Abstract
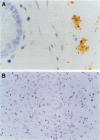
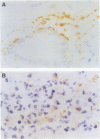
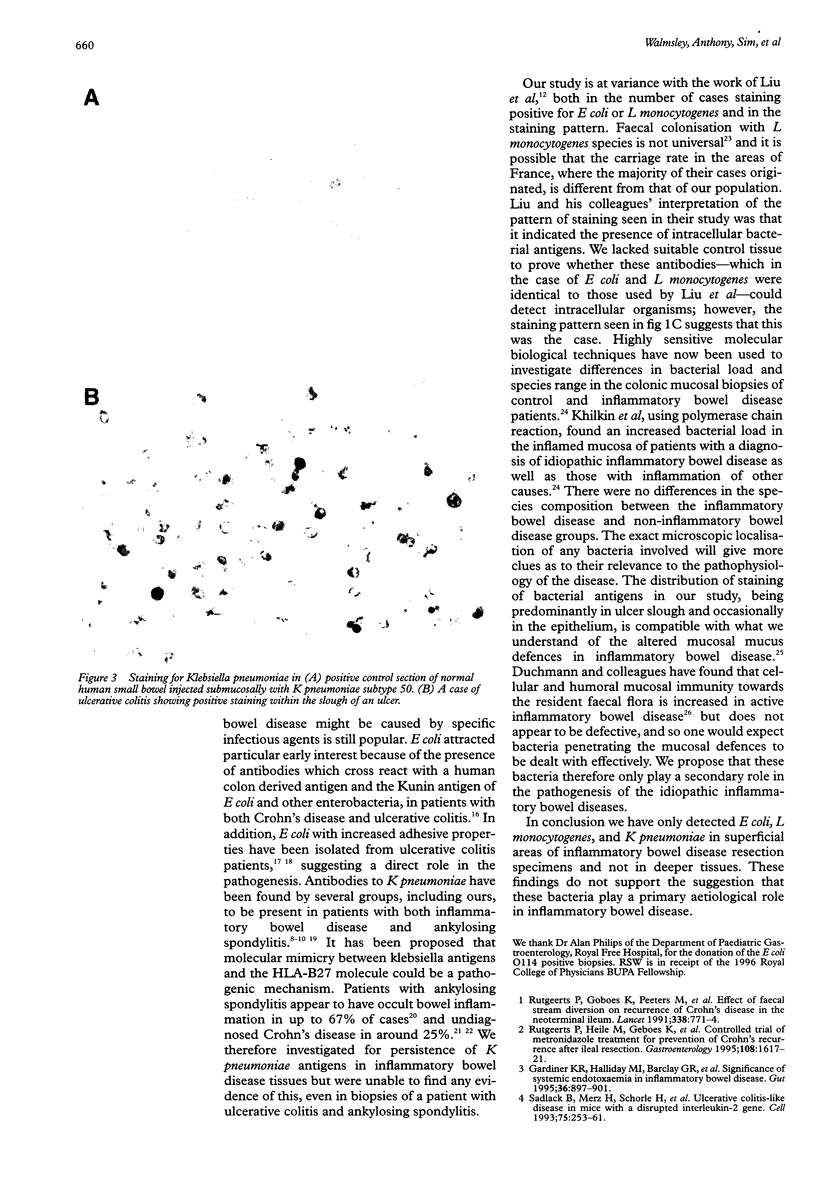
BACKGROUND: Escherichia coli, listeria, and streptococcal antigens have been found in Crohn's disease tissues. Antibodies to Klebsiella pneumoniae have been found in patients with inflammatory bowel disease and ankylosing spondylitis. The presence of these bacterial antigens in Crohn's granulomas would be of aetiological interest, while their presence in ulcers alone would be more likely to indicate secondary infection. AIM: To investigate inflammatory bowel disease tissues for the presence of these bacteria. METHODS: Formalin fixed, paraffin processed sections from 53 patients (19 ulcerative colitis, 23 Crohn's disease; 11 normal tissues from cancer resections) were studied by immunohistochemistry. Control tissue consisted of normal human small bowel injected submucosally with either E coli, Listeria monocytogenes, Proteus mirabilis, or Klebsiella pneumoniae serotypes K2, 3, 17, 21, 26, 36, and 50, and colonic biopsies from a child with E coli 0114 infection. Tissues were stained by Gram-Twort, and with specific antibodies for E coli (Dako B357), L monocytogenes (Difco 2302-50), and K pneumoniae (Biogenesis 5580-5208) using an immunoperoxidase technique. RESULTS: Positive staining for E coli was observed on the luminal surface epithelium and in ulcers in 35% of Crohn's disease patients, 26% of ulcerative colitis patients, and no normal controls. Superficial staining for L monocytogenes was observed in one case of ulcerative colitis only. Staining for K pneumoniae was observed in one case of ulcerative colitis and one of Crohn's disease. No granulomas, giant cells, or germinal centres stained positively for any of the three bacterial antigens. CONCLUSIONS: These data do not support a primary role for E coli, L monocytogenes, and K pneumoniae in inflammatory bowel disease. The presence of E coli antigens in ulcers suggests secondary infection in these lesions.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auer I. O., Röder A., Wensinck F., van de Merwe J. P., Schmidt H. Selected bacterial antibodies in Crohn's disease and ulcerative colitis. Scand J Gastroenterol. 1983 Mar;18(2):217–223. doi: 10.3109/00365528309181586. [DOI] [PubMed] [Google Scholar]
- Burke D. A., Axon A. T. Ulcerative colitis and Escherichia coli with adhesive properties. J Clin Pathol. 1987 Jul;40(7):782–786. doi: 10.1136/jcp.40.7.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper R., Fraser S. M., Sturrock R. D., Gemmell C. G. Raised titres of anti-klebsiella IgA in ankylosing spondylitis, rheumatoid arthritis, and inflammatory bowel disease. Br Med J (Clin Res Ed) 1988 May 21;296(6634):1432–1434. doi: 10.1136/bmj.296.6634.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vos M., Mielants H., Cuvelier C., Elewaut A., Veys E. Long-term evolution of gut inflammation in patients with spondyloarthropathy. Gastroenterology. 1996 Jun;110(6):1696–1703. doi: 10.1053/gast.1996.v110.pm8964393. [DOI] [PubMed] [Google Scholar]
- Dickinson R. J., Varian S. A., Axon A. T., Cooke E. M. Increased incidence of faecal coliforms with in vitro adhesive and invasive properties in patients with ulcerative colitis. Gut. 1980 Sep;21(9):787–792. doi: 10.1136/gut.21.9.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchmann R., Kaiser I., Hermann E., Mayet W., Ewe K., Meyer zum Büschenfelde K. H. Tolerance exists towards resident intestinal flora but is broken in active inflammatory bowel disease (IBD) Clin Exp Immunol. 1995 Dec;102(3):448–455. doi: 10.1111/j.1365-2249.1995.tb03836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner K. R., Halliday M. I., Barclay G. R., Milne L., Brown D., Stephens S., Maxwell R. J., Rowlands B. J. Significance of systemic endotoxaemia in inflammatory bowel disease. Gut. 1995 Jun;36(6):897–901. doi: 10.1136/gut.36.6.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gent A. E., Hellier M. D., Grace R. H., Swarbrick E. T., Coggon D. Inflammatory bowel disease and domestic hygiene in infancy. Lancet. 1994 Mar 26;343(8900):766–767. doi: 10.1016/s0140-6736(94)91841-4. [DOI] [PubMed] [Google Scholar]
- Ibbotson J. P., Pease P. E., Allan R. N. Serological studies in Crohn's disease. Eur J Clin Microbiol. 1987 Jun;6(3):286–290. doi: 10.1007/BF02017614. [DOI] [PubMed] [Google Scholar]
- Kühn R., Löhler J., Rennick D., Rajewsky K., Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993 Oct 22;75(2):263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- Lagercrantz R., Hammarström S., Perlmann P., Gustafsson B. E. Immunological studies in ulcerative colitis. IV. Origin of autoantibodies. J Exp Med. 1968 Dec 1;128(6):1339–1352. doi: 10.1084/jem.128.6.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leirisalo-Repo M., Turunen U., Stenman S., Helenius P., Seppälä K. High frequency of silent inflammatory bowel disease in spondylarthropathy. Arthritis Rheum. 1994 Jan;37(1):23–31. doi: 10.1002/art.1780370105. [DOI] [PubMed] [Google Scholar]
- Liu Y., van Kruiningen H. J., West A. B., Cartun R. W., Cortot A., Colombel J. F. Immunocytochemical evidence of Listeria, Escherichia coli, and Streptococcus antigens in Crohn's disease. Gastroenterology. 1995 May;108(5):1396–1404. doi: 10.1016/0016-5085(95)90687-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielants H., Veys E. M., Cuvelier C., de Vos M. Ileocolonoscopic findings in seronegative spondylarthropathies. Br J Rheumatol. 1988;27 (Suppl 2):95–105. doi: 10.1093/rheumatology/xxvii.suppl_2.95. [DOI] [PubMed] [Google Scholar]
- Müller H. E. Listeria isolations from feces of patients with diarrhea and from healthy food handlers. Infection. 1990 Mar-Apr;18(2):97–99. doi: 10.1007/BF01641423. [DOI] [PubMed] [Google Scholar]
- O'Mahony S., Anderson N., Nuki G., Ferguson A. Systemic and mucosal antibodies to Klebsiella in patients with ankylosing spondylitis and Crohn's disease. Ann Rheum Dis. 1992 Dec;51(12):1296–1300. doi: 10.1136/ard.51.12.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes J. M. Mucins and inflammatory bowel disease. QJM. 1997 Feb;90(2):79–82. doi: 10.1093/qjmed/90.2.79. [DOI] [PubMed] [Google Scholar]
- Rutgeerts P., Goboes K., Peeters M., Hiele M., Penninckx F., Aerts R., Kerremans R., Vantrappen G. Effect of faecal stream diversion on recurrence of Crohn's disease in the neoterminal ileum. Lancet. 1991 Sep 28;338(8770):771–774. doi: 10.1016/0140-6736(91)90663-a. [DOI] [PubMed] [Google Scholar]
- Rutgeerts P., Hiele M., Geboes K., Peeters M., Penninckx F., Aerts R., Kerremans R. Controlled trial of metronidazole treatment for prevention of Crohn's recurrence after ileal resection. Gastroenterology. 1995 Jun;108(6):1617–1621. doi: 10.1016/0016-5085(95)90121-3. [DOI] [PubMed] [Google Scholar]
- Sadlack B., Merz H., Schorle H., Schimpl A., Feller A. C., Horak I. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 1993 Oct 22;75(2):253–261. doi: 10.1016/0092-8674(93)80067-o. [DOI] [PubMed] [Google Scholar]
- Satsangi J., Welsh K. I., Bunce M., Julier C., Farrant J. M., Bell J. I., Jewell D. P. Contribution of genes of the major histocompatibility complex to susceptibility and disease phenotype in inflammatory bowel disease. Lancet. 1996 May 4;347(9010):1212–1217. doi: 10.1016/s0140-6736(96)90734-5. [DOI] [PubMed] [Google Scholar]
- Tiwana H., Wilson C., Walmsley R. S., Wakefield A. J., Smith M. S., Cox N. L., Hudson M. J., Ebringer A. Antibody responses to gut bacteria in ankylosing spondylitis, rheumatoid arthritis, Crohn's disease and ulcerative colitis. Rheumatol Int. 1997;17(1):11–16. doi: 10.1007/pl00006845. [DOI] [PubMed] [Google Scholar]
- Wensinck F., Van de Merwe J. P. Serum agglutinins to Eubacterium and Peptostreptococcus species in Crohn's and other diseases. J Hyg (Lond) 1981 Aug;87(1):13–24. doi: 10.1017/s0022172400069199. [DOI] [PMC free article] [PubMed] [Google Scholar]