Abstract
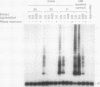
AIMS: To investigate the pattern of telomerase activity in hydatidiform mole as compared with normal placenta and choriocarcinoma, and to determine the prognostic significance of telomerase activity in hydatidiform mole. METHODS: Telomerase activity in 35 cases of hydatidiform mole, 35 normal placentas, one choriocarcinoma sample, and two choriocarcinoma cell lines (JAR, JEG3) was determined using the sensitive polymerase chain reaction based telomeric repeat amplification protocol (TRAP) assay. Two cases of breast carcinoma and two cases of ovarian carcinoma were also included as positive controls in the telomerase assay. RESULTS: Telomerase activity was detected in 11 of 30 early placentas (36.7%), one of five term placentas (20%), five of 27 hydatidiform moles which regressed spontaneously (18.5%), and six of eight hydatidiform moles which developed persistent trophoblastic disease (75%) (including three which developed metastases). Hydatidiform moles which subsequently developed persistent disease, especially those which metastasised, were more likely to express telomerase activity (p < 0.01). However, there was no significant difference in the frequency of telomerase activity between early placentas and hydatidiform mole. Strong telomerase activity was observed in choriocarcinoma tissue, choriocarcinoma cell lines, and ovarian and breast carcinomas. CONCLUSIONS: Telomerase activation occurs in hydatidiform mole with a similar incidence to early normal placentas. This supports the concept that hydatidiform mole is essentially an abnormal conceptus. There is an association between telomerase activation and the development of persistent trophoblastic disease. Further study is warrant to confirm the prognostic significance of telomerase activity in hydatidiform mole.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albanell J., Han W., Mellado B., Gunawardane R., Scher H. I., Dmitrovsky E., Moore M. A. Telomerase activity is repressed during differentiation of maturation-sensitive but not resistant human tumor cell lines. Cancer Res. 1996 Apr 1;56(7):1503–1508. [PubMed] [Google Scholar]
- Bednarek A. K., Sahin A., Brenner A. J., Johnston D. A., Aldaz C. M. Analysis of telomerase activity levels in breast cancer: positive detection at the in situ breast carcinoma stage. Clin Cancer Res. 1997 Jan;3(1):11–16. [PubMed] [Google Scholar]
- Blasco M. A., Rizen M., Greider C. W., Hanahan D. Differential regulation of telomerase activity and telomerase RNA during multi-stage tumorigenesis. Nat Genet. 1996 Feb;12(2):200–204. doi: 10.1038/ng0296-200. [DOI] [PubMed] [Google Scholar]
- Cheng R. Y., Yuen P. W., Nicholls J. M., Zheng Z., Wei W., Sham J. S., Yang X. H., Cao L., Huang D. P., Tsao S. W. Telomerase activation in nasopharyngeal carcinomas. Br J Cancer. 1998;77(3):456–460. doi: 10.1038/bjc.1998.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung A. N., Ngan H. Y., Chen W. Z., Loke S. L., Collins R. J. The significance of proliferating cell nuclear antigen in human trophoblastic disease: an immunohistochemical study. Histopathology. 1993 Jun;22(6):565–568. doi: 10.1111/j.1365-2559.1993.tb00177.x. [DOI] [PubMed] [Google Scholar]
- Cheung A. N., Ngan H. Y., Collins R. J., Wong Y. L. Assessment of cell proliferation in hydatidiform mole using monoclonal antibody MIB1 to Ki-67 antigen. J Clin Pathol. 1994 Jul;47(7):601–604. doi: 10.1136/jcp.47.7.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung A. N., Sit A. S., Chung L. P., Ngan H. Y., O'Hanlan K., Wong L. C., Ma H. K. Detection of heterozygous XY complete hydatidiform mole by chromosome in situ hybridization. Gynecol Oncol. 1994 Dec;55(3 Pt 1):386–392. doi: 10.1006/gyno.1994.1311. [DOI] [PubMed] [Google Scholar]
- Counter C. M., Gupta J., Harley C. B., Leber B., Bacchetti S. Telomerase activity in normal leukocytes and in hematologic malignancies. Blood. 1995 May 1;85(9):2315–2320. [PubMed] [Google Scholar]
- Feng J., Funk W. D., Wang S. S., Weinrich S. L., Avilion A. A., Chiu C. P., Adams R. R., Chang E., Allsopp R. C., Yu J. The RNA component of human telomerase. Science. 1995 Sep 1;269(5228):1236–1241. doi: 10.1126/science.7544491. [DOI] [PubMed] [Google Scholar]
- Gupta J., Han L. P., Wang P., Gallie B. L., Bacchetti S. Development of retinoblastoma in the absence of telomerase activity. J Natl Cancer Inst. 1996 Aug 21;88(16):1152–1157. doi: 10.1093/jnci/88.16.1152. [DOI] [PubMed] [Google Scholar]
- Hiyama E., Gollahon L., Kataoka T., Kuroi K., Yokoyama T., Gazdar A. F., Hiyama K., Piatyszek M. A., Shay J. W. Telomerase activity in human breast tumors. J Natl Cancer Inst. 1996 Jan 17;88(2):116–122. doi: 10.1093/jnci/88.2.116. [DOI] [PubMed] [Google Scholar]
- Hiyama E., Hiyama K., Yokoyama T., Matsuura Y., Piatyszek M. A., Shay J. W. Correlating telomerase activity levels with human neuroblastoma outcomes. Nat Med. 1995 Mar;1(3):249–255. doi: 10.1038/nm0395-249. [DOI] [PubMed] [Google Scholar]
- Hiyama E., Yokoyama T., Tatsumoto N., Hiyama K., Imamura Y., Murakami Y., Kodama T., Piatyszek M. A., Shay J. W., Matsuura Y. Telomerase activity in gastric cancer. Cancer Res. 1995 Aug 1;55(15):3258–3262. [PubMed] [Google Scholar]
- Hiyama K., Hirai Y., Kyoizumi S., Akiyama M., Hiyama E., Piatyszek M. A., Shay J. W., Ishioka S., Yamakido M. Activation of telomerase in human lymphocytes and hematopoietic progenitor cells. J Immunol. 1995 Oct 15;155(8):3711–3715. [PubMed] [Google Scholar]
- Härle-Bachor C., Boukamp P. Telomerase activity in the regenerative basal layer of the epidermis inhuman skin and in immortal and carcinoma-derived skin keratinocytes. Proc Natl Acad Sci U S A. 1996 Jun 25;93(13):6476–6481. doi: 10.1073/pnas.93.13.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim N. W., Piatyszek M. A., Prowse K. R., Harley C. B., West M. D., Ho P. L., Coviello G. M., Wright W. E., Weinrich S. L., Shay J. W. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994 Dec 23;266(5193):2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- Kyo S., Kunimi K., Uchibayashi T., Namiki M., Inoue M. Telomerase activity in human urothelial tumors. Am J Clin Pathol. 1997 May;107(5):555–560. doi: 10.1093/ajcp/107.5.555. [DOI] [PubMed] [Google Scholar]
- Kyo S., Takakura M., Kohama T., Inoue M. Telomerase activity in human endometrium. Cancer Res. 1997 Feb 15;57(4):610–614. [PubMed] [Google Scholar]
- Kyo S., Takakura M., Tanaka M., Kanaya T., Sagawa T., Kohama T., Ishikawa H., Nakano T., Shimoya K., Inoue M. Expression of telomerase activity in human chorion. Biochem Biophys Res Commun. 1997 Dec 18;241(2):498–503. doi: 10.1006/bbrc.1997.7767. [DOI] [PubMed] [Google Scholar]
- Langford L. A., Piatyszek M. A., Xu R., Schold S. C., Jr, Shay J. W. Telomerase activity in human brain tumours. Lancet. 1995 Nov 11;346(8985):1267–1268. doi: 10.1016/s0140-6736(95)91865-5. [DOI] [PubMed] [Google Scholar]
- Langford L. A., Piatyszek M. A., Xu R., Schold S. C., Jr, Wright W. E., Shay J. W. Telomerase activity in ordinary meningiomas predicts poor outcome. Hum Pathol. 1997 Apr;28(4):416–420. doi: 10.1016/s0046-8177(97)90029-0. [DOI] [PubMed] [Google Scholar]
- Rhyu M. S. Telomeres, telomerase, and immortality. J Natl Cancer Inst. 1995 Jun 21;87(12):884–894. doi: 10.1093/jnci/87.12.884. [DOI] [PubMed] [Google Scholar]
- Saito T., Schneider A., Martel N., Mizumoto H., Bulgay-Moerschel M., Kudo R., Nakazawa H. Proliferation-associated regulation of telomerase activity in human endometrium and its potential implication in early cancer diagnosis. Biochem Biophys Res Commun. 1997 Feb 24;231(3):610–614. doi: 10.1006/bbrc.1997.6164. [DOI] [PubMed] [Google Scholar]
- Sallinen P., Miettinen H., Sallinen S. L., Haapasalo H., Helin H., Kononen J. Increased expression of telomerase RNA component is associated with increased cell proliferation in human astrocytomas. Am J Pathol. 1997 Apr;150(4):1159–1164. [PMC free article] [PubMed] [Google Scholar]
- Sharma H. W., Sokoloski J. A., Perez J. R., Maltese J. Y., Sartorelli A. C., Stein C. A., Nichols G., Khaled Z., Telang N. T., Narayanan R. Differentiation of immortal cells inhibits telomerase activity. Proc Natl Acad Sci U S A. 1995 Dec 19;92(26):12343–12346. doi: 10.1073/pnas.92.26.12343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerfeld H. J., Meeker A. K., Piatyszek M. A., Bova G. S., Shay J. W., Coffey D. S. Telomerase activity: a prevalent marker of malignant human prostate tissue. Cancer Res. 1996 Jan 1;56(1):218–222. [PubMed] [Google Scholar]
- Strahl C., Blackburn E. H. Effects of reverse transcriptase inhibitors on telomere length and telomerase activity in two immortalized human cell lines. Mol Cell Biol. 1996 Jan;16(1):53–65. doi: 10.1128/mcb.16.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahara H., Nakanishi T., Kitamoto M., Nakashio R., Shay J. W., Tahara E., Kajiyama G., Ide T. Telomerase activity in human liver tissues: comparison between chronic liver disease and hepatocellular carcinomas. Cancer Res. 1995 Jul 1;55(13):2734–2736. [PubMed] [Google Scholar]
- Taylor R. S., Ramirez R. D., Ogoshi M., Chaffins M., Piatyszek M. A., Shay J. W. Detection of telomerase activity in malignant and nonmalignant skin conditions. J Invest Dermatol. 1996 Apr;106(4):759–765. doi: 10.1111/1523-1747.ep12345811. [DOI] [PubMed] [Google Scholar]
- Tsao J. l., Zhao Y., Lukas J., Yang X., Shah A., Press M., Shibata D. Telomerase activity in normal and neoplastic breast. Clin Cancer Res. 1997 Apr;3(4):627–631. [PubMed] [Google Scholar]
- Wright W. E., Piatyszek M. A., Rainey W. E., Byrd W., Shay J. W. Telomerase activity in human germline and embryonic tissues and cells. Dev Genet. 1996;18(2):173–179. doi: 10.1002/(SICI)1520-6408(1996)18:2<173::AID-DVG10>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Yasumoto S., Kunimura C., Kikuchi K., Tahara H., Ohji H., Yamamoto H., Ide T., Utakoji T. Telomerase activity in normal human epithelial cells. Oncogene. 1996 Jul 18;13(2):433–439. [PubMed] [Google Scholar]
- Zhang D. K., Ngan H. Y., Cheng R. Y., Cheung A. N., Liu S. S., Tsao S. W. Clinical significance of telomerase activation and telomeric restriction fragment (TRF) in cervical cancer. Eur J Cancer. 1999 Jan;35(1):154–160. doi: 10.1016/s0959-8049(98)00303-7. [DOI] [PubMed] [Google Scholar]
- Zhang W., Piatyszek M. A., Kobayashi T., Estey E., Andreeff M., Deisseroth A. B., Wright W. E., Shay J. W. Telomerase activity in human acute myelogenous leukemia: inhibition of telomerase activity by differentiation-inducing agents. Clin Cancer Res. 1996 May;2(5):799–803. [PubMed] [Google Scholar]