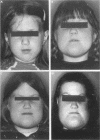
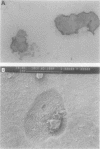
Abstract
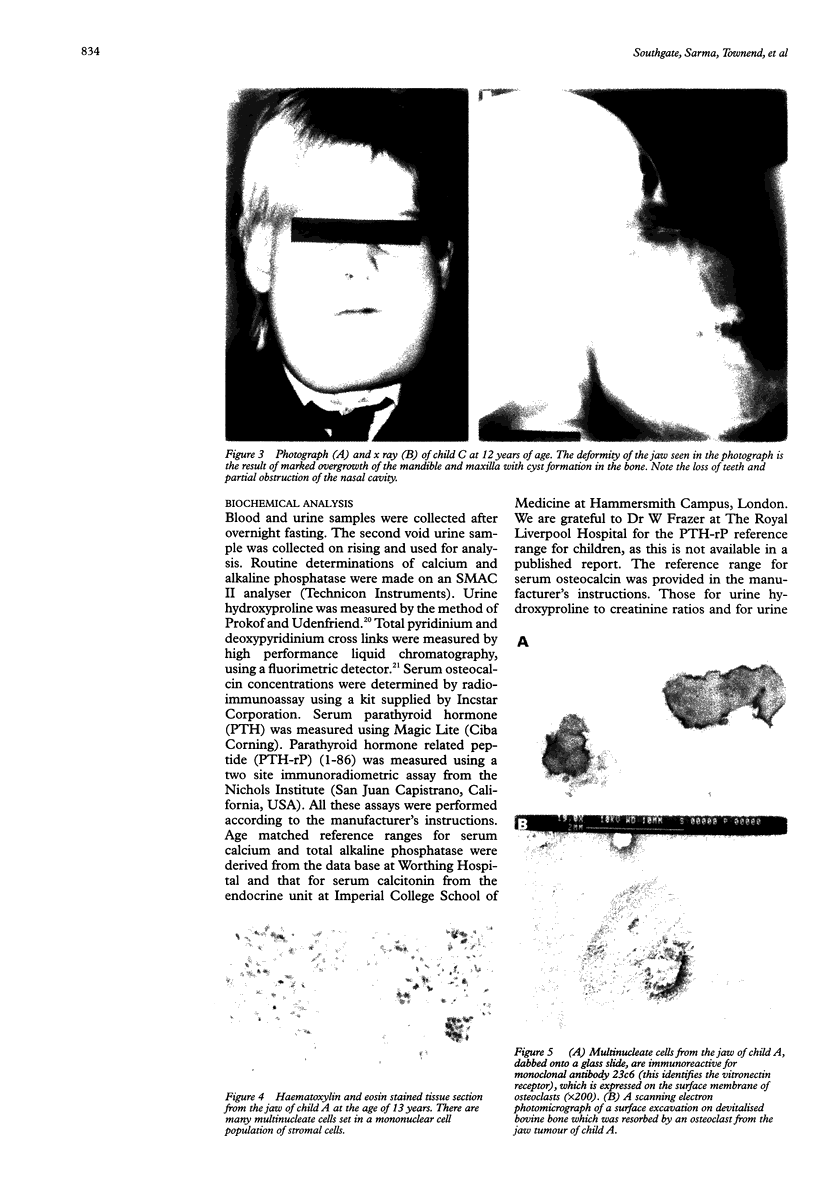
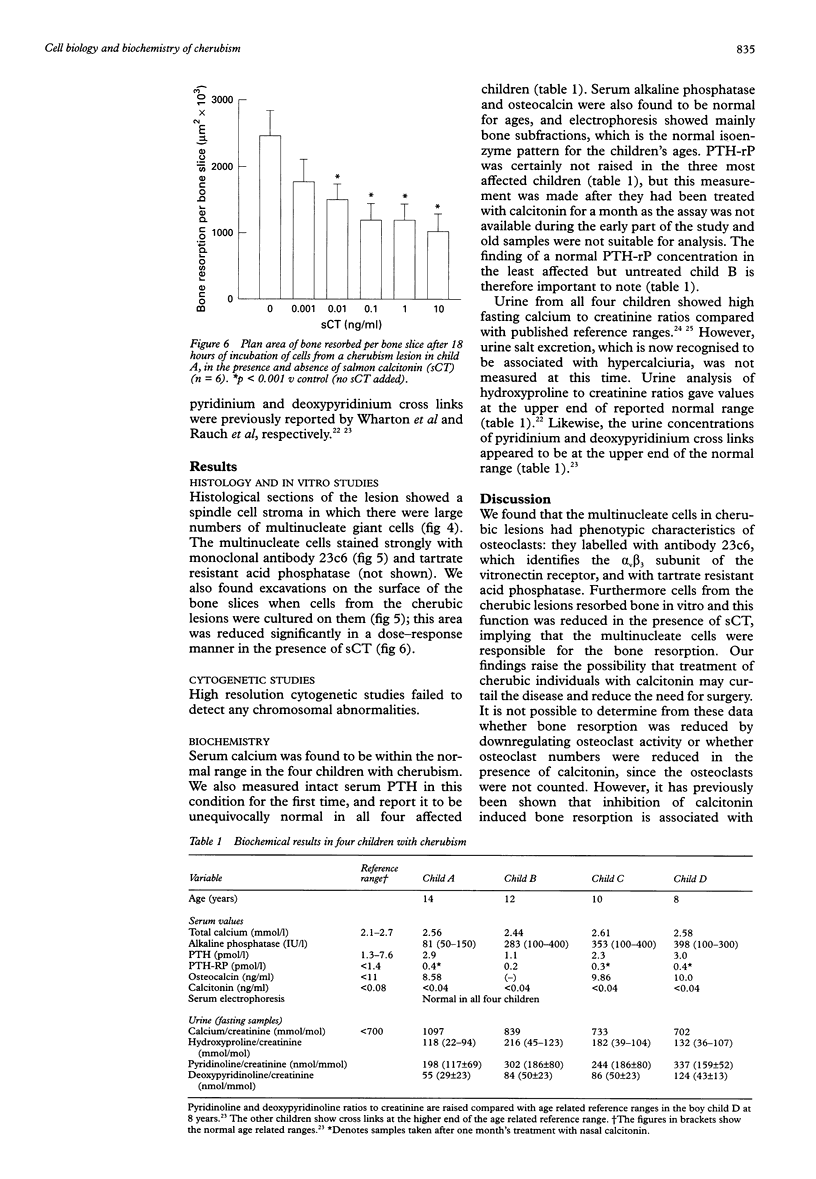
AIMS: To establish whether the multinucleate cells in lesions of patients with cherubism are also osteoclasts and if this is the case whether they were responsive to calcitonin; to carry out cytogenetic studies on two members of the same family affected by cherubism in an attempt to identify any major chromosomal defects; and to perform an in-depth modern biochemical study of four children in the same family. SUBJECTS AND METHODS: Four related children with cherubism were studied. Tissue taken from one of the children at elective decompression of an optic nerve was submitted to in vitro bone resorption studies. Cytogenetic studies were done on two of the children and biochemical studies on all four. RESULTS: The multinucleate cells in the cherubic lesions were shown to be osteoclasts since they synthesised tartrate resistant acid phosphatase, expressed the vitronectin receptor, and resorbed bone. Bone resorption by the cultured multinucleate cells was significantly inhibited by calcitonin. High resolution cytogenetic studies failed to detect any chromosomal abnormalities in two children with cherubism. The biochemistry profile of all four children with cherubism showed that serum calcium, parathyroid hormone, parathyroid related hormone, calcitonin, and alkaline phosphatase were within normal levels. Urine analysis of pyridinium and deoxypyridinium cross links, hydroxyproline, and calcium in relation to urine creatinine were measured to assess bone resorption in these children, and the values were at the upper end of the normal range in all four. CONCLUSIONS: Further studies are required to determine whether calcitonin treatment will control this grossly deforming disease until the time when the physiological changes that occur at puberty rectify the pathology. It is not recommended that biochemical markers of bone resorption are used in isolation to monitor the activity of cherubism in individuals because the results are based on a small number of children and because of reports of marked interindividual variation in the levels of these markers, particularly in children.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chambers T. J., Fuller K., McSheehy P. M., Pringle J. A. The effects of calcium regulating hormones on bone resorption by isolated human osteoclastoma cells. J Pathol. 1985 Apr;145(4):297–305. doi: 10.1002/path.1711450403. [DOI] [PubMed] [Google Scholar]
- Chambers T. J., Magnus C. J. Calcitonin alters behaviour of isolated osteoclasts. J Pathol. 1982 Jan;136(1):27–39. doi: 10.1002/path.1711360104. [DOI] [PubMed] [Google Scholar]
- Chambers T. J., Revell P. A., Fuller K., Athanasou N. A. Resorption of bone by isolated rabbit osteoclasts. J Cell Sci. 1984 Mar;66:383–399. doi: 10.1242/jcs.66.1.383. [DOI] [PubMed] [Google Scholar]
- Chambers T. J. The cellular basis of bone resorption. Clin Orthop Relat Res. 1980 Sep;(151):283–293. [PubMed] [Google Scholar]
- Davies J., Warwick J., Totty N., Philp R., Helfrich M., Horton M. The osteoclast functional antigen, implicated in the regulation of bone resorption, is biochemically related to the vitronectin receptor. J Cell Biol. 1989 Oct;109(4 Pt 1):1817–1826. doi: 10.1083/jcb.109.4.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan A. M., Nui B., Tinkler S. M., Horton M. A., Williams D. M., Chambers T. J. The multinucleate cells in giant cell granulomas of the jaw are osteoclasts. Cancer. 1988 Sep 15;62(6):1139–1145. doi: 10.1002/1097-0142(19880915)62:6<1139::aid-cncr2820620617>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Grigoriadis A. E., Wang Z. Q., Cecchini M. G., Hofstetter W., Felix R., Fleisch H. A., Wagner E. F. c-Fos: a key regulator of osteoclast-macrophage lineage determination and bone remodeling. Science. 1994 Oct 21;266(5184):443–448. doi: 10.1126/science.7939685. [DOI] [PubMed] [Google Scholar]
- Grünebaum M., Tiqva P. Non familial cherubism: report of two cases. J Oral Surg. 1973 Aug;31(8):632–635. [PubMed] [Google Scholar]
- Harris M. Central giant cell granulomas of the jaws regress with calcitonin therapy. Br J Oral Maxillofac Surg. 1993 Apr;31(2):89–94. doi: 10.1016/0266-4356(93)90168-v. [DOI] [PubMed] [Google Scholar]
- Horton M. A., Lewis D., McNulty K., Pringle J. A., Chambers T. J. Monoclonal antibodies to osteoclastomas (giant cell bone tumors): definition of osteoclast-specific cellular antigens. Cancer Res. 1985 Nov;45(11 Pt 2):5663–5669. [PubMed] [Google Scholar]
- Ibrahim S., Mojiminiyi S., Barron J. L. High-performance liquid chromatographic determination of pyridinium crosslinks in serum, urine and dialysate of patients in chronic renal failure. Ann Clin Biochem. 1996 Jan;33(Pt 1):31–35. doi: 10.1177/000456329603300104. [DOI] [PubMed] [Google Scholar]
- JAFFE H. L. Giant-cell reparative granuloma, traumatic bone cyst, and fibrous (fibro-oseous) dysplasia of the jawbones. Oral Surg Oral Med Oral Pathol. 1953 Jan;6(1):159–175. doi: 10.1016/0030-4220(53)90151-0. [DOI] [PubMed] [Google Scholar]
- James I. T., Walne A. J., Perrett D. The measurement of pyridinium crosslinks: a methodological overview. Ann Clin Biochem. 1996 Sep;33(Pt 5):397–420. doi: 10.1177/000456329603300503. [DOI] [PubMed] [Google Scholar]
- Jones W. A. Cherubism. A thumbnail sketch of its diagnosis and a conservative method of treatment. Oral Surg Oral Med Oral Pathol. 1965 Nov;20(5):648–653. doi: 10.1016/0030-4220(65)90111-8. [DOI] [PubMed] [Google Scholar]
- Kaugars G. E., Niamtu J., 3rd, Svirsky J. A. Cherubism: diagnosis, treatment, and comparison with central giant cell granulomas and giant cell tumors. Oral Surg Oral Med Oral Pathol. 1992 Mar;73(3):369–374. doi: 10.1016/0030-4220(92)90137-f. [DOI] [PubMed] [Google Scholar]
- Matos V., van Melle G., Boulat O., Markert M., Bachmann C., Guignard J. P. Urinary phosphate/creatinine, calcium/creatinine, and magnesium/creatinine ratios in a healthy pediatric population. J Pediatr. 1997 Aug;131(2):252–257. doi: 10.1016/s0022-3476(97)70162-8. [DOI] [PubMed] [Google Scholar]
- Overgaard K., Riis B. J., Christiansen C., Hansen M. A. Effect of salcatonin given intranasally on early postmenopausal bone loss. BMJ. 1989 Aug 19;299(6697):477–479. doi: 10.1136/bmj.299.6697.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PROCKOP D. J., UDENFRIEND S. A specific method for the analysis of hydroxyproline in tissues and urine. Anal Biochem. 1960 Nov;1:228–239. doi: 10.1016/0003-2697(60)90050-6. [DOI] [PubMed] [Google Scholar]
- Peters W. J. Cherubism: a study of twenty cases from one family. Oral Surg Oral Med Oral Pathol. 1979 Apr;47(4):307–311. doi: 10.1016/0030-4220(79)90251-2. [DOI] [PubMed] [Google Scholar]
- Rauch F., Schönau E., Woitge H., Remer T., Seibel M. Urinary excretion of hydroxy-pyridinium cross-links of collagen reflects skeletal growth velocity in normal children. Exp Clin Endocrinol. 1994;102(2):94–97. doi: 10.1055/s-0029-1211269. [DOI] [PubMed] [Google Scholar]
- Riefkohl R., Georgiade G. S., Georgiade N. G. Cherubism. Ann Plast Surg. 1985 Jan;14(1):85–90. doi: 10.1097/00000637-198501000-00016. [DOI] [PubMed] [Google Scholar]
- Rodan G. A., Martin T. J. Role of osteoblasts in hormonal control of bone resorption--a hypothesis. Calcif Tissue Int. 1981;33(4):349–351. doi: 10.1007/BF02409454. [DOI] [PubMed] [Google Scholar]
- Sarma U., Edwards M., Motoyoshi K., Flanagan A. M. Inhibition of bone resorption by 17beta-estradiol in human bone marrow cultures. J Cell Physiol. 1998 Apr;175(1):99–108. doi: 10.1002/(SICI)1097-4652(199804)175:1<99::AID-JCP11>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Silva O. L., Becker K. L. Salmon calcitonin in the treatment of hypercalcemia. Arch Intern Med. 1973 Sep;132(3):337–339. [PubMed] [Google Scholar]
- Soriano P., Montgomery C., Geske R., Bradley A. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell. 1991 Feb 22;64(4):693–702. doi: 10.1016/0092-8674(91)90499-o. [DOI] [PubMed] [Google Scholar]
- Sweid H. A., Bagga A., Vaswani M., Vasudev V., Ahuja R. K., Srivastava R. N. Urinary excretion of minerals, oxalate, and uric acid in north Indian children. Pediatr Nephrol. 1997 Apr;11(2):189–192. doi: 10.1007/s004670050257. [DOI] [PubMed] [Google Scholar]
- TOPAZIAN R. G., COSTICH E. R. FAMILIAL FIBROUS DYSPLASIA OF THE JAWS (CHERUBISM): REPORT OF CASE. J Oral Surg. 1965 Sep;23:559–568. [PubMed] [Google Scholar]
- Wada S., Udagawa N., Nagata N., Martin T. J., Findlay D. M. Calcitonin receptor down-regulation relates to calcitonin resistance in mature mouse osteoclasts. Endocrinology. 1996 Mar;137(3):1042–1048. doi: 10.1210/endo.137.3.8603572. [DOI] [PubMed] [Google Scholar]
- Wayman J. B. Cherubism: a report on three cases. Br J Oral Surg. 1978 Jul;16(1):47–56. doi: 10.1016/s0007-117x(78)80055-9. [DOI] [PubMed] [Google Scholar]
- Wharton B. A., Gough G., Williams A., Kitts S., Pennock C. A. Urinary total hydroxyproline: creatinine ratio. Range of normal, and clinical application in British children. Arch Dis Child. 1972 Feb;47(251):74–79. doi: 10.1136/adc.47.251.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H., Hayashi S., Kunisada T., Ogawa M., Nishikawa S., Okamura H., Sudo T., Shultz L. D., Nishikawa S. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature. 1990 May 31;345(6274):442–444. doi: 10.1038/345442a0. [DOI] [PubMed] [Google Scholar]