yang YC, Yatsuya H, Yoon J, Yoon SJ, Zhao Y, Zhou M, Zhu S, Lopez AD, Murray CJ, Gakidou E. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 384: 766–781, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nickelson KJ, Stromsdorfer KL, Pickering RT, Liu TW, Ortinau LC, Keating AF, Perfield JW 2nd. A comparison of inflammatory and oxidative stress markers in adipose tissue from weight-matched obese male and female mice. Exp Diabetes Res 2012: 859395, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Onat A, Karadeniz Y, Tusun E, Yüksel H, Kaya A. Advances in understanding gender difference in cardiometabolic disease risk. Expert Rev Cardiovasc Ther 14: 513–523, 2016. [DOI] [PubMed] [Google Scholar]
- 37.Pettersson US, Waldén TB, Carlsson PO, Jansson L, Phillipson M. Female mice are protected against high-fat diet induced metabolic syndrome and increase the regulatory T cell population in adipose tissue. PLoS One 7: e46057, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ribas V, Drew BG, Le JA, Soleymani T, Daraei P, Sitz D, Mohammad L, Henstridge DC, Febbraio MA, Hewitt SC, Korach KS, Bensinger SJ, Hevener AL. Myeloid-specific estrogen receptor alpha deficiency impairs metabolic homeostasis and accelerates atherosclerotic lesion development. Proc Natl Acad Sci USA 108: 16457–16462, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ribas V, Drew BG, Zhou Z, Phun J, Kalajian NY, Soleymani T, Daraei P, Widjaja K, Wanagat J, de Aguiar Vallim TQ, Fluitt AH, Bensinger S, Le T, Radu C, Whitelegge JP, Beaven SW, Tontonoz P, Lusis AJ, Parks BW, Vergnes L, Reue K, Singh H, Bopassa JC, Toro L, Stefani E, Watt MJ, Schenk S, Akerstrom T, Kelly M, Pedersen BK, Hewitt SC, Korach KS, Hevener AL. Skeletal muscle action of estrogen receptor α is critical for the maintenance of mitochondrial function and metabolic homeostasis in females. Sci Transl Med 8: 334ra54, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ribas V, Nguyen MT, Henstridge DC, Nguyen AK, Beaven SW, Watt MJ, Hevener AL. Impaired oxidative metabolism and inflammation are associated with insulin resistance in ERα-deficient mice. Am J Physiol Endocrinol Metab 298: E304–E319, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Romero-Aleshire MJ, Diamond-Stanic MK, Hasty AH, Hoyer PB, Brooks HL. Loss of ovarian function in the VCD mouse-model of menopause leads to insulin resistance and a rapid progression into the metabolic syndrome. Am J Physiol Regul Integr Comp Physiol 297: R587–R592, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rubinow KB, Wang S, den Hartigh LJ, Subramanian S, Morton GJ, Buaas FW, Lamont D, Gray N, Braun RE, Page ST. Hematopoietic androgen receptor deficiency promotes visceral fat deposition in male mice without impairing glucose homeostasis. Andrology 3: 787–796, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sartipy P, Loskutoff DJ. Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc Natl Acad Sci USA 100: 7265–7270, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schiebinger L. Women's health and clinical trials. J Clin Invest 112: 973–977, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Singer K, DelProposto J, Morris DL, Zamarron B, Mergian T, Maley N, Cho KW, Geletka L, Subbaiah P, Muir L, Martinez-Santibanez G, Lumeng CN. Diet-induced obesity promotes myelopoiesis in hematopoietic stem cells. Mol Metab 3: 664–675, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Singer K, Maley N, Mergian T, DelProposto J, Cho KW, Zamarron BF, Martinez-Santibanez G, Geletka L, Muir L, Wachowiak P, Demirjian C, Lumeng CN. Differences in hematopoietic stem cells contribute to sexually dimorphic inflammatory responses to high fat diet-induced obesity. J Biol Chem 290: 13250–13262, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sutton-Tyrrell K, Wildman RP, Matthews KA, Chae C, Lasley BL, Brockwell S, Pasternak RC, Lloyd-Jones D, Sowers MF, Torréns JI, Investigators S, Investigators SWAN . Sex-hormone-binding globulin and the free androgen index are related to cardiovascular risk factors in multiethnic premenopausal and perimenopausal women enrolled in the Study of Women Across the Nation (SWAN). Circulation 111: 1242–1249, 2005. [DOI] [PubMed] [Google Scholar]
- 49.Tooze JA, Schoeller DA, Subar AF, Kipnis V, Schatzkin A, Troiano RP. Total daily energy expenditure among middle-aged men and women: the OPEN Study. Am J Clin Nutr 86: 382–387, 2007. [DOI] [PubMed] [Google Scholar]
- 50.Vieira Potter VJ, Strissel KJ, Xie C, Chang E, Bennett G, Defuria J, Obin MS, Greenberg AS. Adipose tissue inflammation and reduced insulin sensitivity in ovariectomized mice occurs in the absence of increased adiposity. Endocrinology 153: 4266–4277, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wade GN, Gray JM, Bartness TJ. Gonadal influences on adiposity. Int J Obes 9, Suppl 1: 83–92, 1985. [PubMed] [Google Scholar]
- 52.Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, Charo I, Leibel RL, Ferrante AW Jr. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest 116: 115–124, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112: 1796–1808, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Westcott DJ, Delproposto JB, Geletka LM, Wang T, Singer K, Saltiel AR, Lumeng CN. MGL1 promotes adipose tissue inflammation and insulin resistance by regulating 7/4hi monocytes in obesity. J Exp Med 206: 3143–3156, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wiedemann MS, Wueest S, Item F, Schoenle EJ, Konrad D. Adipose tissue inflammation contributes to short-term high-fat diet-induced hepatic insulin resistance. Am J Physiol Endocrinol Metab 305: E388–E395, 2013. [DOI] [PubMed] [Google Scholar]
- 56.Winer S, Chan Y, Paltser G, Truong D, Tsui H, Bahrami J, Dorfman R, Wang Y, Zielenski J, Mastronardi F, Maezawa Y, Drucker DJ, Engleman E, Winer D, Dosch HM. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med 15: 921–929, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wittert GA, Chapman IM, Haren MT, Mackintosh S, Coates P, Morley JE. Oral testosterone supplementation increases muscle and decreases fat mass in healthy elderly males with low-normal gonadal status. J Gerontol A Biol Sci Med Sci 58: 618–625, 2003. [DOI] [PubMed] [Google Scholar]