Abstract
Background: The aim of this study was to evaluate the effects of targeted silencing of forkhead box C1 (FOXC1) gene with small interfering RNA (siRNA) on the proliferation and in vitro migration of human non-small-cell lung carcinoma (NSCLC) A549 and NCIH460 cells, and to explore the molecular mechanism. Methods: These cells were divided into FOXC1 siRNA groups and negative control groups. Results: Quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR) showed that compared with normal cells and paracancerous tissues, FOXC1 mRNA expressions in NSCLC cells and tissues were significantly higher (P<0.05). qRT-PCR and Western blot showed that FOXC1 siRNA effectively silenced FOXC1 gene expression in NSCLC cells. EdU labeling assay revealed that the proliferative capacity significantly decreased compared with that of normal control group after FOXC1 silencing (P<0.05). Significantly fewer cells in the transfected group migrated than those in negative control group did. After FOXC1 silencing, NSCLC cells were arrested in the G0/G1 phase, which were significantly different from those in negative control group (P<0.05). Compared with negative control group, the expression of cyclin D1 decreased and that of E-cadherin increased. Meanwhile, vimentin and MMP-2 expressions significantly reduced (P<0.05). FOXC1 siRNA effectively silenced FOXC1 gene expressions in NSCLC cells, inhibited their proliferation and invasion, and arrested them in the G0/G1 phase, suggesting that FOXC1 affected proliferation probably by regulating the expression of cell cycle-related protein cyclin D1. Conclusion: Silencing FOXC1 may evidently inhibit the migration of these cells by reversing the EMT process through suppressing cadherin, being associated with the expressions of extracellular MMPs.
Keywords: Forkhead box C1 gene, proliferation, migration, non-small-cell lung carcinoma, silencing
Introduction
Non-small-cell lung carcinoma (NSCLC), which accounts for 80% of all lung cancers, is the most common type. Due to the lack of effective screening or diagnosis protocols, 80% of NSCLC patients are diagnosed in the advanced stage, losing the optimum treatment timing [1]. NSCLC is lowly sensitive to traditional radiotherapy or chemotherapy, with its onset, progression and metastasis closely associated with angiogenesis. Therefore, NSCLC has been clinically treated by resisting angiogenesis. Tumor cells are biologically typified by abnormal proliferation as an important parameter for determining the malignant phenotypes of cancers. Abnormal proliferation of tumor cells is closely related to their invasion and metastasis as well as cancer relapses. Thus, inhibition of tumor proliferation has recently been highlighted.
Forkhead box C1 (FOXC1), as a member of the FOX family, plays crucial roles in cellular biological processes, embryonic development and tumor onset and progression (e.g. breast cancer [2,3] and gastric cancer [4]). However, its role in NSCLC has seldom been studied. Herein, we detected FOXC1 gene expression by quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR) and silenced it in NSCLC cells with small interfering RNA (siRNA). The effects of such silencing on cell proliferation and in vitro proliferation were assessed, and the possible molecular mechanism was explored, aiming to provide experimental evidence for the genetic diagnosis of NSCLC.
Materials and methods
Patient materials
Primary NSCLC tissues and corresponding paracancerous tissues (n=18) were collected from the patients who were surgically treated in our hospital. All samples were confirmed by histological examination. This study has been approved by the ethics committee of our hospital.
Cell culture
Human NSCLC cell lines A549 and NCIH460 as well as primary human lung epithelial cells BEAS-2B and MRC-5 were purchased from China Center for Type Culture Collection. A549 and NCIH460 were routinely cultured, while BEAS-2B and MRC-5 cells were cultured in CS-C culture medium containing 10% FBS [5,6]. They were cultured at 37°C in 50 mL/L CO2. The culture medium was refreshed 2-3 d for further culture or subculture.
Detection of FOXC1 mRNA expressions in NSCLC cells and tissues by qRT-PCR
According to a previous literature [7], total RNA was extracted from A549 and NCIH460 cells and 18 pairs of primary NSCLC tissues by the TRIzol method. Residual DNA was removed using DNA-free DNase (Ambion, Austin, TX). RNAs were reverse transcribed into cDNA using Moloney murine leukemia virus reverse transcriptase (Invitrogen, Carlsbad, CA). FOXC1 mRNA expression was detected according to the instructions of PCR-related SYBR Premix reagent together with FOXC1 upstream and downstream primers (Sigma-Aldrich, St Louis, MO). PCR was performed by a two-step method on a BIO-RED PCR system. After the reaction, amplification and melting curves were plotted. Relative expression of FOXC1 mRNA was calculated by the 2-ΔΔCt method: ΔCt=Cttarget gene-Ct18S rRNA. 2-ΔΔCt represents relative expression of target gene.
FOXC1 siRNA transfection
A549 and NCI-H460 cells in the logarithmic growth phase were digested by trypsin, resuspended in 10% FBS solution and inoculated into 6-well plates at a density of 1×105/well in 2 ml of complete culture medium. After 24 h, the cells were transfected by using DharmaFECT 2 (Thermo Fisher Scientific, Lafayette, CO) with a siRNA pool containing 4 siRNAs targeting FOXC1 (Thermo Fisher Scientific) or with a pool of 4 non-targeting siRNAs (Thermo Fisher Scientific) at a final concentration of 25 nM according to the manufacturer’s instructions. After 48 h, fresh medium was added or the cells were seeded to the following experiments. The silencing efficiency of FOXC1 was verified by qRT-PCR and Western blot.
For the knockdown and knockdown-resistance experiments, cells were first transfected with FOXC1 siRNA pool plasmids. After 4 h, cells were transfected with either FOXC1-resistant plasmids (Thermo Fisher Scientific) or vector control of resistance plasmids (Thermo Fisher Scientific) at a final concentration of 20 nM according to the manufacturer’s instructions. After 48 h, fresh medium was added or the cells were seeded to the following experiments.
Cell proliferation assay
As a synthetic analog to thymidine, EdU can penetrate DNA molecules undergoing synthesis during replication, linking an alkynyl group that is scarce in natural compounds. EdU specifically reacts with fluorescent dye, based on which cellular DNA replication can be directly observed to clarify proliferation changes [8,9]. According to EdU kit instruction (Santa Cruz, CA), NC siRNA and FOXC1 siRNA groups were inoculated into confocal dishes respectively, incubated with 100 μL of 50 μmol/L EdU solution for 2 h, fixed in 4% paraformaldehyde, decolorized with 50 μL of 2 mg/mL glycine solution, observed under LSM710 laser scanning confocal microscope (Japan) and photographed. After the images were merged, the ratios of Apollo staining-positive cells to Hoechst staining-positive ones were calculated.
Analysis of cell cycle
NSCLC cells (30-40×105) were plated on 60 mm culture dishes (BD Biosciences, San Jose, CA, USA). Cells were harvested by trypsin in the logarithmic growth phase, centrifuged at room temperature and 1000 r/min for 5 min, washed twice by 4°C PBS, resuspended by adding 200 μL of PBS, slowly dropped 1.5 mL of pre-cooled ethanol and fixed overnight at -20°C. On the next day, the cells were centrifuged at 1000 r/min for 5 min at 4°C, washed twice by 4°C PBS, resuspended by adding 500 μL of PBS, and reacted with 50 μg/mL RNAase and propidium iodide in dark at room temperature for 30 min. Single cell suspension was obtained with a 300 mesh screen, and cell cycle distribution was detected by flow cytometry. The results from 10,000 cells were analyzed with CellQuest and ModFit. Three replicate wells were set for each group.
Cell migration assay
Cells were transfected in 6-well plates, collected 48 h later, counted and adjusted to the density of 1×106/ml. The outer surface of Transwell chamber membrane was coated with 10 μg fibronectin and air-dried. The chambers were put in 24-well plates containing α-MEM medium with 0.1% bovine serum albumin. Then 100 μl of cell suspension was added into each chamber and cultured at 37°C in 5% CO2 for 6 h. Afterwards, the chambers were taken out, and the membrane was fixed in methanol for 1 min and subjected to HE staining, from which the unpenetrating cells were wiped off. The sections were thereafter sealed. The cells penetrating the membrane were counted under a light microscope (×400). Five visual fields were randomly selected for each membrane, and four samples were set for each group.
Western blot
The expressions of P21, P53, cyclin D1, E-cadherin, vimentin and MMP-2 proteins in NSCLC cell growth-related pathways were detected by Western blot. After silencing FOXC1, total protein was extracted by lysis buffer (Thermo Fisher Scientific), and concentration was detected by the BCA method (Thermo Fisher Scientific). Subsequently, 50 μg of the protein was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electronically transferred to a nitrocellulose membrane that was then blocked in 5% skimmed milk, incubated overnight with diluted primary antibodies for FOXC1 (1:1000, Abcam, Cambridge, MA) P21 (1:1200, Santa-Cruz, CA), P53 (1:1000, Santa-Cruz, CA), cyclin D1 (1:900, Santa-Cruz, CA), E-cadherin (1:1200, Santa-Cruz, CA), vimentin (1:1100, Santa-Cruz, CA), MMP-2 (1:1000, Santa-Cruz, CA) and GAPDH (1:1000, Abcam) at 4°C, washed three times with TBST (15 min each time) and finally incubated with horseradish peroxidase (HRP)-labeled goat anti-human IgG (Santa-Cruz, CA) at room temperature for 1 h. Then the membrane was washed three times with TBST (15 min each time), reacted with ECL reagent, exposed and imaged, with the gray values analyzed by Image J. GAPDH (1:500, Santa-Cruz, CA) was used as the internal reference.
Statistical analysis
All experimental data were analyzed by SPSS14.0 and expressed as x̅±s. Inter-group comparisons were performed by using t test. P<0.05 was considered statistically significant. All the experiments were conducted in triplicate.
Results
FOXC1 mRNA levels were significantly up-regulated in human NSCLC cells and tissues
Based on our preliminary gene array studies from NSCLC tissues and normal lung tissues, we found that FOXC1 but not FOXC2 was up-regulated in NSCLC tissues compared to normal lung tissues. qRT-PCR analysis revealed that FOXC1 mRNA level in NSCLC tissues is significantly upregulated than that normal lung tissues (Figure 1A). FOXC1 mRNA levels were also analyzed by qRT-PCR in NSCLC cells (A549 and NCI-H460) and normal lung epithelial cells. qRT-PCR results showed that FOXC1 mRNA level in NSCLC cells was significantly higher than that in normal lung epithelial cells (Figure 1B).
Figure 1.
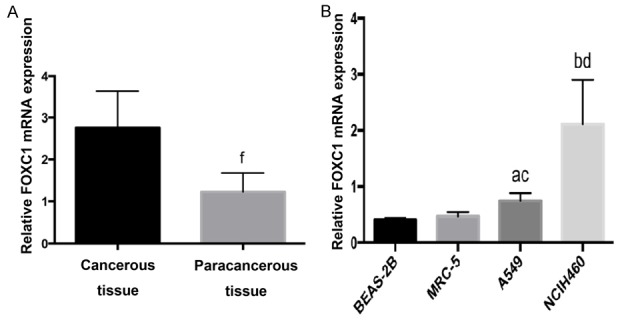
FOXC1 mRNA expressions in human NSCLC cells and normal lung tissues. A: FOXC1 mRNA expressions in normal lung epithelial cells and NSCLC A549 and NCIH460 cells; B: FOXC1 mRNA expressions in 18 pairs of NSCLC and paracancerous tissues. aP<0.05, bP<0.01 vs. BEAS-2B; cP<0.05, dP<0.01 vs. MRC-5; fP<0.01 vs. NSCLC tissues.
FOXC1 silencing inhibited cell proliferation
To assess the role of FOXC1 in cell proliferation, we silenced FOXC1 using a FOXC1 siRNA. After 48 h of transfection with FOXC1 siRNA, FOXC1 mRNA levels in A549 and NCI-H460 cells were reduced by 53±3.5% and 63±4.8% respectively (P<0.05, Figure 2). In the meantime, FOXC1 siRNA significantly reduced the expressions of FOXC1 protein in A549 and NCI-H460 cells. ImageJ calculation revealed that FOXC1 protein expressions in A549 and NCI-H460 cells decreased by 72.56±2.8% and 78.23±3.1% respectively for 3 independent replicates (P<0.05, Figure 3).
Figure 2.
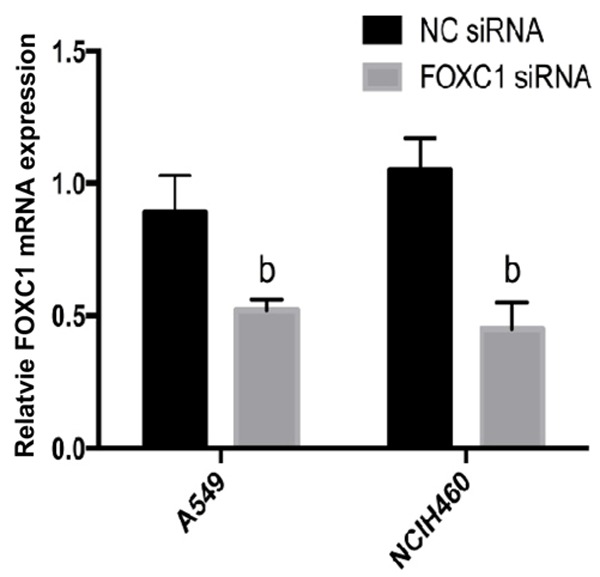
FOXC1 mRNA expressions after FOXC1 siRNA transfection detected by qRT-PCR. bP<0.05 vs. NC siRNA.
Figure 3.
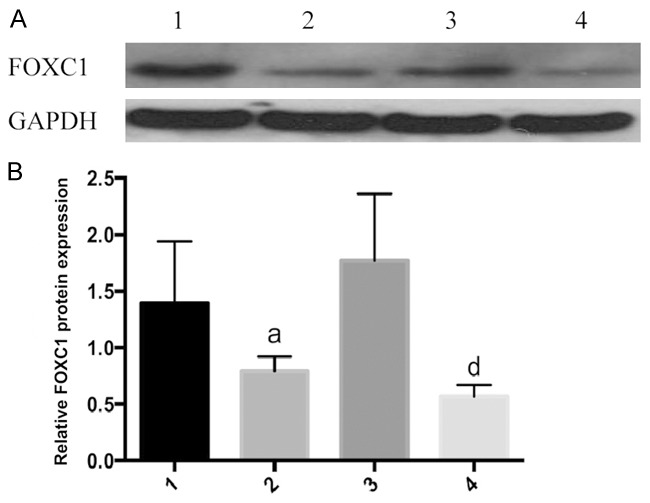
FOXC1 protein expressions after FOXC1 siRNA transfection detected by Western blot. A: Western blot results; B: FOXC1 protein expressions, 1: A549 negative control group; 2: A549 FOXC1 siRNA group; 3: NCIH460 negative control group; 4: NCIH460 FOXC1 siRNA group. aP<0.05 vs. A549 negative control group; dP<0.01 vs. NCIH460 negative control group.
After FOXC1 silencing, the proliferation of A549 and NCI-H460 cells decreased by 38.23±5.6% and 42.17±3.7% respectively compared to that of the NC group by calculating the ratios of Apollo staining-positive cells to Hoechst staining-positive ones (P<0.05, Figure 4).
Figure 4.
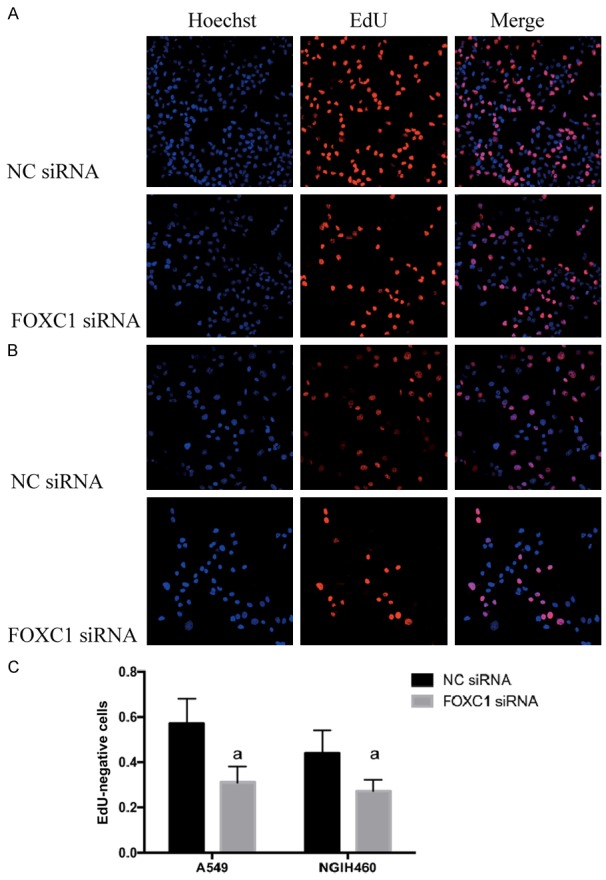
Proliferation of NSCLC cells after FOXC1 gene silencing detected by EdU labeling. A: A549 cells; B: NCIH460 cells; C: quantitative results of three replicate experiments. aP<0.05 vs. NC siRNA. Red: Apollo staining-positive, proliferative cells; blue: Hoechst staining-positive, all cells.
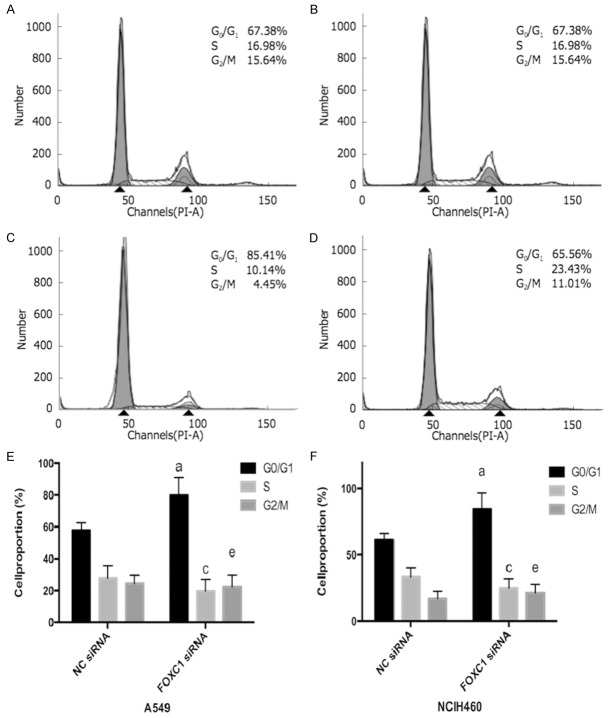
FOXC1 silencing increased G0/G1 phase arrest
As shown in Figure 5, FOXC1 silencing induces more G0/G1 phase arrest and reduces S and G2/M phases in A549 and NCI-H460 cells compared to related NC group.
Figure 5.
NSCLC cell cycle distribution after FOXC1 gene silencing detected by flow cytometry. A: A549 negative control group; B: A549 FOXC1 siRNA group; C: NCIH460 negative control group; D: NCIH460 FOXC1 siRNA group; E: A549 cell cycle distribution; F: NCIH460 cell cycle distribution. aP<0.05 vs. NC siRNA G0/G1 phase; cP<0.05 vs. NC siRNA S phase; eP<0.05 vs. NC siRNA G2/M phase.
FOXC1 silencing inhibited cell migration
Cell migration potential was examined using a Boyden chamber. Microscopy images of the Boyden chamber assay are shown in Figure 6. The migrations of A549 and NCI-H460 cells in FOXC1 siRNA groups were inhibited by (52.71±4.3)% and (61.22±3.7)% compared with related NC groups, respectively. Accordingly, the migrations of A549 and NCI-H460 cells were both significantly decreased after FOXC1 silencing (P<0.05).
Figure 6.
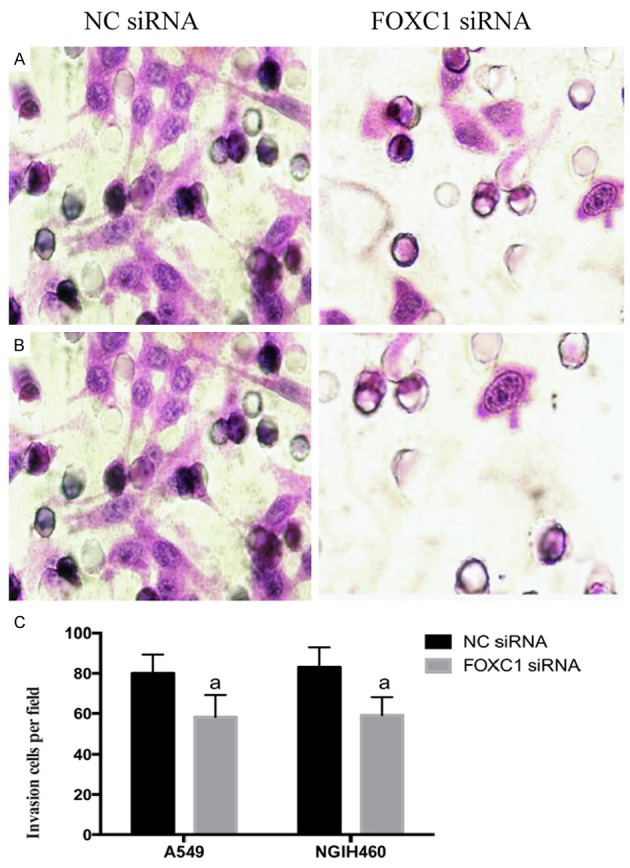
Effects of FOXC1 gene silencing on the in vitro migration of NSCLC cells (HE staining, ×400). A: A549 cells; B: NCIH460 cells; C: Quantitative results of three replicate experiments. aP<0.05 vs. NC siRNA.
Effects of FOXC1 gene silencing on P21, P53, cyclin D1, vimentin, MMP-2 and E-cadherin proteins related to NSCLC cell proliferation and migration
To clarify the mechanism by which FOXC1 gene silencing suppressed the proliferation, endogenous protein expressions of P21, P53 and cyclin D1 in NSCLC cells were detected by Western blot. Compared with NC groups, cyclin D1 protein expressions in FOXC1 siRNA groups reduced by 86.23±1.5% and 52.71±2.6% in A549 and NCI-H460 cells respectively (Figure 6). However, there were no changes in protein expressions of P21 and P53 after FOXC1 silencing. Meanwhile, vimentin and MMP-2 levels dropped by 71.03±3.8%, 53.29±2.5% and 69.22±4.2%, 55.22±3.7% in A549 and NCI-H460 cells compared to related NC groups, respectively (Figures 7, 8). However, the protein expressions of E-cadherin significantly increased by 55.12±1.9% and 54.23±2.4% in A549 and NCI-H460 cells in comparison with related NC groups, respectively.
Figure 7.
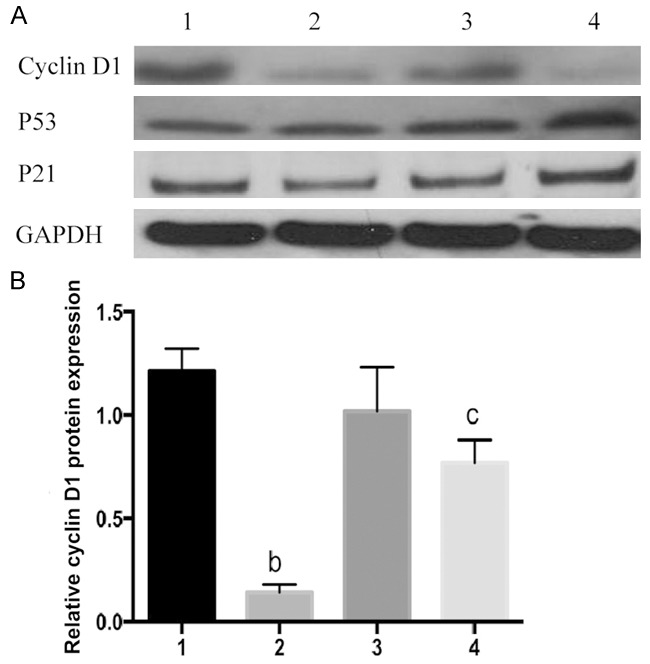
Effects of FOXC1 gene silencing on proteins related to cell cycle. A: Western blot results; B: Cyclin D1 protein expressions, 1: A549 negative control group; 2: A549 FOXC1 siRNA group; 3: NCIH460 negative control group; 4: NCIH460 FOXC1 siRNA group. bP<0.01 vs. A549 negative control group; cP<0.05 vs. NCIH460 negative control group.
Figure 8.
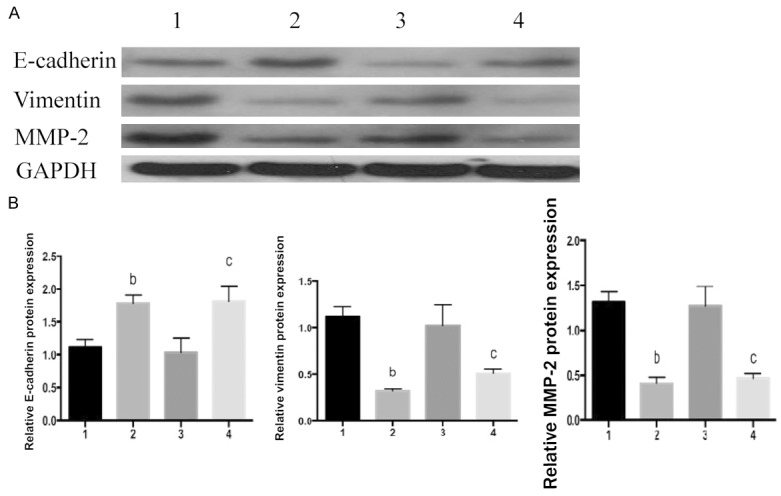
Effects of FOXC1 gene silencing on proteins related to cell migration. A: Western blot results; B: E-cadherin, vimentin and MMP-2 protein expressions, 1: A549 negative control group; 2: A549 FOXC1 siRNA group; 3: NCIH460 negative control group; 4: NCIH460 FOXC1 siRNA group. bP<0.01 vs. A549 negative control group; cP<0.05 vs. NCIH460 negative control group.
To further confirm mechanistic relation between FOXC1 and the down-stream molecules (P21, P53, cyclin D1, vimentin, MMP-2 and E-cadherin), we knocked down endogenous FOXC1 from A549 and NCI-H460 cells by siRNA and reintroduced it by transfecting siRNA-resistant plasmids or vector control plasmids (VC) with mutations in all the FOXC1-binding sites. After siRNA-resistant FOXC1 was reintroduced, cyclin D, vimentin and MMP-2 protein expressions were fully restored (Figure 9) and E-cadherin was completely depleted compared with VC groups, which was able to reverse the pattern with FOXC1 gene silencing. However, reintroduction of siRNA-resistant FOXC1 had no effect on either P21 or P53. These results indicate that cyclin D1, vimentin, MMP-2 and E-cadherin but not P21 or P53 are involved in the FOXC1 regulatory network.
Figure 9.
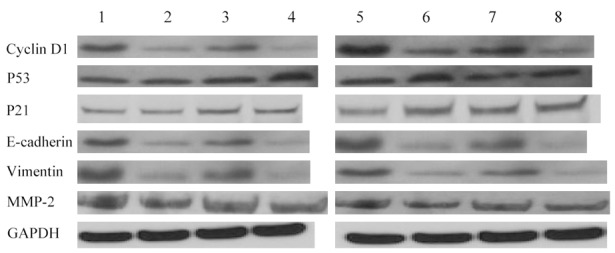
siRNA-resistant versions of FOXC1 proteins reverse FOXC1 gene silencing pattern of proteins related to cell cycle. Western blot analysis of cyclin D1, P21, P53, E-cadherin, vimentin and MMP-2 in lysates of A549 and NCI-H460 cells with transfection of mixtures of contained plasmids encoding siRNA-resistant versions of all FOXC1 binding sites or siRNA-resistant vector control as indicated. 1: A549 NC group; 2: A549 FOXC1 siRNA group; 3: A549 FOXC1 siRNA-resistant group; 4: A549 vector control siRNA-resistant group; 5: NCI-H460 group; 6: NCI-H460 FOXC1 siRNA group; 7: NCI-H460 vector control siRNA-resistant group; 8: NCI-H460 vector control siRNA-resistant group.
Discussion
Transcription factor FOXC1 belongs to the FOXC subfamily that includes two members (i.e. FOXC1 and FOXC2). This gene is located on chromosome 6p25 with the whole length of 3500 bp, only containing one 1600 bp exon. With 553 amino acid residues, its encoded protein, from the N terminal to the C terminal, consists of active domain 1, forkhead domain and active domain 2 in sequence [10]. There are two nuclear localization signals at both terminals of the forkhead domain, with a typical motif at the C terminal. FOXC1 has mainly been associated with Axenfeld-Rieger syndrome and embryo development [11-14]. Recently, it has been proved to play important roles in cancer onset and progression. Hayashi et al. [15] found that the interactions between FOXC1 and Notch signals and vascular endothelial growth factor regulated the expressions of vascular genes and induced tumor angiogenesis in multi-steps. Ray et al. [3] reported that the intracranial-free survival was significantly shortened in patients with high FOXC1 mRNA expressions. Besides, ectropic FOXC1 expression enhanced tumor invasion such as the EMT process during which tumors cells in situ undergo distal migration and invasion [16,17]. After endogenous FOXC1 expression in basal-like breast cancer cells was knocked down by shRNA, they failed to invade.
Comparatively, the role of FOXC1 gene in NSCLC remains largely unknown. First, we detected the expressions of FOXC1 gene in NSCLC tissues and cells by qRT-PCR. Since this gene was highly expressed in primary NSCLC tissues and cells, it may be related to the onset and progression of this cancer. Subsequently, we found that the proliferation of NSCLC cells was significantly inhibited after FOXC1 gene expression was silenced by siRNA technique. Proliferation is closely associated with cell cycle [18-20]. Like normal cells, tumor cells undergo G0, G1, S, G2 and M phases successively. DNA is synthesized in the S phase and cells divide in the M phase. G1-S phase transition is the key point of the entire cell cycle, which, when disrupted by external factors, may inhibit the proliferation of tumor cells [21,22]. In this study, NSCLC cells were arrested in the G0/G1 phase after FOXC1 gene expression was silenced by siRNA. Western blot showed that the expression of cell cycle-related protein cyclin D1 was significantly suppressed. As a member of cell cycle-related protein, cyclin D1 is mainly responsible for G1-S phase transition, also known as an oncogene [23-25]. Therefore, FOXC1 affected the proliferation of NSCLC cells probably by regulating cyclin D1.
EMT predominantly controls tumor cell migration [26,27], which is mainly typified by phenotype changes, including 1) decrease or depletion of epithelial phenotypes (e.g. E-cadherin) [28], and 2) overexpression of mesenchymal phenotypes (e.g. vimentin, MMP and TIMPs) [29,30]. To clarify the influence of FOXC1 expression silencing on the metastasis of NSCLC cells and the molecular mechanism, we examined the changes in metastatic capability as well as expression levels of EMT-related marker protein and MMP-2. Significantly more cells in the FOXC1-siRNA group penetrated the basal membrane of chambers than those in the control group, suggesting that silencing FOXC1 gene in NSCLC cells significantly inhibited their migration and invasion in vitro. Additionally, silencing FOXC1 expression in A549 and NCIH460 cells significantly reduced those of vimentin and MMP-2, whereas E-cadherin expression was significantly elevated. It is well-documented that down-regulating E-cadherin expression and function can induce EMT [31].
To reach the conclusion that suppressing the promoter of E-cadherin gene, FOXC1 can substantially promote tumor invasion and metastasis, and maintain the normal proliferation and differentiation of cells, other transcription factors which are known to control EMT gene transcription, such as Snail, Slug, ZEB and Twist, will be examined. Therefore, our results leave us with an interesting direction to follow in future studies. Additionally, FOXC1 has no effect on P21 or P53 protein expression, suggesting that P21 or P53 is not involved in the FOXC1 regulatory network.
In conclusion, FOXC1 functioned in NSCLC cells as an oncogene, which enhanced their proliferation and migration by regulating cell cycle-related protein cyclin D1 and inhibiting associated migration proteins. All these results suggest that FOXC1 has potentially important value in the genotherapy and biological targeted therapy of NSCLC.
Disclosure of conflict of interest
None.
References
- 1.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, Campos D, Maoleekoonpiroj S, Smylie M, Martins R, van Kooten M, Dediu M, Findlay B, Tu D, Johnston D, Bezjak A, Clark G, Santabárbara P, Seymour L. Erlotinib in previously treated non-small-cell lung cancer. New Engl J Med. 2005;353:123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 2.Ray PS, Bagaria SP, Wang J, Shamonki JM, Ye X, Sim MS, Steen S, Qu Y, Cui X, Giuliano AE. Basal-like breast cancer defined by FOXC1 expression offers superior prognostic value: a retrospective immunohistochemical study. Ann Surg Oncol. 2011;18:3839–3847. doi: 10.1245/s10434-011-1657-8. [DOI] [PubMed] [Google Scholar]
- 3.Ray PS, Wang J, Qu Y, Sim MS, Shamonki J, Bagaria SP, Ye X, Liu B, Elashoff D, Hoon DS, Walter MA, Martens JW, Richardson AL, Giuliano AE, Cui X. FOXC1 is a potential prognostic biomarker with functional significance in basal-like breast cancer. Cancer Res. 2010;70:3870–3876. doi: 10.1158/0008-5472.CAN-09-4120. [DOI] [PubMed] [Google Scholar]
- 4.Xu Y, Shao QS, Yao HB, Jin Y, Ma YY, Jia LH. Overexpression of FOXC1 correlates with poor prognosis in gastric cancer patients. Histopathology. 2014;64:963–970. doi: 10.1111/his.12347. [DOI] [PubMed] [Google Scholar]
- 5.Xiao J, Peng F, Yu C, Wang M, Li X, Li Z, Jiang J, Sun C. microRNA-137 modulates pancreatic cancer cells tumor growth, invasion and sensitivity to chemotherapy. Int J Clin Exp Pathol. 2014;7:7442–7450. [PMC free article] [PubMed] [Google Scholar]
- 6.Li Z, Li X, Yu C, Wang M, Peng F, Xiao J, Tian R, Jiang J, Sun C. MicroRNA-100 regulates pancreatic cancer cells growth and sensitivity to chemotherapy through targeting FGFR3. Tumour Biol. 2014;35:11751–11759. doi: 10.1007/s13277-014-2271-8. [DOI] [PubMed] [Google Scholar]
- 7.Yu C, Yang SL, Fang X, Jiang JX, Sun CY, Huang T. Hypoxia disrupts the expression levels of circadian rhythm genes in hepatocellular carcinoma. Mol Med Rep. 2015;11:4002–4008. doi: 10.3892/mmr.2015.3199. [DOI] [PubMed] [Google Scholar]
- 8.Qiu S, Huang D, Yin D, Li F, Li X, Kung HF, Peng Y. Suppression of tumorigenicity by microRNA-138 through inhibition of EZH2-CDK4/6-pRb-E2F1 signal loop in glioblastoma multiforme. Biochim Biophys Acta. 2013;1832:1697–1707. doi: 10.1016/j.bbadis.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 9.Yang S, Luo A, Hao X, Lai Z, Ding T, Ma X, Mayinuer M, Shen W, Wang X, Lu Y, Ma D, Wang S. Peroxiredoxin 2 inhibits granulosa cell apoptosis during follicle atresia through the NFKB pathway in mice. Biol Reprod. 2011;84:1182–1189. doi: 10.1095/biolreprod.110.087569. [DOI] [PubMed] [Google Scholar]
- 10.Berry FB, Saleem RA, Walter MA. FOXC1 transcriptional regulation is mediated by N- and C-terminal activation domains and contains a phosphorylated transcriptional inhibitory domain. J Biol Chem. 2002;277:10292–10297. doi: 10.1074/jbc.M110266200. [DOI] [PubMed] [Google Scholar]
- 11.Nishimura DY, Searby CC, Alward WL, Walton D, Craig JE, Mackey DA, Kawase K, Kanis AB, Patil SR, Stone EM, Sheffield VC. A spectrum of FOXC1 mutations suggests gene dosage as a mechanism for developmental defects of the anterior chamber of the eye. Am J Hum Genet. 2001;68:364–372. doi: 10.1086/318183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanwar M, Kumar M, Dada T, Sihota R, Dada R. MYOC and FOXC1 gene analysis in primary congenital glaucoma. Mol Vis. 2010;16:1996–2006. [PMC free article] [PubMed] [Google Scholar]
- 13.Aldinger KA, Lehmann OJ, Hudgins L, Chizhikov VV, Bassuk AG, Ades LC, Krantz ID, Dobyns WB, Millen KJ. FOXC1 is required for normal cerebellar development and is a major contributor to chromosome 6p25.3 Dandy-Walker malformation. Nat Genet. 2009;41:1037–1042. doi: 10.1038/ng.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kume T. The cooperative roles of Foxc1 and Foxc2 in cardiovascular development. Adv Exp Med Biol. 2009;665:63–77. doi: 10.1007/978-1-4419-1599-3_5. [DOI] [PubMed] [Google Scholar]
- 15.Hayashi H, Kume T. Forkhead transcription factors regulate expression of the chemokine re ceptor CXCR4 in endothelial cells and CXCL12-induced cell migration. Biochem Biophys Res Commun. 2008;367:584–589. doi: 10.1016/j.bbrc.2007.12.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jechlinger M, Grunert S, Tamir IH, Janda E, Lüdemann S, Waerner T, Seither P, Weith A, Beug H, Kraut N. Expression profiling of epithelial plasticity in tumor progression. Oncogene. 2003;22:7155–7169. doi: 10.1038/sj.onc.1206887. [DOI] [PubMed] [Google Scholar]
- 17.Strizzi L, Bianco C, Normanno N, Seno M, Wechselberger C, Wallace-Jones B, Khan NI, Hirota M, Sun Y, Sanicola M, Salomon DS. Epithelial mesenchymal transition is a characteristic of hyperplasias and tumors in mammary gland from MMTV-Cripto-1 transgenic mice. J Cell Physiol. 2004;201:266–276. doi: 10.1002/jcp.20062. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz GK, Shah MA. Targeting the cell cycle: a new approach to cancer therapy. J. Clin. Oncol. 2005;23:9408–9421. doi: 10.1200/JCO.2005.01.5594. [DOI] [PubMed] [Google Scholar]
- 19.Olivera-Martinez I, Schurch N, Li RA, Song J, Halley PA, Das RM, Burt DW, Barton GJ, Storey KG. Major transcriptome re-organisation and abrupt changes in signalling, cell cycle and chromatin regulation at neural differentiation in vivo. Development. 2014;141:3266–3276. doi: 10.1242/dev.112623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galderisi U, Jori FP, Giordano A. Cell cycle regulation and neural differentiation. Oncogene. 2003;22:5208–5219. doi: 10.1038/sj.onc.1206558. [DOI] [PubMed] [Google Scholar]
- 21.Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature. 2001;411:342–348. doi: 10.1038/35077213. [DOI] [PubMed] [Google Scholar]
- 22.Massague J. G1 cell-cycle control and cancer. Nature. 2004;432:298–306. doi: 10.1038/nature03094. [DOI] [PubMed] [Google Scholar]
- 23.Motokura T, Bloom T, Kim HG, Jüppner H, Ruderman JV, Kronenberg HM, Arnold A. A novel cyclin encoded by a bcl1-linked candidate oncogene. Nature. 1991;350:512–515. doi: 10.1038/350512a0. [DOI] [PubMed] [Google Scholar]
- 24.Lew DJ, Dulic V, Reed SI. Isolation of three novel human cyclins by rescue of G1 cyclin (Cln) function in yeast. Cell. 1991;66:1197–1206. doi: 10.1016/0092-8674(91)90042-w. [DOI] [PubMed] [Google Scholar]
- 25.Russell A, Thompson MA, Hendley J, Trute L, Armes J, Germain D. Cyclin D1 and D3 associate with the SCF complex and are coordinately elevated in breast cancer. Oncogene. 1999;18:1983–1991. doi: 10.1038/sj.onc.1202511. [DOI] [PubMed] [Google Scholar]
- 26.Mafra RS, Alberti LR, Vasconcelos BM, De Oliveira RS. Metastatic urethral melanoma: case report and review of the literature. Rev Urol. 2014;16:47–49. [PMC free article] [PubMed] [Google Scholar]
- 27.Yang T, Zhang H, Qiu H, Li B, Wang J, Du G, Ren C, Wan X. EFEMP1 is repressed by estrogen and inhibits the epithelial-mesenchymal transition via Wnt/beta-catenin signaling in endometrial carcinoma. Oncotarget. 2016;7:25712–25. doi: 10.18632/oncotarget.8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun L, Kokura K, Izumi V, Koomen JM, Seto E, Chen J, Fang J. MPP8 and SIRT1 crosstalk in E-cadherin gene silencing and epithelial-mesenchymal transition. EMBO Rep. 2015;16:689–699. doi: 10.15252/embr.201439792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu CY, Lin HH, Tang MJ, Wang YK. Vimentin contributes to epithelial-mesenchymal transition cancer cell mechanics by mediating cytoskeletal organization and focal adhesion maturation. Oncotarget. 2015;6:15966–15983. doi: 10.18632/oncotarget.3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang SH, Law CH, Kuo PH, Hu RY, Yang CC, Chung TW, Li JM, Lin LH, Liu YC, Liao EC, Tsai YT, Wei YS, Lin CC, Chang CW, Chou HC, Wang WC, Chang MD, Wang LH, Kung HJ, Chan HL, Lyu PC. MMP-13 is involved in oral cancer cell metastasis. Oncotarget. 2016;7:17144–17161. doi: 10.18632/oncotarget.7942. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Ray ME, Mehra R, Sandler HM, Daignault S, Shah RB. E-cadherin protein expression predicts prostate cancer salvage radiotherapy outcomes. J Urol. 2006;176:1409–1414. doi: 10.1016/j.juro.2006.06.014. discussion 14. [DOI] [PubMed] [Google Scholar]