Abstract
We isolated the kexB gene, which encodes a subtilisin-like processing enzyme, from a filamentous fungus, Aspergillus oryzae. To examine the physiological role of kexB in A. oryzae, we constructed a kexB disruptant (ΔkexB), which formed shrunken colonies with poor generation of conidia on Czapek-Dox (CD) agar plates and hyperbranched mycelia in CD liquid medium. The phenotypes of the ΔkexB strain were restored under high osmolarity in both solid and liquid culture conditions. We found that transcription of the mpkA gene, which encodes a putative mitogen-activated protein kinase involved in cell integrity signaling, was significantly higher in ΔkexB cells than in wild-type cells. The ΔkexB cells also contained higher levels of transcripts for cell wall-related genes encoding β-1,3-glucanosyltransferase and chitin synthases, which is presumably attributable to cell integrity signaling through the increased gene expression of mpkA. As expected, constitutively increased levels of phosphorylated MpkA were observed in ΔkexB cells on the CD plate culture. High osmotic stress greatly downregulated the increased levels of both transcripts of mpkA and the phosphorylated form of MpkA in ΔkexB cells, concomitantly suppressing the morphological defects. These results suggest that the upregulation of transcription levels of mpkA and cell wall biogenesis genes in the ΔkexB strain is autoregulated by phosphorylated MpkA as the active form through cell integrity signaling. We think that KexB is required for precise proteolytic processing of sensor proteins in the cell integrity pathway or of cell wall-related enzymes under transcriptional control by the pathway and that the KexB defect thus induces disordered cell integrity signaling.
Some secretory proteins of eukaryotic cells are converted from the precursor proteins to the mature proteins after modification through processes such as various glycosylation reactions and limited proteolysis within the Golgi apparatus. The modification process is well conserved from yeast to mammals, and the target proteins of the process are expanded to peptide hormones, neuropeptides, serum proteins, cell growth factors, and cell growth factor receptors. Therefore, elucidation of the protein modification process serves a clue to understand the physiological meaning of the posttranslational process. Kexin is a Ca2+-dependent transmembrane serine protease that cleaves the secretory proproteins on the carboxyl side of Lys-Arg and Arg-Arg in a late Golgi compartment (15, 46). Kexin-like enzymes have been found from yeast to mammals (16, 19, 54, 65). Because filamentous fungi, including Aspergillus species, secrete large amounts of proteins that are predicted to be modified through proteolysis in a Golgi compartment, kexins also are thought to be key enzymes of the proteolytic process in the Golgi compartment of the fungi. Aspergillus kexins are found in Aspergillus nidulans (39) and A. niger (29).
Disruption of the A. niger kexB gene causes various morphological alterations such as shorter and multibranched hyphae (29, 60). Recent studies have shown that Kex2p in the dimorphic yeast Candida glabrata is required for cell surface integrity (3). A bioinformatics approach, using the C. albicans genome database, assigned 147 open reading frames (ORFs) as encoding potential substrates for Kex2p in C. albicans (56). Among these ORF products, some predicted Kex2p targets were cell wall-related proteins and sensor proteins including GAS1 and WSC2 homologs. In light of the prediction from the bioinformatics approach using C. albicans and the morphological defects of the A. niger kexB disruptant, kexin activity in Aspergillus species also seems to be concerned primarily with formation of the cell wall and morphogenesis. Therefore, maintenance of morphologenesis in fungi is likely to be one of the biologically important functions in which kexin plays some roles. However, the mechanism underlying the involvement of kexB in morphogenesis in fungi is still unclear.
Although the Saccharomyces cerevisiae kex2-null mutant (kex2Δ) does not exhibit morphological deffects compared with the phenotypes of other filamentous fungi, additional genetic defects in MPK1 (SLT2), together with the kex2Δ mutation, cause lethality (62). Since the yeast MPK1 gene encodes a mitogen-activated protein (MAP) kinase in the cell integrity pathway that plays an essential role in maintaining the biogenesis and integrity of the cell wall (41), kexin seems to be involved in cell wall integrity in S. cerevisiae. The cell integrity pathway is induced in response to several environmental stimuli, resulting in the increased expression of numerous genes, many of which encode integral cell wall proteins (glycosylphosphatidylinositol proteins, PIR [proteins with internal repeats] family proteins, and others) or enzymes, including β-1,3-glucan synthases (Fks1p and Fks2p) and chitin synthases, required for cell wall biogenesis in S. cerevisiae (13, 31, 67, 68). The A. nidulans mpkA gene that is a counterpart of the yeast MPK1 (SLT2) was cloned and characterized (7). An mpkA deletion mutant (ΔmpkA) was constructed, and its morphological defects suggested that the kinase is involved in germination of conidial spores and polarized growth. As described above, the apparent morphologic changes observed in the A. niger kexB disruptant suggest the possibility that involvement of kexin in cell integrity is more significant in Aspergillus fungi than S. cerevisiae. Consequently, our studies focus on gaining an insight into the roles of kexin in morphogenesis and cell wall integrity in Aspergillus fungi.
A. oryzae, which is an economically important filamentous fungus, as well as A. niger, is used in the manufacture of fermented foods and in the production of enzymes for medical and food-grade use (10, 27, 71). Because its industrial importance has fortunately accelerated the establishment of A. oryzae genomics, including an expressed sequence tag (EST) database and genome sequence information, A. oryzae genomics is now one of the most advanced platforms of fungal genomics and thus is also valuable for cell biology research on filamentous fungi (43; http://www.aist.go.jp/RIODB/ffdb/index.html). As a part of the A. oryzae genome projects, we manufactured A. oryzae cDNA microarrays consisting of approximately 2,000 cDNA clones by using information in the A. oryzae EST database (44). In the present study, we isolated an A. oryzae kexB knockout (ΔkexB) strain that had remarkable morphological defects, and the mutant phenotypes, surprisingly, were suppressed under highly osmotic conditions. We investigated the function of kexB in morphogenesis by using the kexB knockout strain and our cDNA microarrays. Here we report (i) cloning of the A. oryzae kexin gene (kexB) and enzymatic characterization of KexB in the membrane fraction isolated from a KexB-overexpressing A. oryzae strain, (ii) construction of the ΔkexB strain and observation of its phenotype, (iii) comparison of gene expression profiles between the ΔkexB strain and the wild type under solid-culture conditions by using our cDNA microarrays and Northern blot analysis, and (iv) demonstration of constitutive upregulation of both transcription levels of the mpkA gene and phosphorylation levels of MpkA in the A. oryzae ΔkexB strain on the solid culture. Our results suggest that disruption of kexB in A. oryzae leads to morphological changes attributable to disordered cell integrity signaling. We discuss the contribution of A. oryzae kexB to the cell integrity pathway and morphogenesis.
MATERIALS AND METHODS
Strains, media, and growth conditions.
We used A. oryzae RIB40 (ATCC 42149) as the wild type and for constructing the kexB knockout mutant. This strain was also used for the A. oryzae EST and genome sequencing projects (43). A. oryzae niaD300 (niaD), a niaD mutant derived from RIB40, was used as a recipient strain for transformation and protein expression. These strains were grown in YPD complete medium (1% yeast extract, 2% polypeptone, 2% dextrin) or Czapek-Dox minimal medium (CD) (52). Instead of glucose, maltose was added to CD medium as an inducer for overexpression by recombinant A. oryzae. CD medium supplemented with 0.1 μg of pyrithiamine (TaKaRa Bio Inc., Tokyo, Japan) per ml was used as the selection medium for the kexB knockout derivatives of A. oryzae. To isolate niaD mutants from the kexB knockout strain of A. oryzae, 500 mM chlorate and either nitrite (NO2), hypoxanthine, glutamate, or ammonium chloride as a nitrogen source were added to CD medium.
Molecular cloning and sequencing of kexB.
For subcloning, Escherichia coli XL1-blue (hsdR17 supE44 recA1 endA1 gryA6 thi relA1 lac [F′ proAB+ lacIq lacZΔM15::Tn10, Terr]) cells and pBluescript II SK(+) plasmid (TOYOBO Inc., Tokyo, Japan) were used as host and vector, respectively, for DNA manipulation. The vector pGEM-T Easy (Promega Co., Tokyo, Japan) was used for TA cloning of PCR products. All basic molecular biology procedures were carried out as described by Sambrook et al. (63). To clone the kexin gene of A. oryzae, we searched the A. nidulans EST database of the University of Oklahoma (http://www.genome.ou.edu/fungal.html) with the yeast (S. cerevisiae) KEX2 gene and found an approximately 600-bp homologous sequence. The nucleotide identity between the yeast KEX2 gene and the obtained A. nidulans DNA fragment was about 40%. PCR primers (5′-CGCTTCTGGGAAGATCTACAAATC-3′ and 5′-GGCCCGAACTTCTCCCGCGCATC-3′) were designed on the basis of the sequence of the fragment, and PCR was performed using genomic DNA from A. nidulans as the template. We obtained a 581-bp fragment, which we used as a probe for screening an A. nidulans cDNA library. Three positive clones were obtained from 2,000 plaques, and the positive clone with the longest insert was sequenced. The insert contains a 2,460-bp ORF encoding a single polypeptide comprising 819 amino acid residues. A FASTA search against the A. oryzae EST database (http://www.aist.go.jp/RIODB/ffdb/index.html) was performed with the full-length sequence of the A. nidulans kexB gene (DDBJ/EMBL/GenBank accession no. AB056726). Clone 6-58 in the A. oryzae EST database was homologous, and the DNA sequencing of this clone was completed using the ABI PRISM BigDye Terminator cycle-sequencing ready reaction kit version 2.0 (Applied Biosystems Japan Ltd., Tokyo, Japan) and an ABI PRISM 377 sequencer (Applied Biosystems Japan). The initiation codon of the A. oryzae kexB gene was predicted by comparison with that of the A. niger kexB gene (29) and on the basis of the discovery of a stop codon 33 bp upstream of the initiation codon in the A. oryzae kexB cDNA.
Creation of the kexB-overexpressing strain.
The overexpression plasmid pNAKX1 was constructed as follows. The kexB gene was PCR amplified using Z-Taq DNA polymerase (TaKaRa) and a pair of primers, 5′-AAGCTTATAATGCGGCTTTCCGAAAG-3′ and 5′-AAGCTTGTAGAAGCAAATGCAAAGCC-3′. Each primer was designed to introduce a HindIII site (underlined). EST clone 6-58 of A. oryzae was used as the template. The amplified fragment was inserted into the pGEM-T Easy vector (Promega) and sequenced. The plasmid was digested with HindIII, and the digested fragment was ligated into the HindIII sites of the pNGA142 vector that has the glaA142 promoter (24). The constructed expression plasmid was named pNAKX1. Transformation of A. oryzae niaD300 (niaD) was performed using the modified protoplast-polyethylene glycol method (20) and pNAKX1 digested with BamHI. The BamHI-digested pNGA142 also was introduced into the niaD300 strain as a control (pNGA strain). For protoplast formation, 5 mg of lysing enzyme (Sigma Chemical Co., St. Louis, Mo.) per ml, 10 mg of cellulase Onozuka R-10 (Yakult Co., Tokyo, Japan) per ml, and 10 mg of Yatalase (TaKaRa) per ml were used. Transformants were subcultured at least three times on CD agar plates to obtain homokaryotic strains.
Enzyme assay.
The cells were grown in 50 ml of CD liquid medium with shaking for 2 days at 30°C. They were then transferred to CD liquid medium containing 2% maltose and cultured for 17 h at 30°C. They were collected using glass filters (Asahi Techno Glass Co., Funabasi, Japan) and ground in a mortar on ice. The ground cells were resuspended in 0.2 M HEPES (pH 7.6) containing 1 mM EDTA and centrifuged at 10,000 × g for 10 min at 4°C. The supernatant was further centrifuged at 100,000 × g for 90 min at 4°C. The precipitate was resuspended in 50 mM HEPES (pH 7.6) containing 1 mM EDTA, 50 mM NaCl, 2% sodium deoxycholate, and 20% glycerol, and the mixture was centrifuged for 90 min at 4°C and 100,000 × g. The supernatants were pooled as the membrane protein fraction. Enzyme assays were performed as described previously (45), but 20 mM Tris-HCl (pH 7.0) was used as the assay buffer.
Creation of the kexB disruption mutant.
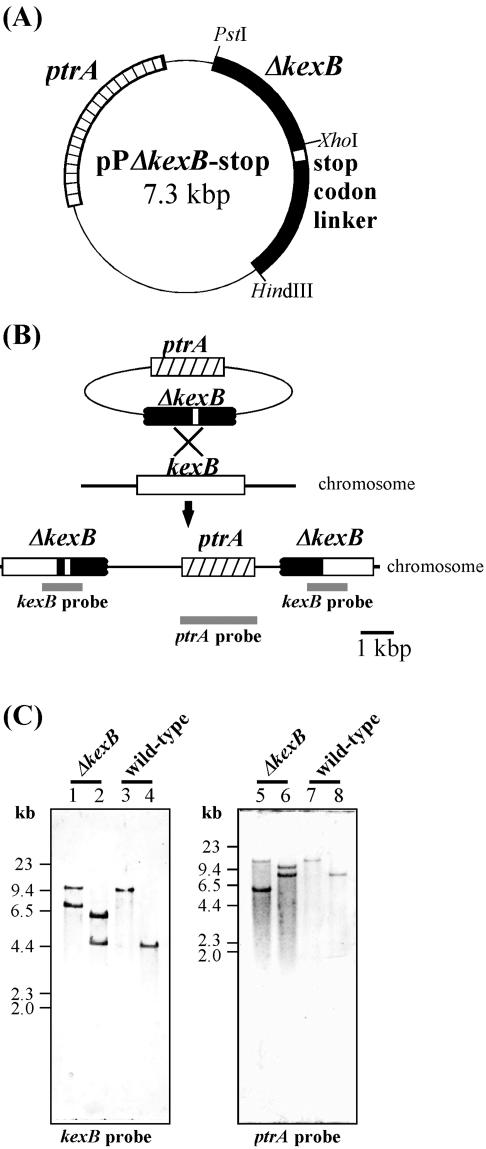
The plasmid for kexB gene disruption (pPΔkexB-stop) was created as follows. The kexB fragment was obtained by PCR with primers 1 (5′-TAATGCGGCTTTCCGAAAcTGCAgCGGTAG-3′) and 2 (5′-TAGGGTAGAAGCAAATGCAAAGCtTAGTCC-3′) by using EST clone 6-58 of A. oryzae as a template. These primers were designed to introduce PstI and HindIII sites (underlined; nucleotides that were changed to create the restriction sites are lowercase), respectively. The fragment was digested with PstI and HindIII and ligated into the PstI-HindIII fragment of the pPTRI vector (TaKaRa), which contains the pyrithiamine resistance gene (ptrA). The constructed plasmid was named pPΔkexB. In addition, a stop codon linker (5′-CTAGTGCTGTGATCACTAGGATCCTTACA-3′) was inserted into the NheI site of pPΔkexB. The linker had terminator codons in every frame, and the resulting plasmid was named pPΔkexB-stop (Fig. 1A). Transformation of A. oryzae RIB40 (wild type) was performed as described above by using pPΔkexB-stop digested with XhoI (Fig. 1B). A. oryzae transformants were screened for resistance to pyrithiamine (0.1 μg/ml) and were subcultured at least three times on CD agar plates containing pyrithiamine.
FIG. 1.
Generation of the kexB gene disruptant. (A) Construction of the plasmid for kexB gene disruption. The plasmid contains a truncated kexB gene and ptrA, which encodes a thiazole biosynthetic enzyme (37). The open box indicates a stop codon linker inserted in the kexB ORF. (B) Strategy for homologous recombination of A. oryzae for kexB gene disruption. The solid box indicates the kexB ORF with the stop codon linker. The gray bars indicate the hybridization positions of the probes to confirm gene disruption by Southern blot analysis. The upper bars indicate the kexB probe. The lower bar indicates the ptrA probe. (C) Southern blot analyses of the genomic DNA from transformant. Each lane contained 20 μg of restriction enzyme-digested genomic DNA of the ΔkexB disruptant (lanes 1, 2, 5, and 6) and wild type (lanes 3, 4, 7, and 8). The enzymes used were PstI and SphI (lanes 1 and 3), ApaLI and NspV (lanes 2 and 4), SpeI (lanes 5 and 7), and PstI (lanes 6 and 8). Hybridization was performed with the kexB probe (see Materials and Methods) (left) and the ptrA probe (right).
Candidates for the knockout strain were selected by colony PCR by using primer sets 1 (Δ-sense 1 [5′-GTTGGGTAACGCCAGGGTTTTCCC-3′] and Δ-antisense 1 [5′-GACTGACAACGAAAACTAAATTACGGGAGC-3′]) and 2 (Δ-sense 2 [5′-CCCATAATGCGGCTTTCCGAAAGTGC-3′] and Δ-antisense 1) and A. oryzae genomic DNA as a template. A. oryzae genomic DNA was isolated from mycelia grown in CD medium for 40 h at 30°C as described previously (66). If the XhoI-digested pPΔkexB-stop fragment was inserted into the kexB coding region, a 1,861-bp fragment was amplified by using primer set 1, and if the fragment was not inserted into the kexB coding region, a 1,783-bp product was amplified by using primer set 2. Recombinants were confirmed by Southern analysis. Two probes were prepared. One was the 1.0-kb EcoRI fragment of A. oryzae EST clone 6-58 (kexB probe), and the other was a full-length ptrA fragment PCR amplified using primers 5′-GATTTCGGTCTATTGGCTAGCAAATGAGCT-3′ and 5′-CACCAGCGTTTCTGGGCTAGCAAAAACAGG-3′ and pPTRI as a template (ptrA probe).
Electron microscopy.
Samples were prepared for electron microscopy by a modification of the method of Nakamura et al. (53). ΔkexB and wild-type colonies grown on CD plates for 4 days at 30°C were fixed with 2.5% (vol/vol) glutaraldehyde, washed in 0.1 M sodium phosphate buffer (pH 7.0), and dehydrated in 50 to 100% (vol/vol) ethanol and then in 100% isoamyl alcohol. Residual isoamyl alcohol in the dehydrated colonies was removed by the critical-point drying method. Dried colonies were coated with platinum-vanadium and observed under an S-700 scanning electron microscope (Hitachi Ltd., Tokyo, Japan) at an accelerating voltage of 15.0 kV.
mRNA isolation from mycelia in solid and liquid culture.
Spores (104) were inoculated on a sterile nylon mesh filter (Nippon Rikagaku Kikai Co. Ltd., Tokyo, Japan) on a CD plate and grown at 30°C for 4 days. The nylon mesh filter with mycelia of A. oryzae was then removed from the plate and frozen in liquid N2. The mycelia were collected with a spatula and ground to a fine powder in a mortar. Total RNA was prepared from powdered cells by using Isogen (Nippongene Co. Ltd., Tokyo, Japan) as specified by the manufacturer. mRNA was isolated from the total RNA by using Message Maker (Invitrogen Co., Tokyo, Japan) as specified by the manufacturer. mRNA also was prepared from liquid cultures. Conidia (5 × 106) were inoculated into 100 ml of CD medium and cultured at 30°C for 22 h, and mRNA was isolated as described above.
Microarray analysis.
Details of A. orzyae cDNA microarrays were described by Maeda et al. (44), and 2,000 cDNA clones on the cDNA microarray were chosen as highly expressed genes from approximately 5,000 nonredundant EST clones of the A. oryzae EST libraries (http://www.aist.go.jp/RIODB/ffdb/index.html) (43). Comparative microarray analyses between the ΔkexB and wild-type strains cultured on CD agar plates and on CD agar plates containing 0.8 M NaCl were performed. First, we examined the transcription levels of the histone H2B and H4 genes by using Northern blot analysis. Under both conditions, the transcription levels of the histone genes were the same in both the ΔkexB and wild-type strains (data not shown), suggesting that these genes are appropriate controls for microarray analyses. Fluorescently labeled cDNA was prepared from each mRNA by using Cy3-dUTP or Cy5-dUTP (Amersham Biosciences Inc., Tokyo, Japan) and the CyScribe first-strand cDNA labeling kit (Amersham Biosciences) as specified by the manufacturer. Cy3- and Cy5-labeled cDNAs (20 μl each) were mixed and purified using the Microcon-30 sample reservoir (Millipore Co., Tokyo, Japan). Human Cot-1 DNA (60 μg; Invitrogen) was added to the cDNA, which was then concentrated to 28 μl. Blocking reagents, including 4 μl of yeast tRNA (10 μg/μl; Invitrogen), 16 μl of poly(dA) (1 μg/μl; Invitrogen), 10.2 μl of 20× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), and 1.8 μl of 10% sodium dodecyl sulfate (SDS), were added to the mixture and boiled for 1 min. The mixture was cooled to room temperature. The labeled DNA was applied to a microarray slide (Asahi Techno Glass Co.) in a cassette chamber, and the chamber was incubated at 55°C for 9 h. The slide was washed sequentially with 2× SSC to 0.1% SDS at room temperature, 0.2× SSC-0.1% SDS at 40°C, and 0.2× SSC at room temperature for 5 min per wash. The slide was dried by centrifugation for 1 min at 300 × g.
Fluorescent DNA bound to the microarray was detected and quantified with a GenePix 4000B microarray scanner (Axon Instruments, Foster City, Calif.), using the GenePix Pro 3.0 software package to locate spots in the microarray. Data are means of triplicate determinations from three different experiments. Spots were quantified as the median of all pixel values in the spot region. The background was quantified for each spot separately as the median of all pixel values in the background region. Background values were subtracted from the respective spot values to obtain net signal values. Net signal values of 1 and below were set to 1. Probes with net signal values below 1.000 for both channels were eliminated from further calculation. Spots that showed net signal values less than 5% of that of histone H2 were also omitted. The merged signal values were used for subsequent comparisons and assessed with the Genomic Profiler software package (Mitsui Knowledge Industry, Tokyo, Japan) and Student's t test by the method of Arfin et al. (2). We performed both global normalization and internal normalization with histones H2B and H4 as the internal standards and detected only a small difference between the results from the two normalization methods (44).
Northern blot hybridization.
mRNAs were prepared as described above from the kexB disruptant and wild-type strains cultured on CD agar plates at 30°C for 50, 70, and 105 h or a CD agar plate plus 0.8 M NaCl at 30°C for 105 h. mRNAs (500 ng each) were electrophoresed through an agarose-formaldehyde gel (64) and transferred to Hybond-N+ nylon membranes (Amersham Biosciences) by using 7.5 mM NaOH. Blotted membranes were hybridized with the probes for chsC, chsB, gelA, or gelB of A. oryzae. The probe for the histone H2B gene was used as a quantitative control. Probes for chsC, chsB, gelA, and gelB were prepared by PCR using the primers 5′-GAGTCTTATGGGGAGGACGACCATAGTC-3′ (forward) and 5′-CTTGGTATTCATCGCTGTAGGGATCGGG-3′ (reverse), 5′-ATGGCCTACCAACCGCCTGGTAAAG-3′ (forward) and 5′-ATGTTTCACTGCGAGCGTAGGGAGAAGC-3′ (reverse), 5′-ATATTAAATATACTTCAGACGGATCTCGCC-3′ (forward) and 5′-TCGAAGAAATTCATGAAACAACAATGACCC-3′ (reverse), and 5′-CGATATAGATCCACAGATTACCCGAAAAGG-3′ (forward) and 5′-GAAAAGCAATACATGAGGTTAATTCAACGC-3′ (reverse), respectively, against A. oryzae genomic DNA. The mpkA probe was prepared from the cDNA library (17) of A. nidulans. The A. oryzae probe for the histone H2B gene was prepared by PCR using the M13 primer set (TaKaRa) against A. oryzae EST clone JZ5168 (http://www.aist.go.jp/RIODB/ffdb/). All probes were labeled with [α-32P]dCTP by using the Rediprime II kit (Amersham Biosciences). Each blot was hybridized at 60°C (55°C for the mpkA probe) with [α-32P]dCTP-labeled probe. The blots were washed in 2× SSC-0.1% SDS at room temperature for 20 min and then twice in 1× SSC-0.1% SDS for 15 min at 60°C (55°C for the mpkA probe) and detected by autoradiography.
Molecular cloning and sequencing of mpkA and plasmid construction.
A FASTA search against the A. oryzae EST database (http://www.nrib.go.jp/ken/EST/db/index.html) was performed with the full-length sequence of the A. nidulans mpkA gene (7) (DDBJ/EMBL/GenBank accession no. AAD24428). AoEST02842 in the A. oryzae EST database was found to be homologous to the A. nidulans mpkA gene. We predicted the initiation codon of A. oryzae mpkA in comparison with that of the A. nidulans mpkA gene (7). The mpkA gene was amplified by the PCR method using Z-Taq DNA polymerase (TaKaRa) and a pair of primers, 5′-AAGCTTATGGCTGATCTACGTAAGATC-3′ and 5′-TCTAGATTATTGTACGTCCATTCCACGC-3′. Each primer was designed to have a HindIII or XbaI site at the 5′ end (underlined). The A. oryzae cDNA library, which was kindly provided by Katsuya Gomi, was used as the template. After TA cloning of the PCR products, the DNA sequencing of this clone was completed using the ABI PRISM BigDye Terminator cycle-sequencing ready reaction kit version 2.0 and an ABI PRISM 377 sequencer. To examine the in vivo functionality of A. oryzae MpkA, S. cerevisae strain TNP46 (MATa mpk1D::HIS3 ura3 leu2 trp1 his3 ade2 can1) with a temperature-sensitive MPK1 allele (41) was used for complementation analysis. Expression plasmids used in this experiment were constructed with the expression vector pYES2 (Invitrogen), in which expression is under the control of the galactose-inducible GAL1 promoter (30). A fragment containing the complete ORF of the A. oryzae mpkA cDNA was digested with HindIII and XbaI and ligated into the HindIII-XbaI fragment of pYES2, resulting in the mpkA expression vector pYmpkA.
Creation of the mychis-tagged MpkA-expressing strain.
The plasmids (pNmpkAmh and pYmpkAmh) for expression of MpkA possessing a c-myc epitope and a polyhistidine tag (mychis-tagged) were created as follows. To construct the mychis-tagged vectors, a mychis fragment was digested with XhoI and AgeI from pPITZαC (Invitrogen) and ligated to the fragment of pSL1180 (Amersham Biosciences) digested with XhoI and AgeI, resulting in pSLmychis. The mychis fragment derived from pSLmychis by SphI-SpeI double digestion was ligated to the fragment from pNGA142 digested with SphI and SpeI to generate pNGmychis. The mpkA fragment was obtained by PCR with primers 5′-AAGCTTATGGCTGATCTACGTAAGATC-3′ and 5′-CGGCCCTCTACTagtTTGTACGTCCATTCC-3′ (stop codon [TAA] replaced by the codon for Thr [ACT]; nucleotides that were changed to remove a nonsense codon are lowercase). These primers were designed to introduce HindIII and SpeI sites (underlined), respectively. Plasmid pYmpkA was used as the template. The amplified fragment was inserted into the pGEM-T Easy vector and sequenced. The resulting plasmid was digested with HindIII and SpeI, and the digested fragment was ligated into the HindIII and SpeI sites of the pNGmychis vector, resulting in the vector pNmpkAmh expressing mychis-tagged MpkA. The fragment containing the ORF for mychis-tagged MpkA was digested with HindIII and NheI from the pNmpkAmh and ligated into the HindIII and XbaI sites of pYES2, resulting in the vector pYmpkAmh expressing mychis-tagged MpkA.
To add a niaD mutation to the A. oryzae ΔkexB strain, the nitrite auxotrophs derived from the ΔkexB strain were screened for resistance to chlorate by using a CD agar plate containing nitrite (NO2) instead of nitrate (NO3) as a nitrogen source in the presence of 500 mM chlorate. We further screened the niaD-mutating ΔkexB strains that were unable to utilize nitrate but could use nitrite, hypoxanthine, glutamate, or ammonium chloride as nitrogen sources. Strains (ΔkexB niaD) obtained from the selection were subcultured at least three times on the above-mentioned selection agar plates. Transformations of A. oryzae ΔkexB niaD and niaD300 (niaD) strains were performed as described above by using pNmpkAmh digested with BamHI. A. orzyae transformants (ΔkexB-mpkAmh and wt-mpkAmh strains) were screened as prototrophs.
Transformants, ΔkexB-mpkAmh and wt-mpkAmh strains of A. oryzae, were obtained by colony PCR by using primers 5′-GCAAACGAAGTCGAAGCAGTCG-3′ and 5′-GTAGAATCACGAATGAGACCTTTGACGACC-3′ to confirm that the BamHI-digested pNmpkAmh fragment was inserted into the niaD locus of the genome. Genomic DNAs isolated from the transformants as described above were used as templates for colony PCR. When the BamHI-digested pNmpkAmh fragment was inserted into the niaD locus, a 2,218-bp fragment was amplified by these primers.
Preparation of cell extracts and immunoblot analysis.
Cell extracts were prepared by the same method as described for the mRNA isolation from solid cultures of the ΔkexB, ΔkexB-mpkAmh, wild-type, and wt-mpkAmh strains grown on CD agar plates at 30°C for 50, 70, and 105 h or a CD agar plate plus 0.8 M NaCl at 30°C for 105 h. The mycelia were ground to a fine powder in a mortar and immediately suspended in prewarmed SDS sample buffer (120 mM Tris HCl [pH 8.8], 5% SDS, 5% mercaptoethanol, 10% glycerol, 1 mM sodium vanadate) without dye. The samples were vortexed at 10 s quickly and boiled at 100°C for 10 min, and the cell debris was removed by centrifugation for 10 min at 15,000 × g. Each sample (50 μg of protein) was subject to SDS-polyacrylamide gel electrophoresis analysis. Protein concentrations were determined by the method of Schaffner and Weissmann (64) with bovine serum albumin as a standard. After the transfer of proteins to a Pall Fluoro Trans W membrane (NIPPON Genetics Co. Ltd., Tokyo, Japan) using a semidry blotting apparatus (Bio-Rad), the membrane was used for immunoblotting with anti-phospho-p44/42 MAP kinase (Cell Signalling Technology, Inc., Beverly, Calif.) antibodies followed by immunoconjugation with the alkaline phosphatase-conjugated goat anti-rabbit immunoglobulin G antibody (NACALAI TESQUE, Inc., Kyoto, Japan) and visualization of the immune complexes with the chromogenic alkaline phosphatase substrate 5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium. To detect the MpkA MAP kinase containing the mychis tag, immunoblots were probed with the anti-myc monoclonal antibody or anti-His monoclonal antibody (Covance Laboratories Inc., Vienna, Va.) and then an alkaline phosphatase-conjugated horse anti-mouse IgG antibody (Vector Laboratories Inc., Burlingame, Calif.). Immune complexes were detected as described above.
Nucleotide sequence accession number.
The sequence data of the kexB genes of A. oryzae and A. nidulans have been submitted to the DDBJ/EMBL/GenBank database under accession no. AB056727 and AB056726, respectively. The sequence data of the mpkA gene of A. orzyae have been submitted to the DDBJ/EMBL/GenBank database under accession no. AB167718.
RESULTS
Cloning and characterization of the kexB gene from A. oryzae.
To clone the kexin gene of A. oryzae, we searched for A. oryzae EST sequences homologous to the A. nidulans kexin-like gene by using the A. oryzae EST database (http://www.aist.go.jp/RIODB/ffdb/) and identified clone 6-58. We determined the complete nucleotide sequence of this EST clone, which contained a 2,511-bp ORF encoding a protein of 836 amino acid residues. The putative amino acid sequence showed about 70% identity to those of the putative kexin identified in A. nidulans (AB056726) and A. niger KexB (29), and thus the A. oryzae and A. nidulans genes both were designated kexB. The catalytic triad, Ser-Asp-His, and several amino acids upstream and downstream from the catalytic domain were well conserved. The putative A. oryzae KexB also has a P-domain, which is necessary for enzymatic activities in other proteases (42, 55). Hydropathy analysis suggested the presence of two highly hydrophobic regions that are a signal peptide and transmembrane domain required for anchoring to Golgi membranes, similar to those in the amino acid sequence of yeast kexin. A propeptide with a Lys-Arg dibasic autocleavage site follows the signal peptide. In the putative cytoplasmic tail, 15 amino acids downstream from the transmembrane domain, we found the conserved peptide sequence YDFEMI, similar to that found in KexB of A. niger (29). The underlined amino acid residues are identical to the late-Golgi retention signal (consensus, YXFXXI) in the cytoplasmic tail of the S. cerevisiae Kex2p (69).
To confirm whether the A. oryzae kexB gene product has protein-processing activities like those of known kexins, kexB was integrated at the niaD locus in the A. oryzae niaD300 strain and expressed using the A. oryzae glaA142 promoter. Southern blot analysis confirmed, in addition to the authentic kexB locus, the integration of an extra copy of kexB at the niaD locus in the transformant (data not shown). The transformed strain in which kexB was overexpressed did not show a detectable or noteworthy phenotype for hyphal development or morphology under induced conditions in either liquid or agar plate culture. We fractionated a cell lysate of the kexB-overexpressing strain and found kexin-like enzymatic activities in the membrane fraction. The solubilized membrane fraction from the kexB-overexpressing strain showed higher hydrolysis of fluorogenic peptides than did that from the control strain harboring the expression vector lacking the kexB insert (Table 1). In particular, A. oryzae KexB has a preference for peptides containing dibasic residues, as follows: Boc-Gln-Arg-Arg-MCA (4-methylcoumaryl-7-amide), Boc-Leu-Lys-Arg-MCA, Boc-Leu-Arg-Arg-MCA, and Boc-Arg-Val-Arg-Arg-MCA. The substrate specificity of A. oryzae KexB is similar to that of yeast Kex2p (5). KexB of A. oryzae also recognizes Arg-Arg and Lys-Arg at the P1 and P2 positions but hardly ever recognizes Lys-Lys. According to the substrate specificity, we concluded that the cloned kexB gene encodes kexin of A. oryzae.
TABLE 1.
Enzymatic activities for peptidyl-MCA substrates
| Peptide substratea | Cleavage activity (nkat/ml)
|
|
|---|---|---|
| P4 P3 P2 P1↓ P1′ | pNGA142 | pNAKX1 |
| Boc-Gln-Arg-Arg-MCA | 0.07 | 3.47 |
| Boc-Leu-Lys-Arg-MCA | 0.06 | 3.15 |
| Boc-Leu-Arg-Arg-MCA | 0.07 | 2.91 |
| Boc-Arg-Val-Arg-Arg-MCA | 0.05 | 2.39 |
| Boc-Val-Pro-Arg-MCA | 0.04 | 1.74 |
| Boc-Gly-Lys-Arg-MCA | 0.03 | 1.30 |
| Boc-Gly-Arg-Arg-MCA | 0.01 | 0.56 |
| Boc-Leu-Ser-Thr-Arg-MCA | 0.02 | 0.55 |
| Boc-Leu-Gly-Arg-MCA | 0.00 | 0.11 |
| Boc-Gln-Gly-Arg-MCA | 0.01 | 0.20 |
| Boc-Glu-Lys-Lys-MCA | 0.00 | 0.01 |
The arrow indicates the cleavage site by A. oryzae KexB. Boc, t-butyloxycarbonyl-; MCA, 4-methylcoumaryl-7-amide. Basic amino acids are in bold.
Construction and characterization of the kexB disruptant.
To further study the in vivo functionality of A. oryzae kexB, we constructed the kexB disruptant (ΔkexB) strain by homologous recombination with pPΔkexB-stop (Fig. 1A), which contained ptrA and a truncated kexB fragment carrying a stop codon linker downstream of the XhoI site (Fig. 1B). The stop codon linker had multiple stop codons to cover all reading frames. The ΔkexB strain was isolated from about 400 colonies of the transformants by the colony PCR method as described by van Zeijl et al. (66) with primers 1 and 2, as described in Materials and Methods. The ΔkexB candidate was further confirmed by PCR and Southern analysis (Fig. 1C). The probes for both kexB and ptrA indicated the expected two hybridization signals to digested genomic DNA isolated from the ΔkexB candidate and the single hybridization signal to that from the wild-type strain, suggesting that the homologous recombination successfully took place at the authentic kexB locus. Reverse transcription-PCR failed to reveal transcripts derived from kexB in the ΔkexB strain (data not shown).
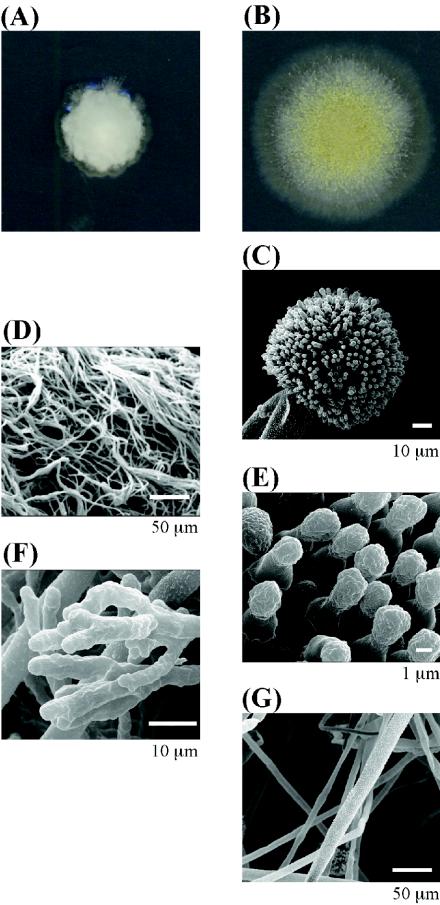
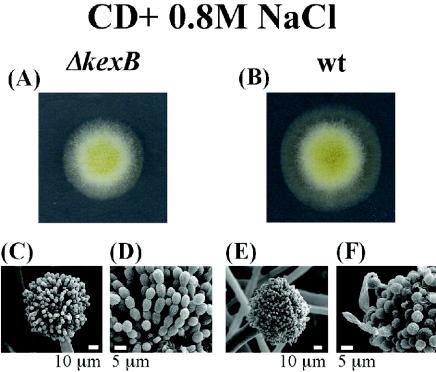
The ΔkexB strain formed shrunken colonies and scarcely differentiated conidia on CD agar plates (Fig. 2A). The detailed morphology of the ΔkexB strain was further compared with that of the wild type by using scanning electron microscopy (Fig. 2C to G). ΔkexB cells grown for 4 days on CD agar plates formed neither conidiophores nor conidia. The hyphae of the ΔkexB strain became finer, denser and hyperbranched in comparison with those of the wild type. Hyphal tips of the ΔkexB strain were thicker and multibranched (Fig. 2F). The disruptant grown in CD liquid culture medium also showed highly branched mycelia (data not shown). We carried out a rescue experiment in which the mutant phenotypes of the A. oryzae ΔkexB (ΔkexB niaD) strain were almost fully restored by transformation with the pNAKX1 plasmid containing the wild-type kexB gene (see Fig. S1 in the supplemental material). However, the A. oryzae ΔkexB (ΔkexB niaD) strain transformed with the pNGA142 vector without the kexB insert maintained the mutant phenotypes (see Fig. S1 in the supplemental material). These results suggested that the phenotypes of the ΔkexB strain are attributable to the defect of the kexB gene. Surprisingly, although the ΔkexB strain exhibited the various described morphological defects on CD agar plates, the defects were suppressed on high-osmolarity CD agar plates containing 0.8 M sodium chloride (Fig. 3), 0.8 M potassium chloride, or 1.2 M sorbitol (data not shown). The morphological defects of the ΔkexB strain also were restored in a high-osmolarity CD liquid culture medium (data not shown).
FIG. 2.
Morphological phenotypes of the ΔkexB strain on CD agar plates. ΔkexB (A, D, and F) and wild-type (B, C, E, and G) cells were cultivated on CD agar plates at 30°C for 4 days. (A and B) Colony growth of the ΔkexB (A) and wild-type (B) strains. (C through G) Scanning electron micrographs. Conidiophores and conidia of the wild type are shown (C and E, respectively); the ΔkexB strain did not form conidiophores or conidia. Hyphae of the ΔkexB and wild-type strains are visible (D and G, respectively); hyphal tips of the ΔkexB strain can also be seen (F).
FIG. 3.
Restoration of the ΔkexB phenotypes on CD agar plates with high osmotic pressure. ΔkexB (A) and wild-type (B) strains were cultivated on CD agar plus 0.8 M NaCl at 30°C for 4 days. Conidiophores of ΔkexB and wild-type strains (C and E, respectively) and conidia of ΔkexB and wild-type strains (D and F, respectively) are also visible.
cDNA microarray analysis of the ΔkexB disruptant.
Because the ΔkexB strain showed the various described phenotypes, we wondered how transcription profiles reflected the phenotypes and therefore compared the gene expression profiles of the ΔkexB and wild-type strains by using A. oryzae cDNA microarrays. We analyzed transcripts from the two strains cultured on CD agar plates for 105 h at 30°C as well as on CD agar plates containing 0.8 M NaCl (Table 2). On CD agar plates, a large number of genes were more upregulated in the ΔkexB cells than in wild-type cells whereas gene expression levels of the ΔkexB strain were similar to those of the wild type in the presence of high osmotic pressure (∼0.8 M NaCl). The ΔkexB strain exhibited 4.9- and 55-fold-lower levels of brlA (70) and rodA (57) transcripts, respectively, than did the wild type. Because brlA encodes a transactivator to promote the formation of conidia and rodA encodes a hydrophobic protein necessary to form conidiophores, the downregulation of brlA expression in the ΔkexB strain might be one of the reasons for its poor generation of conidia. We were unable to assign identities to other genes that showed markedly reduced transcript levels in the ΔkexB strain, because the annotations of some genes in the cDNA microarray remain unknown.
TABLE 2.
Number of genes whose expression levels were altered by deletion of kexB in A. oryzae
| Relative expression ratios (ΔkexB/wild type) (fold) | No. of genes in cells grown ona:
|
|
|---|---|---|
| CD | CD + 0.8 M NaCl | |
| >4.0 | 68 | 0 |
| 2.0-4.0 | 291 | 25 |
| 0.5-2.0 | 1,087 | 1,736 |
| 0.25-0.5 | 48 | 1 |
| <0.25 | 27 | 0 |
After three independent analyses, 1,521 spots on the cDNA microarrays for CD plates and 1,762 spots on the cDNA microarrays for CD plates with 0.8 M NaCl were statistically verified by using the Genomic profiler program.
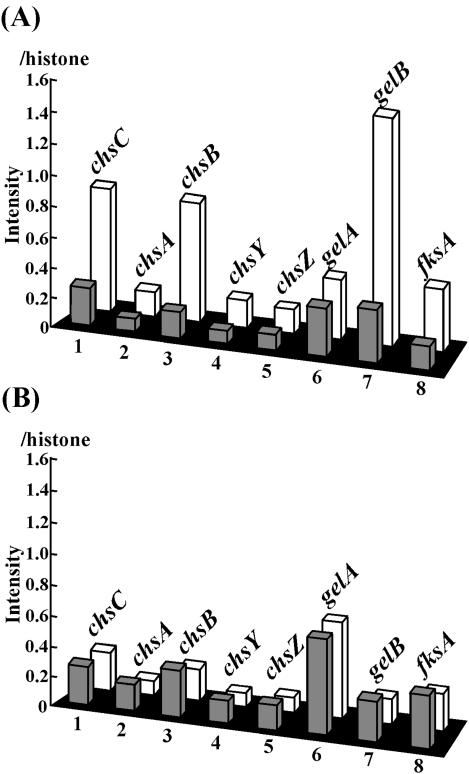
Because of the morphological defects of the ΔkexB strain, we paid further attention to the following eight genes which are involved in cell wall biogenesis: chsC (50), chsA (unpublished data; DDBJ/ENBL/GenBank accession no. BAB85683), chsB (51), chsY, chsZ (9), gelA, gelB (48), and fksA (34) (Fig. 4). chsC, chsA and -B, chsY, and chsZ encode type I, III, V, and VI chitin synthases, respectively (9). According to tBlastx analysis, gelA and gelB are predicted to be the A. oryzae counterparts of the A. fumigatus gel1 and gel2 genes, which encode putative glucanosyltransferases that are thought to be involved in cell wall biosynthesis (49). fksA is the putative A. oryzae β-1,3-glucan synthase gene (34). On CD agar plates, transcription of chsC, chsB, and gelB of the ΔkexB strain was markedly upregulated and their transcription levels were 3.2, 4.6, and 4.3 times higher, respectively, than those of the wild type. In contrast, high osmotic pressure (0.8 M NaCl) in the culture plate suppressed the upregulation of these three genes in the ΔkexB strain.
FIG. 4.
Expression levels of genes involved in cell wall biogenesis. The bar graphs indicate the expression levels of the genes encoding cell wall synthesis-related proteins from wild-type and ΔkexB strains grown on CD agar plates (A) and CD agar plates containing 0.8 M NaCl (B). The gray and white bars indicate the relative intensities of transcription of the genes in the wild-type and ΔkexB strains, respectively, on the basis of the cDNA microarray analyses. The relative intensities of the examined genes were calculated using the intensity of histone H2B as an internal standard (1.0). The genes were as follows: 1, chsC (chitin synthase C) (50); 2, chsA (chitin synthase A) (our unpublished data; DDBJ/ENBL/GenBank accession no. BAB85683); 3, chsB (chitin synthase B) (51); 4, chsY (chitin synthase Y) (9); 5, chsZ (chitin synthase Z) (9); 6, gelA (glycosylphosphatidylinositol-anchored glucanosyltransferase) (48); 7, gelB (glycosylphosphatidylinositol-anchored glycanosyltransferases) (48); and 8, fksA [β(1-3) glucan synthesis] (34).
Northern blot analysis of the ΔkexB strain.
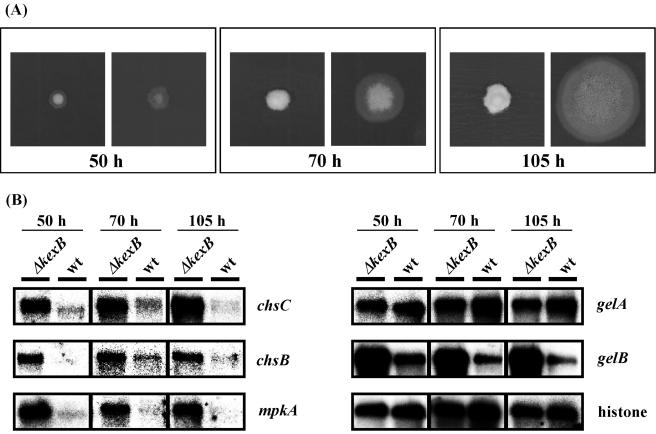
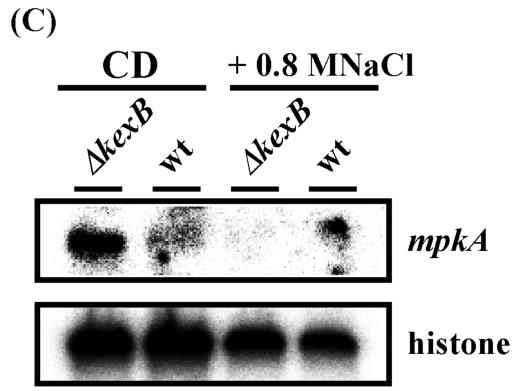
Cell wall biogenesis in S. cerevisiae is thought to be under the control of signal transduction pathways including the cell integrity pathway. The S. cerevisiae MPK1 (SLT2) gene encodes a MAP kinase in the cell integrity pathway (26). In S. cerevisiae, when cell wall biosynthesis is inhibited or the cell wall is damaged, transcription levels of MPK1 and genes required for cell wall biogenesis are simultaneously upregulated by the transcription factor Rlm1p, which is a phosphorylation target of Mpk1p (13, 31, 67, 68). Bussink et al. (7) isolated the A. nidulans mpkA gene which is the counterpart of yeast MPK1. Recently we confirmed that expression of A. nidulans mpkA cDNA in a temperature-sensitive S. cerevisiae mpk1 disruptant suppressed the mpk1 disruption mutation, suggesting that A. nidulans mpkA functionally complements yeast MPK1 (T. Fujioka, K. Furukawa, O. Mizutani, K. Abe, and T. Nakajima, unpublished data). We also found an A. oryzae mpkA gene homolog in the A. oryzae EST database and A. oryzae genome information through homology searches with the nucleotide sequence of A. nidulans mpkA. Because our cDNA microarray analyses showed upregulation of the transcription levels of genes involved in cell wall biogenesis in A. oryzae ΔkexB, we further examined whether transcription of mpkA is also upregulated in this strain. We performed transcriptional analyses of mpkA and cell wall-related genes such as chsC, chsB, gelA, and gelB by Northern blotting at various times after inoculation (Fig. 5A and B). The wild-type strain began to form conidiophores and conidia 70 h after inoculation and had formed fully mature conidiophores with plenty of conidia at 105 h on CD plates. Transcription of the histone H2B gene seemed to be constitutive and was used as a control at all time points. Transcription levels of chsC, chsB, and gelB were higher in the ΔkexB strain than in the wild type at the three time points assayed. As expected, the transcription of mpkA in the ΔkexB strain also was increased at all time points. However, the transcription levels of gelA were almost same in both strains at all time points. Because cell integrity signaling is inactivated by high osmotic stress in S. cerevisiae (11, 25, 26), we examined whether high osmotic stress downregulates the high level of transcription of mpkA in the ΔkexB strain to the transcription level of mpkA in the wild-type strain. We performed transcriptional analyses of mpkA by Northern blotting under conditions of high osmosis (Fig. 5C). As expected, high osmotic stress apparently downregulated the transcription level of mpkA in the ΔkexB strain to a level similar to that in the wild-type strain. Therefore, the morphological defects concomitant with the marked upregulation of mpkA and the osmoresponsive suppression of the morphological defects with the simultaneous downregulation of transcription of mpkA and genes for cell wall biogenesis may indicate that kexB is involved in a signal transduction pathway, particularly cell integrity signaling.
FIG. 5.
Gene expression analysis of chsC, chsB, gelA, gelB, and mpkA by Northern blotting. (A) Time course of the phenotypic change of the ΔkexB and wild-type strains on CD agar plates. The left, middle, and right panels show colonies cultivated for 50, 70, and 105 h, respectively. (B) Gene expression analysis of chsC, chsB, gelA, gelB, and mpkA over time (50, 70, and 105 h) by Northern blotting. A histone gene was used as a control. wt, wild type. (C) Gene expression analysis of mpkA on CD agar plates plus 0.8 M NaCl for 105 h by Northern blotting. A histone gene was used as a control.
Cloning of the mpkA gene from A. oryzae and time course of MpkA phosphorylation in the ΔkexB strain.
Since transcription levels of the mpkA gene in the ΔkexB strain are constitutively upregulated under normal culture conditions and downregulated in the presence of osmotic stress, we further examined phosphorylation levels of MpkA protein in the ΔkexB strain under normal and high-osmosis conditions. From the A. oryzae cDNA library, we isolated a positive clone by using PCR. We determined the complete nucleotide sequence of this clone, which contained a 1,272-bp ORF encoding an MpkA protein of 423 amino acid residues. The catalytic domain of MpkA possesses all the subdomains found in protein kinases (23). In addition, MpkA has a TEY tripeptide dual phosphorylation motif characteristic of MAP kinases, which is known to be required for activation of MAP kinases. The putative amino acid sequence showed about 90% identity to that of the putative MpkA identified in A. nidulans (7), and thus the A. oryzae gene was designated mpkA.
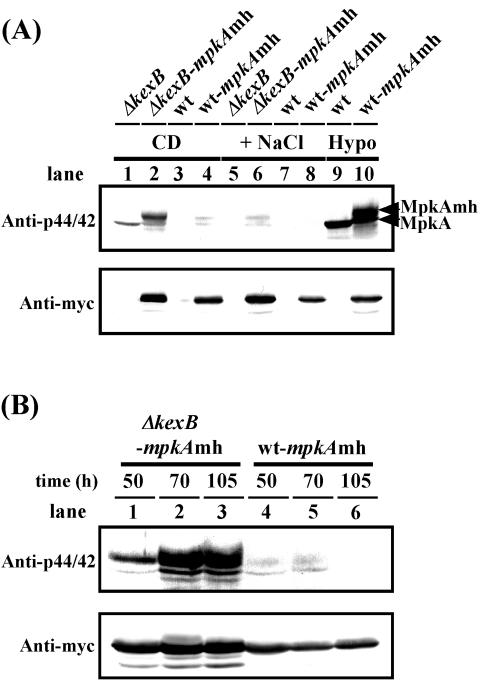
Because MpkA of A. oryzae is also related to the yeast Mpk1p, we investigated the in vivo functionality of MpkA and its derivative mychis-tagged MpkA by using an S. cerevisiae mpk1 mutant. We confirmed that expression of both A. oryzae mpkA and mpkAmh genes in the S. cerevisae mpk1 disruptant suppressed the temperature sensitivity attributed to the mpk1 mutation, suggesting that both mpkA and mpkAmh functionally complement the yeast MPK1 (data not shown). To demonstrate whether MpkA is phosphorylated in the ΔkexB strain, we examined the phosphorylation levels of MpkA and MpkAmh expressed in ΔkexB cells grown on the CD agar plates. We detected a significant increase in the phosphorylation levels of both MpkA and MpkAmh in the A. oryzae ΔkexB cells grown for 105 h on the CD agar plates by using anti-phospho-p44/42 MAP kinase antibodies (Fig. 6A, lanes 1 and 2), although MpkA in the wild-type strain was scarcely phosphorylated, regardless of osmotic stress (lanes 3, 4, 7, and 8). The phosphorylation levels of MpkA and MpkAmh in the ΔkexB strain were apparently downregulated by high osmotic stress (lanes 5 and 6), with concomitant downregulation of the transcription levels of mpkA (Fig. 5C). Phosphorylation of MpkA was observed when the wild-type cells were subjected to hypotonic stress (Fig. 6A, lanes 9 and 10), indicating that MpkA is capable of transducing the same types of stress signals through the putative cell integrity pathway as well as that of S. cerevisiae. The ΔkexB-mpkAhm and wt-mpkAhm strains used as controls of expression quantity of MpkA showed the same phenotypes as the ΔkexB and the wild-type strains on the CD agar plate culture, respectively. Furthermore, the phosphorylation levels of MpkAmh and the authentic MpkA in the ΔkexB-mpkAmh cells significantly increased at the three culture time points examined whereas MpkA and its derivative in the wt-mpkAmh strain were poorly phosphorylated (Fig. 6B). The phosphorylation levels of MpkAmh and the authentic MpkA in the ΔkexB-mpkAmh strains at 70 and 105 h of cultivation were higher than those at 50 h. Although the phosphorylation levels of MpkAs at each growth stage were different in the ΔkexB-mpkAmh cells, the MpkAs in the ΔkexB-mpkAmh were remarkably phosphorylated at all time points after inoculation, suggesting that the KexB defect in A. oryzae causes constitutive activation of the cell integrity pathway.
FIG. 6.
The cell integrity pathway MAP kinase MpkA is constitutively phosphorylated in the A. oryzae ΔkexB strain on the CD plate. Upper panels show immunoblotting with anti-phospho-p44/42 MAP kinase antibodies (Anti-p44/42). Lower panels show immunoblotting with anti-Myc antibodies (Anti-myc). (A) ΔkexB (lanes 1 and 5), ΔkexB with mpkAmh (ΔkexB-mpkAmh; lanes 2 and 6), wild type (wt; lanes 3 and 7) and wild type with mpkAmh (wt-mpkAmh; lanes 4 and 8) were grown for 105 h at 30°C on the CD plate (CD) and CD plate with an osmolyte (+NaCl) before preparation of cell extracts (see Materials and Methods). As controls, wild-type (lane 9) and wt-mpkAmh (lane 10) cells were exposed for 1 h to a hypotonic condition (Hypo) by addition of water after cultivation for 50 h on the CD plate. (B) The ΔkexB-mpkAmh (lanes 1 to 3) and wt-mpkAmh (lanes 4 to 6) strains were grown at 30°C on the CD plate for 50, 70, and 105 h, respectively, before preparation of cell extracts.
DISCUSSION
In the present study, we cloned the A. oryzae kexB gene, which encodes 836 amino acid residues and confirmed that the encoded protein has processing activity (Table 1). To study kexB function, we constructed a kexB gene disruptant strain (ΔkexB; Fig. 1). The ΔkexB strain showed shrunken colonies with poor generation of conidia on CD agar plates (Fig. 2), and hyperbranched mycelia occurred in liquid culture. Interestingly, the morphological defects derived from the ΔkexB genotype were restored under conditions of high osmosis (Fig. 3). In A. oryzae ΔkexB, the transcription levels of genes for cell wall biogenesis and an mpkA homolog that presumably encodes a MAP kinase involved in cell integrity signaling were higher than those in the wild type (Table 2; Fig. 4 and 5), and high osmotic pressure downregulated the transcription levels of A. oryzae mpkA and the cell wall-related genes in the ΔkexB strain to levels similar to those in the wild type (Fig. 4 and 5). Then we cloned the A. oryzae mpkA gene and confirmed that its expression suppressed the temperature sensitivity of the S. cerevisiae mpk1 disruptant (data not shown), suggesting the in vivo functionality of A. oryzae MpkA. As expected, constitutively elevated phosphorylation levels of MpkA in ΔkexB cells on the CD agar plate culture were demonstrated by using anti-phospho-p44/42 MAP kinase antibodies, and high osmotic stress downregulated the increased phosphorylation levels of MpkA in the ΔkexB strain to the same as those observed in the wild type (Fig. 6). These results suggest that the upregulation of transcription levels of mpkA and cell wall-related genes in the ΔkexB strain is mediated by phosphorylated MpkA as an active form through cell integrity signaling.
The phenotypes of A. oryzae ΔkexB are different from those of kexB disruptants of A. niger (29, 60) and A. nidulans (K. Furukawa, O. Mizutani, T. Fujioka, Y. Yamagata, K. Abe, K. Gomi, and T. Nakajima, unpublished data). The A. niger kexB disruptant formed conidiophores and conidia on agar plates (29). The A. nidulans ΔkexB strain showed shrunken colonies on agar plates but had differentiated conidiophores and conidia. According to the phenotypes of the ΔkexB strains of these three Aspergillus species, the function of the kexB gene in A. oryzae is probably more essential for cell growth and especially for cell wall biogenesis. The differences of the ΔkexB phenotypes among these three Aspergillus species may be attributable to the processing targets of KexB in each species but not to the substrate specificity of each KexB protein, because the substrate specificity of the A. oryzae product was similar to those of A. niger (29) and A. nidulans (39). Moreover, it is noteworthy that the various morphological phenotypes derived from the ΔkexB genotype in A. oryzae were restored under high osmotic stress in both solid and liquid culture. However, the number of conidia in the ΔkexB strain was 70% of that in the wild type even under high osmotic pressure, suggesting that osmotic suppression of ΔkexB phenotypes is incomplete. Although high osmotic pressure did not suppress the phenotypes of kexin gene disruptants of S. cerevisiae or C. albicans (36, 56), the osmotic restoration of ΔkexB phenotypes occurs in A. oryzae and A. nidulans (Furukawa et al., unpublished). It remains unknown whether the ΔkexB phenotypes of A. niger are suppressed under high osmotic conditions.
In our comprehensive and comparative analysis of gene expression between the A. oryzae ΔkexB and wild-type strains by using cDNA microarrays, the transcription levels of a large number of genes were higher in the ΔkexB strain than in the wild type on CD agar plates (Table 2). These results imply that disruption of the kexB gene affects the transcription levels of a broad range of genes and that KexB of A. oryzae probably processes key proteins required for maintenance of normal morphogenesis and cell growth. In addition, we found that the expression levels of chsC, chsB, and gelB were markedly higher in the A. oryzae ΔkexB strain than in the wild type (Fig. 4).
Cell wall biogenesis in S. cerevisiae is thought to be under the control of various signal transduction pathways including the cell integrity pathway (26). Cell integrity signaling is activated, with different timing and kinetics, by hypoosmotic shock (11, 32), by heat shock (32), during bud emergence (72), on exposure to mating pheromone (6, 72), and on various treatments leading to perturbation of the cell wall (4, 35, 67). The pathway organizes changes in cellular morphology by controlling the expression of genes encoding enzymes involved in cell wall metabolism. The central pathway concerned with cell integrity is the Mpk1p MAP kinase cascade, and Mpk1p phosphorylates and consequently activates the transcription factor Rlm1p, whose transcriptional targets are genes encoding cell wall biogenesis proteins, cell wall proteins, and Mpk1p itself (13, 31, 68). Northern blot analyses of cell wall-related genes and mpkA revealed that transcription levels of the examined genes were simultaneously upregulated in A. oryzae ΔkexB. Western blot (immunoblot) analysis by using anti-phospho-p44/42 MAP kinase antibodies also revealed the constitutive phosphorylation of MpkA in A. oryzae ΔkexB. If cell integrity signaling similar to that of S. cerevisiae is functional in Aspergillus fungi, including A. oryzae, the results shown in Fig. 4 to 6 suggest that cell integrity signal transduction is constitutively activated by disruption of the kexB gene in A. oryzae.
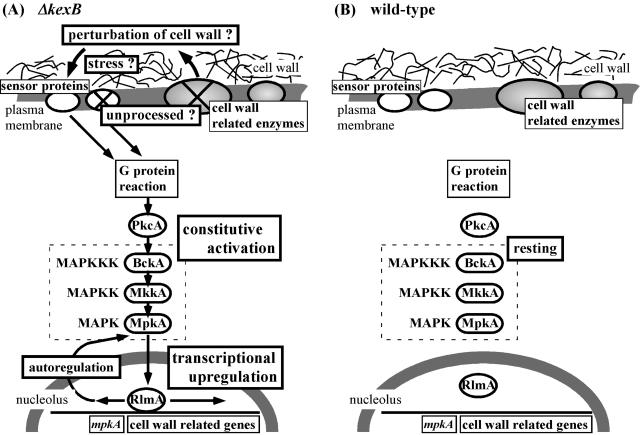
We propose the following two scenarios to explain why and how cell integrity signaling is activated in the A. oryzae ΔkexB strain. In the first scenario, if KexB processes enzymes required for cell wall biogenesis, then the ΔkexB strain fails to process these enzymes, resulting in perturbation of cell wall assembly. Various sensor proteins might detect perturbation of the cell wall as a stressor in A. oryzae ΔkexB and consequently activate the cell integrity pathway that upregulates the transcription of some cell wall-related genes required for restoration of the damaged cell wall. Sensor proteins such as Mid2p and Wsc1p in S. cerevisiae are thought to directly sense alterations of certain cell wall properties to mediate the activation of the cell integrity pathway via G-protein reactions (26). Putative ORFs homologous to the yeast sensors occur among A. oryzae genome sequences (data not shown). In the second scenario, if KexB processes putative cell surface sensors of the cell integrity pathway, incorrect processing of the sensor proteins by the ΔkexB mutation might be responsible for the constitutive activation of cell integrity signaling and for the consequent upregulation of the transcription levels of cell wall-related genes. The morphological defects of the ΔkexB strain might be caused by upregulation of cell wall-related genes through activation of cell integrity signaling. A bioinformatics approach using the C. albicans genome database assigned 147 ORFs as putative Kex2p substrates, and these ORFs included a gene encoding a Wsc2p homologue that seems to be a sensor protein in the cell integrity pathway (56). Applying this analogy to A. oryzae, the sensor/signal-like proteins of the pathway in A. oryzae might be processed by KexB.
Although KexB may process cell wall-related enzymes and/or cell surface sensors (Fig. 7), we currently prefer the first scenario in light of the following predictions. Although the yeast KEX2 disruptant (kex2Δ) has no noteworthy morphological phenotype, the Pkc1-Mpk1 pathway is known to be activated in the kex2Δ strain, resulting in perturbation of cell wall structure (62). Consequently, additional defects of MPK1, together with the kex2Δ mutation, cause cell death (62). This synthetic lethality suggests that the perturbation of cell wall assembly in the kex2Δ strain is probably suppressed by activation of cell integrity signaling, which consequently upregulates the transcription of cell wall-related genes required for restoration and maintenance of the putative damaged cell wall (12, 31, 61). Furthermore, disruption of genes encoding various cell wall-related enzymes causes activation of the pathway in S. cerevisiae (8, 40), whereas, to our knowledge, evidence for activation of the cell integrity pathway by impaired sensors is unavailable even for S. cerevisiae (21, 28, 35, 67). However, we cannot exclude the possibility that the transcription level of mpkA might be upregulated by the influence of another signal transduction pathway.
FIG. 7.
Schematic model for implication of the cell integrity pathway in the ΔkexB (A) and wild-type (B) strains. The cell integrity MAP kinase pathway is shown by the dotted box. pkcA (47), mpkA (7), and rlmA (Fujioka et al., unpublished; accession no. BAD01583) were isolated from Aspergillus fungi. The yeast BCK1 and MKK1 orthologs have not yet been isolated from Aspergillus species; however, putative ORFs (bckA, mkkA) homologous to the two genes are found in A. oryzae genome sequences (O. Mizutani, K. Abe, and T. Nakajima, unpublished data). The cell integrity pathway in the ΔkexB strain is constitutively activated (A), although the pathway is not activated (resting) unless the wild-type strain senses some stress such as hypoosmolarity (B). A. oryzae KexB is predicted to be required for precise proteolytic processing of sensor proteins in the cell integrity pathway and/or of cell wall-related enzymes whose genes are under transcriptional control by the pathway.
The kex2Δ mpk1Δ phenotype of S. cerevisiae is rescued by growth on high-osmolality medium (62). The phenotype of the A. oryzae ΔkexB strain also was restored under high osmotic pressure (Fig. 3). We propose the following two explanations for why the phenotypes of the ΔkexB strain were suppressed under high osmotic pressure. First, instead of KexB, perhaps other processing proteases inducible under high osmotic pressure suppress the ΔkexB mutation. YPS1 and YPS2, which encode aspartyl proteases, are multicopy suppressors of the kex2Δ mutation in S. cerevisiae (14, 36). The expression level of YPS1 in S. cerevisiae cells treated for 10 min with 0.4 M NaCl is 15.7 times higher than that in cells without the NaCl treatment (58). We found two genes homologous to YPS1 and YPS2 in A. oryzae (38), and NaCl may induce these homologs and suppress the ΔkexB mutation. Second, the restoration of the ΔkexB phenotypes might depend on mechanisms mediated by osmotic stress, such as a cascade similar to the yeast Hog1 MAP kinase pathway (22, 26, 33, 59). The cell integrity pathway in S. cerevisiae is thought to be negatively regulated by osmotic pressure (11, 25, 26), and activation of the Hog1p pathway mediates adaptive rearrangements in cell wall composition and architecture in S. cerevisiae and C. albicans (1, 18). A similar scenario might explain the osmotic restoration of the ΔkexB phenotypes in A. oryzae. To verify restoration of the altered phenotypes by increased osmotic pressure in ΔkexB, in vivo functional studies, such as exploration of suppressors of the ΔkexB mutation or construction of a mutant in which the HOG pathway is constitutively activated in the ΔkexB genetic background, are necessary and in progress.
In conclusion, we predict that A. oryzae KexB is required for precise proteolytic processing of sensor proteins in the cell integrity pathway or of cell wall-related enzymes whose genes are under transcriptional control by the pathway. Thus, the KexB defect leads to disordered cell integrity signaling.
Supplementary Material
Acknowledgments
We thank Katsuya Gomi and Hiroyuki Horiuchi for helpful suggestions. We also thank Osamu Hatamoto, Hiroshi Maeda, Junichi Maruyama, Turuji Satou, Takamitsu Maruyama, Yoshihiko Matsuda, Kanako Suzuki, Motoaki Sano, and Masayuki Machida for helpful discussions and/or technical assistance.
This work was supported in part by a Grant-in-Aid (Bio Design Program) from the Ministry of Agriculture, Forestry and Fisheries of Japan (BDP-03-VI-1-7).
Footnotes
Supplemental material for this article may be found at http://ec.asm.org.
REFERENCES
- 1.Alonso-Monge, R., F. Navarro-Garcia, G. Molero, R. Diez-Orejas, M. Gustin, J. Pla, M. Sanchez, and C. Nombela. 1999. Role of the mitogen-activated protein kinase Hog1p in morphogenesis and virulence of Candida albicans. J. Bacteriol. 181:3058-3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arfin, S. M., A. D. Long, E. T. Ito, L. Tolleri, M. M. Riehle, E. S. Paegle, and G. W. Hatfield. 2000. Global gene expression profiling in Escherichia coli K12. The effects of integration host factor. J. Biol. Chem. 275:29672-29684. [DOI] [PubMed] [Google Scholar]
- 3.Bader, O., M. Schaller, S. Klein, J. Kukula, K. Haack, F. Muhlschlegel, H. C. Korting, W. Schafer, and B. Hube. 2001. The KEX2 gene of Candida glabrata is required for cell surface integrity. Mol. Microbiol. 41:1431-1444. [DOI] [PubMed] [Google Scholar]
- 4.Bickle, M., P. A. Delley, A. Schmidt, and M. N. Hall. 1998. Cell wall integrity modulates RHO1 activity via the exchange factor ROM2. EMBO J. 17:2235-2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brenner, C., and R. S. Fuller. 1992. Structural and enzymatic characterization of a purified prohormone-processing enzyme: secreted, soluble Kex2 protease. Proc. Natl. Acad. Sci. USA 89:922-926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buehrer, B. M., and B. Errede. 1997. Coordination of the mating and cell integrity mitogen-activated protein kinase pathways in Saccharomyces cerevisiae. Mol. Cell. Biol. 17:6517-6525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bussink, H. J., and S. A. Osmani. 1999. A mitogen-activated protein kinase (MPKA) is involved in polarized growth in the filamentous fungus, Aspergillus nidulans. FEMS Microbiol. Lett. 173:117-125. [DOI] [PubMed] [Google Scholar]
- 8.Carotti, C., L. Ferrario, C. Roncero, M. H. Valdivieso, A. Duran, and L. Popolo. 2002. Maintenance of cell integrity in the gas1 mutant of Saccharomyces cerevisiae requires the Chs3p-targeting and activation pathway and involves an unusual Chs3p localization. Yeast 19:1113-1124. [DOI] [PubMed] [Google Scholar]
- 9.Chigira, Y., K. Abe, K. Gomi, and T. Nakajima. 2002. chsZ, a gene for a novel class of chitin synthase from Aspergillus oryzae. Curr. Genet. 41:261-267. [DOI] [PubMed] [Google Scholar]
- 10.Christensen, T., H. Woldike, E. Boel, S. B. Mortensen, K. Hjortshoj, L. Thim, and M. T. Hansen. 1988. High level expression of recombinant genes Aspergillus oryzae. Bio/Technology 6:1419-1422. [Google Scholar]
- 11.Davenport, K. R., M. Sohaskey, Y. Kamada, D. E. Levin, and M. C. Gustin. 1995. A second osmosensing signal transduction pathway in yeast. Hypotonic shock activates the PKC1 protein kinase-regulated cell integrity pathway. J. Biol. Chem. 270:30157-30161. [DOI] [PubMed] [Google Scholar]
- 12.de Nobel, H., C. Ruiz, H. Martin, W. Morris, S. Brul, M. Molina, and F. M. Klis. 2000. Cell wall perturbation in yeast results in dual phosphorylation of the Slt2/Mpk1 MAP kinase and in an Slt2-mediated increase in FKS2-lacZ expression, glucanase resistance and thermotolerance. Microbiology 146:2121-2132. [DOI] [PubMed] [Google Scholar]
- 13.Dodou, E., and R. Treisman. 1997. The Saccharomyces cerevisiae MADS-box transcription factor Rlm1 is a target for the Mpk1 mitogen-activated protein kinase pathway. Mol. Cell. Biol. 17:1848-1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Egel-Mitani, M., H. P. Flygenring, and M. T. Hansen. 1990. A novel aspartyl protease allowing KEX2-independent MF alpha propheromone processing in yeast. Yeast 6:127-137. [DOI] [PubMed] [Google Scholar]
- 15.Fuller, R. S., A. Brake, and J. Thorner. 1989. Yeast prohormone processing enzyme (KEX2 gene product) is a Ca2+-dependent serine protease. Proc. Natl. Acad. Sci. USA 86:1434-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuller, R. S., A. J. Brake, and J. Thorner. 1989. Intracellular targeting and structural conservation of a prohormone-processing endoprotease. Science 246:482-486. [DOI] [PubMed] [Google Scholar]
- 17.Furukawa, K., Y. Katsuno, T. Urao, T. Yabe, T. Yamada-Okabe, H. Yamada-Okabe, Y. Yamagata, K. Abe, and T. Nakajima. 2002. Isolation and functional analysis of a gene, tcsB, encoding a transmembrane hybrid-type histidine kinase from Aspergillus nidulans. Appl. Environ. Microbiol. 68:5304-5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia-Rodriguez, L. J., A. Duran, and C. Roncero. 2000. Calcofluor antifungal action depends on chitin and a functional high-osmolarity glycerol response (HOG) pathway: evidence for a physiological role of the Saccharomyces cerevisiae HOG pathway under noninducing conditions. J. Bacteriol. 182:2428-2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gomez-Saladin, E., D. L. Wilson, and I. M. Dickerson. 1994. Isolation and in situ localization of a cDNA encoding a Kex2-like prohormone convertase in the nematode Caenorhabditis elegans. Cell. Mol. Neurobiol. 14:9-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gomi, K., Y. Iimura, and S. Hara. 1987. Integrative transformation of Aspergillus oryzae with a plasmid containing the Aspergillus nidulans argB gene. Agric. Biol. Chem. 51:2549-2555. [Google Scholar]
- 21.Gray, J. V., J. P. Ogas, Y. Kamada, M. Stone, D. E. Levin, and I. Herskowitz. 1997. A role for the Pkc1 MAP kinase pathway of Saccharomyces cerevisiae in bud emergence and identification of a putative upstream regulator. EMBO J. 16:4924-4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han, K. H., and R. A. Prade. 2002. Osmotic stress-coupled maintenance of polar growth in Aspergillus nidulans. Mol. Microbiol. 43:1065-1078. [DOI] [PubMed] [Google Scholar]
- 23.Hanks, S. K., and A. M. Quinn. 1991. Protein kinase catalytic domain sequence database: identification of conserved features of primary structure and classification of family members. Methods Enzymol. 200:38-62. [DOI] [PubMed] [Google Scholar]
- 24.Hata, Y., K. Kitamoto, K. Gomi, C. Kumagai, and G. Tamura. 1992. Functional elements of the promoter region of the Aspergillus oryzae glaA gene encoding glucoamylase. Curr. Genet. 22:85-91. [DOI] [PubMed] [Google Scholar]
- 25.Heinisch, J. J., A. Lorberg, H. P. Schmitz, and J. J. Jacoby. 1999. The protein kinase C-mediated MAP kinase pathway involved in the maintenance of cellular integrity in Saccharomyces cerevisiae. Mol. Microbiol. 32:671-680. [DOI] [PubMed] [Google Scholar]
- 26.Hohmann, S. 2002. Osmotic stress signaling and osmoadaptation in yeasts. Microbiol. Mol. Biol. Rev. 66:300-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ichishima, E. 2000. Unique catalytic and molecular properties of hydrolases from Aspergillus used in Japanese bioindustries. Biosci. Biotechnol. Biochem. 64:675-688. [DOI] [PubMed] [Google Scholar]
- 28.Jacoby, J. J., S. M. Nilius, and J. J. Heinisch. 1998. A screen for upstream components of the yeast protein kinase C signal transduction pathway identifies the product of the SLGI gene. Mol. Gen. Genet. 258:148-155. [DOI] [PubMed] [Google Scholar]
- 29.Jalving, R., P. J. van de Vondervoort, J. Visser, and P. J. Schaap. 2000. Characterization of the kexin-like maturase of Aspergillus niger. Appl. Environ. Microbiol. 66:363-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnston, M. 1987. A model fungal gene regulatory mechanism: the GAL genes of Saccharomyces cerevisiae. Microbiol. Rev. 51:458-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jung, U. S., and D. E. Levin. 1999. Genome-wide analysis of gene expression regulated by the yeast cell wall integrity signalling pathway. Mol. Microbiol. 34:1049-1057. [DOI] [PubMed] [Google Scholar]
- 32.Kamada, Y., U. S. Jung, J. Piotrowski, and D. E. Levin. 1995. The protein kinase C-activated MAP kinase pathway of Saccharomyces cerevisiae mediates a novel aspect of the heat shock response. Genes Dev. 9:1559-1571. [DOI] [PubMed] [Google Scholar]
- 33.Kawasaki, L., O. Sanchez, K. Shiozaki, and J. Aguirre. 2002. SakA MAP kinase is involved in stress signal transduction, sexual development and spore viability in Aspergillus nidulans. Mol. Microbiol. 45:1153-1163. [DOI] [PubMed] [Google Scholar]
- 34.Kelly, R., E. Register, M. J. Hsu, M. Kurtz, and J. Nielsen. 1996. Isolation of a gene involved in 1,3-beta-glucan synthesis in Aspergillus nidulans and purification of the corresponding protein. J. Bacteriol. 178:4381-4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ketela, T., R. Green, and H. Bussey. 1999. Saccharomyces cerevisiae Mid2p is a potential cell wall stress sensor and upstream activator of the PKC1-MPK1 cell integrity pathway. J. Bacteriol. 181:3330-3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Komano, H., and R. S. Fuller. 1995. Shared functions in vivo of a glycosyl-phosphatidylinositol-linked aspartyl protease, Mkc7, and the proprotein processing protease Kex2 in yeast. Proc. Natl. Acad. Sci. USA 92:10752-10756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kubodera, T., N. Yamashita, and A. Nishimura. 2000. Pyrithiamine resistance gene (ptrA) of Aspergillus oryzae: cloning, characterization and application as a dominant selectable marker for transformation. Biosci. Biotechnol. Biochem. 64:1416-1421. [DOI] [PubMed] [Google Scholar]
- 38.Kunihiro, S., Y. Kawanishi, M. Sano, K. Naito, Y. Matsuura, Y. Tateno, T. Gojobori, Y. Yamagata, K. Abe, and M. Machida. 2002. A polymerase chain reaction-based method for cloning novel members of a gene family using a combination of degenerate and inhibitory primers. Gene 289:177-184. [DOI] [PubMed] [Google Scholar]
- 39.Kwon, B. K., K. H. Han, K. Y. Han, S. M. Ju, S. G. Hwang, B. H. Jeon, D. M. Han, and W. S. Kim. 2001. Molecular cloning of kpcA gene encoding a Kex2p-like endoprotease from Aspergillus nidulans. Mol. Cell 12:142-147. [PubMed] [Google Scholar]
- 40.Lagorce, A., N. C. Hauser, D. Labourdette, C. Rodriguez, H. Martin-Yken, J. Arroyo, J. D. Hoheisel, and J. Francois. 2003. Genome-wide analysis of the response to cell wall mutations in the yeast Saccharomyces cerevisiae. J. Biol. Chem. 278:20345-20357. [DOI] [PubMed] [Google Scholar]
- 41.Lee, K. S., K. Irie, Y. Gotoh, Y. Watanabe, H. Araki, E. Nishida, K. Matsumoto, and D. E. Levin. 1993. A yeast mitogen-activated protein kinase homolog (Mpk1p) mediates signalling by protein kinase C. Mol. Cell. Biol. 13:3067-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lipkind, G. M., A. Zhou, and D. F. Steiner. 1998. A model for the structure of the P domains in the subtilisin-like prohormone convertases. Proc. Natl. Acad. Sci. USA 95:7310-7315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Machida, M. 2002. Progress of Aspergillus oryzae genomics. Genomics for fungi. Appl. Microbiol. 51:81-106. [DOI] [PubMed] [Google Scholar]
- 44.Maeda, H., M. Sano, Y. Maruyama, T. Akao, Y. Totsuka, M. Endo, R. Sakurada, Y. Yamagata, M. Machida, O. Akita, F. Hasegawa, K. Abe, K. Gomi, T. Nakajima, and Y. Iguchi. 2004. Transcriptional analysis of genes for energy catabolism and hydrolytic enzymes in the filamentous fungus Aspergillus oryzae using cDNA microarrays and expressed sequence tags (ESTs). Appl. Microbiol. Biotechnol. 65:74-83. [DOI] [PubMed]
- 45.Maeda, H., O. Mizutani, Y. Yamagata, E. Ichishima, and T. Nakajima. 2001. Alkaline-resistance model of subtilisin ALP I, a novel alkaline subtilisin. J. Biochem. 129:675-682. [DOI] [PubMed] [Google Scholar]
- 46.Mizuno, K., T. Nakamura, T. Ohshima, S. Tanaka, and H. Matsuo. 1988. Yeast KEX2 genes encodes an endopeptidase homologous to subtilisin-like serine proteases. Biochem. Biophys. Res. Commun. 156:246-254. [DOI] [PubMed] [Google Scholar]
- 47.Morawetz, R., T. Lendenfeld, H. Mischak, M. Muhlbauer, F. Gruber, J. Goodnight, L. H. de Graaff, J. Visser, J. F. Mushinski, and C. P. Kubicek. 1996. Cloning and characterisation of genes (pkc1 and pkcA) encoding protein kinase C homologues from Trichoderma reesei and Aspergillus niger. Mol. Gen. Genet. 250:17-28. [DOI] [PubMed] [Google Scholar]
- 48.Mouyna, I., M. Monod, T. Fontaine, B. Henrissat, B. Lechenne, and J. P. Latge. 2000. Identification of the catalytic residues of the first family of beta(1-3)glucanosyltransferases identified in fungi. Biochem. J. 347:741-747. [PMC free article] [PubMed] [Google Scholar]
- 49.Mouyna, I., T. Fontaine, M. Vai, M. Monod, W. A. Fonzi, M. Diaquin, L. Popolo, R. P. Hartland, and J. P. Latge. 2000. Glycosylphosphatidylinositol-anchored glucanosyltransferases play an active role in the biosynthesis of the fungal cell wall. J. Biol. Chem. 275:14882-14889. [DOI] [PubMed] [Google Scholar]
- 50.Muller, C., C. M. Hjort, K. Hansen, and J. Nielsen. 2002. Altering the expression of two chitin synthase genes differentially affects the growth and morphology of Aspergillus oryzae. Microbiology 148:4025-4033. [DOI] [PubMed] [Google Scholar]
- 51.Muller, C., K. Hansen, P. Szabo, and J. Nielsen. 2003. Effect of deletion of chitin synthase genes on mycelial morphology and culture viscosity in Aspergillus oryzae. Biotechnol. Bioeng. 81:525-534. [DOI] [PubMed] [Google Scholar]
- 52.Nakajima, K., S. Kunihiro, M. Sano, Y. Zhang, S. Eto, Y. C. Chang, T. Suzuki, Y. Jigami, and M. Machida. 2000. Comprehensive cloning and expression analysis of glycolytic genes from the filamentous fungus, Aspergillus oryzae. Curr. Genet. 37:322-327. [DOI] [PubMed] [Google Scholar]
- 53.Nakamura, K., T. Tomita, N. Abe, and Y. Kamio. 2001. Purification and characterization of an extracellular poly(l-lactic acid) depolymerase from a soil isolate, Amycolatopsis sp. strain K104-1. Appl. Environ. Microbiol. 67:345-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nakayama, K., M. Hosaka, K. Hatsuzawa, and K. Murakami. 1991. Cloning and functional expression of a novel endoprotease involved in prohormone processing at dibasic sites. J. Biochem. 109:803-806. [DOI] [PubMed] [Google Scholar]
- 55.Nakayama, K. 1997. Furin: a mammalian subtilisin/Kex2p-like endoprotease involved in processing of a wide variety of precursor proteins. Biochem. J. 327:625-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Newport, G., A. Kuo, A. Flattery, C. Gill, J. J. Blake, M. B. Kurtz, G. K. Abruzzo, and N. Agabian. 2003. Inactivation of Kex2p diminishes the virulence of Candida albicans. J. Biol. Chem. 278:1713-1720. [DOI] [PubMed] [Google Scholar]
- 57.Parta, M., Y. Chang, S. Rulong, P. Pinto-DaSilva, and K. J. Kwon-Chung. 1994. HYP1, a hydrophobin gene from Aspergillus fumigatus, complements the rodletless phenotype in Aspergillus nidulans. Infect. Immun. 62:4389-4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Posas, F., J. R. Chambers, J. A. Heyman, J. P. Hoeffler, E. de Nadal, and J. Arino. 2000. The transcriptional response of yeast to saline stress. J. Biol. Chem. 275:17249-17255. [DOI] [PubMed] [Google Scholar]
- 59.Posas, F., S. M. Wurgler-Murphy, T. Maeda, E. A. Witten, T. C. Thai, and H. Saito. 1996. Yeast HOG1 MAP kinase cascade is regulated by a multistep phosphorelay mechanism in the SLN1-YPD1-SSK1 “two-component” osmosensor. Cell 86:865-875. [DOI] [PubMed] [Google Scholar]
- 60.Punt, P. J., A. Drint-Kuijvenhoven, B. C. Lokman, J. A. Spencer, D. Jeenes, D. A. Archer, and C. A. van den Hondel. 2003. The role of the Aspergillus niger furin-type protease gene in processing of fungal proproteins and fusion proteins. Evidence for alternative processing of recombinant (fusion) proteins. J. Biotechnol. 106:23-32. [DOI] [PubMed] [Google Scholar]
- 61.Roberts, C. J., B. Nelson, M. J. Marton, R. Stoughton, M. R. Meyer, H. A. Bennett, Y. D. He, H. Dai, W. L. Walker, T. R. Hughes, M. Tyers, C. Boone, and S. H. Friend. 2000. Signaling and circuitry of multiple MAPK pathways revealed by a matrix of global gene expression profiles. Science 287:873-880. [DOI] [PubMed] [Google Scholar]
- 62.Roelants, F. M., P. D. Torrance, N. Bezman, and J. Thorner. 2002. Pkh1 and Pkh2 differentially phosphorylate and activate Ypk1 and Ykr2 and define protein kinase modules required for maintenance of cell wall integrity. Mol. Biol. Cell 13:3005-3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed., vol. 1, Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 64.Schaffner, W., and C. Weissmann. 1973. A rapid, sensitive, and specific method for the determination of protein in dilute solution. Anal. Biochem. 56:502-514. [DOI] [PubMed] [Google Scholar]
- 65.Shennan, K. I., S. P. Smeekens, D. F. Steiner, and K. Docherty. 1991. Characterization of PC2, a mammalian Kex2 homologue, following expression of the cDNA in microinjected Xenopus oocytes. FEBS Lett. 284:277-280. [DOI] [PubMed] [Google Scholar]
- 66.van Zeijl, C. M., E. H. van de Kamp, P. J. Punt, G. C. Selten, B. Hauer, R. F. van Gorcom, and C. A. van den Hondel. 1997. An improved colony-PCR method for filamentous fungi for amplification of PCR-fragments of several kilobases. J. Biotechnol. 59:221-224. [DOI] [PubMed] [Google Scholar]
- 67.Verna, J., A. Lodder, K. Lee, A. Vagts, and R. Ballester. 1997. A family of genes required for maintenance of cell wall integrity and for the stress response in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 94:13804-13809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Watanabe, Y., G. Takaesu, M. Hagiwara, K. Irie, and K. Matsumoto. 1997. Characterization of a serum response factor-like protein in Saccharomyces cerevisiae, Rlm1, which has transcriptional activity regulated by the Mpk1 (Slt2) mitogen-activated protein kinase pathway. Mol. Cell. Biol. 17:2615-2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wilcox, C. A., K. Redding, R. Wright, and R. S. Fuller. 1992. Mutation of a tyrosine localization signal in the cytosolic tail of yeast Kex2 protease disrupts Golgi retention and results in default transport to the vacuole. Mol. Biol. Cell 3:1353-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yamada, O., B. R. Lee, K. Gomi, and Y. Iimura. 1999. Cloning and functional analysis of the Aspergillus oryzae conidiation regulator gene brlA by its disruption and misscheduled expression. J. Biosci. Bioeng. 87:424-429. [DOI] [PubMed] [Google Scholar]
- 71.Yaver, D. S., M. Lamsa, R. Munds, S. H. Brown, S. Otani, L. Franssen, J. A. Johnstone, and H. Brody. 2000. Using DNA-tagged mutagenesis to improve heterologous protein production in Aspergillus oryzae. Fungal Genet. Biol. 29:28-37. [DOI] [PubMed] [Google Scholar]
- 72.Zarzov, P., C. Mazzoni, and C. Mann. 1996. The SLT2(MPK1) MAP kinase is activated during periods of polarized cell growth in yeast. EMBO J. 15:83-91. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.