Abstract
Context
Experimental and observational evidence demonstrates that high density lipoprotein (HDL) can lose its well-documented atheroprotective functions and even adopt a paradoxically proinflammatory nature in certain conditions. Hormonal alterations, especially estradiol reduction, influence the accumulation of risk factors that could potentially impair the quality of HDL during the menopausal transition (MT). Limited data exist to evaluate the relationship between changes in HDL-Cholesterol (HDL-C) and its main carried protein, apolipoprotein-A (apo-A), over the MT and atherosclerosis development.
Objective
To evaluate the associations of changes in HDL-C and apoA with progression of carotid intima-media thickness (cIMT), carotid adventitial diameter (cAD) and presence of carotid plaque relative to the onset of the postmenopause.
Design
Longitudinal study.
Setting
The Study of Women's Health Across the Nation (SWAN).
Participants
213 participants (age (mean (SD)): 45.7(2.5) years at baseline; 70% White).
Main Outcome Measures
Up to 5 measures of cIMT, cAD and carotid plaque over a maximum of 9 years of follow-up.
Results
Adjusting for sociodemographic, CVD risk factors, CVD medication use and C-reactive protein, a larger increase in HDL-C since baseline was significantly associated with a greater cIMT progression (P=0.008). Additionally, a higher apoA level at baseline was significantly associated with a lower cIMT progression (P=0.03). No significant associations were found with cAD or plaque presence.
Conclusions
As women transition through menopause, increases in HDL-C levels are independently associated with greater cIMT progression. Thus, the quality of HDL may be altered over the MT rendering HDL dysfunctional and not providing the expected cardioprotective effect.
Keywords: High-density lipoprotein, Intima-media thickness, Menopaus
Introduction
Experimental and observational evidence demonstrates that high-density lipoprotein (HDL) can lose its well-documented athero-protective functions and even adopt a paradoxically proinflammatory nature under certain conditions (1). Systemic inflammation has been proposed to convert HDL to a dysfunctional form (2). Both oxidative modifications and alterations in the protein composition of HDL may alter its biological activity thereby producing potentially proatherogenic particles (2).
Growing evidence challenges the well-known cardioprotective effect of HDL cholesterol (HDL-C) in postmenopausal women. It has been proposed that menopause-related hormonal alterations, especially estradiol reduction, influence the accumulation of risk factors that in turn may lead to a status of chronic inflammation (3). This chronic status of inflammation could potentially impair the quality of HDL as women transition through menopause. Evidence to support this hypothesis comes from several studies showing that higher level of HDL-C in middle aged and older women is not always protective. In one study, higher levels of HDL-C were associated with a significant increased risk of non-fatal stroke and cerebral infarction in women aged ≥ 52 years, but not in men (4). In postmenopausal women with HDL-C level > 60mg/dL and a very low risk profile for CVD, higher levels of HDL-C were independently associated with increased prevalence of carotid atherosclerosis (5). Additionally, inverse cross-sectional associations between HDL-C levels and aortic calcification and carotid intima-media thickness (cIMT) have been reported in premenopausal women, while the same protective effect of HDL-C was not seen in postmenopausal women (6). Finally, in a sample of 500 midlife men and women, HDL-C was associated with less cIMT progression in men but with more progression in women, who were largely postmenopusal at baseline (7). None of these studies assessed the potential impact of alterations in HDL-C or its main protein carrier, apolipoprotein A (apoA), over the menopausal transition on progression of subclinical atherosclerosis.
In this paper, we evaluate the associations of changes in HDL-C and apoA in women transitioning through menopause with progression of subclinical atherosclerosis, namely cIMT, carotid advential diameter (cAD) and carotid plaque (cPlaque) among women enrolled in the Study of Women's Health Across the Nation (SWAN) at the Pittsburgh site. These women had a maximum of 9 years of follow-up with repeated measures of subclinical atherosclerosis. We hypothesize that increases in HDL-C and apoA over the menopausal transition are associated with greater progression of subclinical measures of atherosclerosis, and that the influence of HDL-C and apoA change varied prior and following the women's final menstrual period (FMP).
Materials and Methods
Study participants
SWAN is an ongoing, multi-racial/ethnic, longitudinal study of biological, physical, psychological, and social changes during the menopausal transition. The study design has been previously described (8). Briefly, between 1996 and 1997, 3,302 participants aged 42-52 years were recruited from seven designated sites (Boston, MA; Detroit, MI; Oakland, CA; Los Angeles, CA; Pittsburgh, PA; Chicago, IL; and Newark, NJ). The eligibility criteria for the SWAN study were 1) an intact uterus and ≥1 ovary, 2) not pregnant or breastfeeding, 3) ≥1 menstrual period within the past 3 months, 4) no hormone therapy (HT) use within the past 3 months.
The current study was an ancillary study to SWAN at the Pittsburgh site where enrollment began between the baseline and 3rd annual visit of the SWAN study (N=257). Each participant had up to 5 carotid scans over a maximum follow-up time of 9-years. For the current analysis, data were censored when women reported a stroke and/or angina, surgical menopause, or using HT. This resulted in excluding 8 participants from the analytical sample. Additionally, participants were excluded if their HDL-C and apoA were missing at all-time points (n=3 participants), if they were on lipid lowering medications (n=1 participants), and if they reported hysterectomy with one or both ovaries intact since the date of FMP cannot be determined (n=5 participants). Based on these criteria 240 women were eligible for the current analyses. The FMP date was known for 131 (55%) of the eligible participants, for the remainder (n=109), FMP was imputed based on an algorithm that utilized data from the SWAN parent study over 13 follow-up visits (Supplemental material). Using this algorithm we were able to estimate FMP date for 82 participants out of the 109. For the current analyses only participants for whom FMP date was known or could be estimated were included. The final sample size for the current longitudinal analyses was 213 participants (531 observations) with data available on any of the evaluated subclinical measures of atherosclerosis. Participants excluded (n=44) were more likely to be nonsmokers and to have higher apoA, and cIMT, and lower LDL-C and cAD at the initial assessment for the ancillary study.
Research protocols were approved by the University of Pittsburgh institutional review board and all the participants provided a written informed consent prior to enrollment in the SWAN study.
Study measures
Ultrasound measures
Carotid intima media thickness (cIMT), carotid adventitial diameter (cAD) and carotid plaque (cPlaque) of the right and left carotid arteries were assessed by B-mode ultrasound using a Toshiba (Toshiba American Medical Systems, Tustin, CA) SSA-270A scanner. For cIMT, B-mode images were obtained from 8 locations – 4 each from the left and right carotid arteries: the near and far walls of the distal common carotid artery (1 cm proximal to the carotid bulb), the far walls of the carotid bulb, and the internal carotid artery (from the flow divider to 1cm distal to this point). IMT measures were obtained by electronically tracing the lumen-intima interface and the media-adventitia interface across a 1-cm segment; one measurement was generated for each pixel over the area, for a total of approximately 140 measures for each segment. The mean value of the average readings at all 8 locations was used for analyses. For AD, the distance from the adventitial-medial interface on the near wall to the medial adventitial interface on the far wall at end-diastole was measured for both right and left common carotid artery. The average value of the readings at both sides was used for the analyses. Presence of cPlaque was identified on the same segments as discrete focal protrusions into the lumen greater than 50% of the surrounding wall thickness. All readings were conducted at the University of Pittsburgh Ultrasound Research Laboratory. Readers were centrally trained with a standardized protocol and recertified annually against the same set of scans to guard against reader drift. Readers were blinded to participants' clinical information. Replicate readings were performed on 20 scans to determine the inter-reader reproducibility of subclinical measures, with an intra-class correlation of 0.98 for cIMT values and 0.99 for cAD.
Blood assays
Phlebotomy was performed in the morning after 10-12 hours of fasting on days 2 to 5 of a spontaneous menstrual cycle when women were premenopausal. If a timed sample could not be obtained, because menstrual cycles became less regular or due to menopause, a random fasting sample was taken within 90 days of the annual visit. Blood was maintained up to 1 hour at 4° C until separated, then frozen at -80°C and shipped on dry ice to the Medical Research Laboratories (Highland Heights, KY). Triglycerides were determined by enzymatic methods (Hitachi 747 analyzer; Boehringer Mannheim Diagnostics, Indianapolis, IN). HDL-C was isolated with heparin-2M manganese chloride. LDL-C was calculated by the Friedewald equation for all subjects with triglycerides < 400mg/dL (9). LDL-C was set to missing for those with triglycerides ≥ 400 mg/dL. apoA-1 and apoB were measured by immunonephelometry (BN1A-100; Behring Diagnostics, Westwood, MA). Glucose was measured using a hexokinase-coupled reaction on a Hitachi 747-200 (Roche Molecular Biochemicals Diagnostics, Indianapolis, IN). Serum insulin was measured using a RIA (DPC Coat-a-Count; Diagnostic Products Corporation, Los Angeles, CA) procedure and monitored as part of the monthly quality assurance program by the Diabetes Diagnostic Laboratory at the University of Missouri. HOMA index, an index derived from glucose and insulin measures, reflected insulin resistance [(fasting insulin*fasting glucose)/22.5] (10). High sensitivity C-reactive protein (hs-CRP) was measured using an ultrasensitive rate immunonephelemetric method (hs-CRP, Dade-Behring, Marburg, Germany). The sensitivity of the assay was 0.03 mg/dL and interassay coefficients of variations (CVs) at hs-CRP concentrations of 0.05 and 2.2 mg/dl were 10–12% and 5–7%, respectively.
Study covariates
Race/ethnicity and education were determined from the SWAN screening interview. Age, income, smoking status, menopausal status, and medication use (Current use of antihypertensive and heart medications) were derived from questionnaires and interviews administered during annual visits. Weight and height were measured at each clinic visit. Body mass index (BMI) was calculated as weight/height2. Blood pressure was averaged from 2 sequential measures in the right arm with the participant seated after at least 5 minutes of rest. Menopause status was determined annually based on reports about frequency, regularity of menstrual bleeding and use of HT as follows: 1) Premenopause: monthly bleeding with no perceived change in cycle interval, 2) Early peri-menopause: monthly bleeding with a perceived change in cycle interval, but at least one menstrual period within the past 3 months, 3) Late peri-menopause: 3 consecutive months of amenorrhea, 4) Postmenopause: 12 consecutive months of amenorrhea.
Statistical Analysis
The distribution of cIMT was slightly skewed. cIMT was evaluated with and without a log-transformation. Since results were comparable, results from models using the original scale were presented for simplicity of interpretation. Triglycerides, glucose, insulin, HOMA-index, and hs-CRP were log-transformed for analyses. Because HDL-C and apoA were not collected at all SWAN visits, values at the missing visits were interpolated using random effect (slope and intercept) models for each lipid measure as a linear function of time in the study. Subject-specific intercepts and slopes were then used to calculate lipid values at missing visits. HDL-C and apoA measures were interpolated for 84 observations out of 531 observations available for these analyses (16%).
Associations between baseline and change since baseline in HDL-C or in apoA and progression of subclinical measures of atherosclerosis relative to FMP were evaluated using linear mixed-effect models with random intercept (cIMT, cAD) or generalized estimating equation models (presence of cPlaque). Repeated measures of cIMT, cAD, and cPlaque (separate models) were modeled as a function of: time since FMP (before and after FMP) in years, baseline and change since baseline of HDL-C or apoA (separate models for each of them with each outcome) and their interactions with time since FMP. The progression rates of cIMT and cAD associated with one SD increases in HDL-C or apoA at baseline or change since baseline per 1 year relative to FMP were estimated from the regression coefficient associated with the interaction terms of baseline HDL-C or apoA and change since baseline with time since FMP (11). A similar approach was applied for odds of presence of cPlaque per SD increase in baseline and changes since baseline in HDL-C or apoA by time since FMP. Baseline HDL-C and apoA were centered on their means. Univariate analyses between each outcome and study covariates were done to determine potential candidates for multivariable analyses. All covariates that were found to be associated with study outcomes at P-value ≤0.1 were considered. BMI and HOMA-index were highly correlated (r=0.70). They were therefore evaluated separately and the model which showed a better fit based on the Akaike information criterion (AIC) criteria was chosen. Models were first adjusted for age at baseline, race and education. Second, time varying BMI and systolic blood pressure (SBP) were added. Finally, LDL-C, hs-CRP, and use of CVD medication were added as time-varying covariates.
To better visualize the significant interaction of change in HDL-C since baseline with time since FMP, predicted means of cIMT were estimated from multivariable mixed-effect models at selected percentiles of time-relative to FMP (5th, 25th, 50th, 75th, and 95th) for 4 percentiles (5th, 50th, 75th, and 95th) of change in HDL-C. Predicted means of cIMT by percentiles of change in HDL-C were plotted at the selected time points (12). Analyses were performed with SAS v9.3 (SAS Institute, Cary, NC). All tests were 2-sided at alpha=0.05.
Results
On average, participants were 45.65 years old with the majority of them (∼95%) pre- or early peri-menopausal at the baseline scan. A summary of the participants' characteristics and study measures were presented in Table 1.
Table 1. Characteristics of Study Population at First Scan.
| Characteristics | N=213 |
|---|---|
| Age, mean (SD), year | 45.65 (2.51) |
| Race, n (%) | |
| Black | 64 (30.05) |
| Caucasian | 149 (69.95) |
| Education, n (%) | |
| <= High school | 51 (23.94) |
| Some college/college | 109 (51.17) |
| Post graduate | 53 (24.88) |
| Smoker, n (%) | |
| No | 176 (83.41) |
| Yes | 35 (16.59) |
| Menopause status, n (%) | |
| Premenopausal | 112 (52.58) |
| Early peri-menopausal | 90 (42.25) |
| Late peri-menopausal | 4 (1.88) |
| Postmenopausal | 7 (3.29) |
| BMI, mean (SD), kg/m2 | 28.23 (6.49) |
| SBP, mean (SD), mmHg | 113.27 (16.01) |
| HDL, mean (SD), mg/dL | 55.25 (11.96) |
| apoA, mean (SD) | 148.44(23.69) |
| LDL, mean (SD), mg/dL | 120.78 (29.51) |
| Triglycerides, median (Q1, Q3), mg/dL | 90.00 (68.00, 123.00) |
| HOMA index, median (Q1, Q3) | 1.72 (1.27, 2.56) |
| hs-CRP, median (Q1, Q3) | 1.60 (0.80,4.20) |
| Current use of CVD medications,a n (%) | 16 (7.51) |
| cIMT, median (Q1, Q3), mm | 0.67 (0.62, 0.71) |
| cAD, mean (SD), mm | 6.73(0.59) |
| Presence of cPlaque, n (%) | 27(12.68) |
CVD medications: Current use of anti-hypertensive and heart medications
The average annualized change in HDL-C was 0.443(SE=0.103) mg/dL and in apoA was 6.077(SE=0.908) mg/dL. Changes in lipid measures were weakly inversely correlated with the baseline lipid value (correlation between HDL-C and changes in HDL-C: r=-0.14 and correlations between apoA and changes in apoA: r=-0.30, P<0.05 for both).
The unadjusted overall rate of change in cIMT relative to FMP was 0.007 (SE=0.001) mm/year, while the unadjusted rate of change in cAD was -0.004(SE=0.004) mm/year. The unadjusted OR(95% CI) for plaque presence for each 1 year increase relative to FMP was 1.18(1.11,1.25).
After adjusting for sociodemographic factors, age at baseline, time since FMP, time varying BMI and SBP, a larger increase in HDL-C since baseline was significantly associated with a greater cIMT progression (P=0.006), while a higher HDL-C level at baseline was significantly associated with a lower cIMT progression (P=0.03), Table 2, Model 2. Additionally, a higher apoA level at baseline was significantly associated with a lower cIMT progression (P=0.03), while a greater apoA increase since baseline tended to be associated with a greater cIMT progression (P=0.10), Table 3, Model 2. Additional adjustment for time varying LDL-C, current use of CVD medications and hs-CRP attenuated the association between baseline HDL-C and cIMT progression (P=0.11), Table 2, Model 3. Results for changes in HDL-C since baseline (Table 2, Model 3), and baseline levels and changes since baseline levels of apoA (Table 3, Model 3) did not change after these additional adjustment. Similar findings were obtained when restricting sample to only those with known FMP (N=131), Supplemental Table 1.
Table 2. Beta Coefficients for the Interactions of Baseline HDL-C and Change in HDL-C and time Relative to FMP on Carotid Intima-Media Thickness, N=212 women / 525 observations.
| HDL-C Measures | Progression in cIMT, mm/year | |
|---|---|---|
|
| ||
| β (SE)a | P value | |
| Model 1 | ||
| Baseline HDL-C | -0.002(0.001) | 0.02 |
| Change in HDL-C | 0.002 (0.001) | 0.008 |
| Model 2 | ||
| Baseline HDL-C | -0.002(0.001) | 0.03 |
| Change in HDL-C | 0.002(0.001) | 0.006 |
| Model 3 | ||
| Baseline HDL-C | -0.001 (0.001) | 0.11 |
| Change in HDL-C | 0.002 (0.001) | 0.004 |
Beta coefficients represent cIMT progression rate (mm/year) per SD unit of baseline HDL-C (SD=11.96 mg/dL) and change in HDL-C since baseline (6.15 mg/dL)
Model 1: Adjusted for baseline age, time since FMP, race and education
Model 2: Adjusted for variables in model 1 plus time varying BMI and SBP
Model 3: Adjusted for variables in model 2 plus LDL-C, current use of CVD medications and log transformed hs-CRP
Table 3. Beta Coefficients for the Interactions of Baseline apoA and Change in apoA and time Relative to FMP on Carotid Intima-Media Thickness,, N=212 women / 525 observations.
| apoA Measures | Progression in cIMT, mm/year | |
|---|---|---|
|
| ||
| β (SE)a | P value | |
| Model 1 | ||
| Baseline apoA | -0.002(0.001) | 0.02 |
| Change in apoA | 0.001 (0.001) | 0.09 |
| Model 2 | ||
| Baseline apoA | -0.002(0.001) | 0.03 |
| Change in apoA | 0.001(0.001) | 0.10 |
| Model 3 | ||
| Baseline apoA | -0.002 (0.001) | 0.03 |
| Change in apoA | 0.001 (0.001) | 0.10 |
Beta coefficients represent cIMT progression rate (mm/year) per SD unit of baseline apoA (SD=23.69 mg/dL) and change in apoA since baseline (18.78 mg/dL)
Model 1: Adjusted for baseline age, time since FMP, race and education
Model 2: Adjusted for variables in model 1 plus time varying BMI and SBP
Model 3: Adjusted for variables in model 2 plus LDL-C, current use of CVD medications and log transformed hs-CRP
Figure 1 presents the interaction between time since FMP and changes in HDL-C on cIMT levels. Of note, a greater increase in HDL-C mainly before the FMP was associated with smaller cIMT whereas an increase in HDL-C after the FMP was associated with larger cIMT.
Figure 1. Predicted cIMT by Change in HDL-C Percentiles at Selected Time Points across the Final Menstrual Period a.
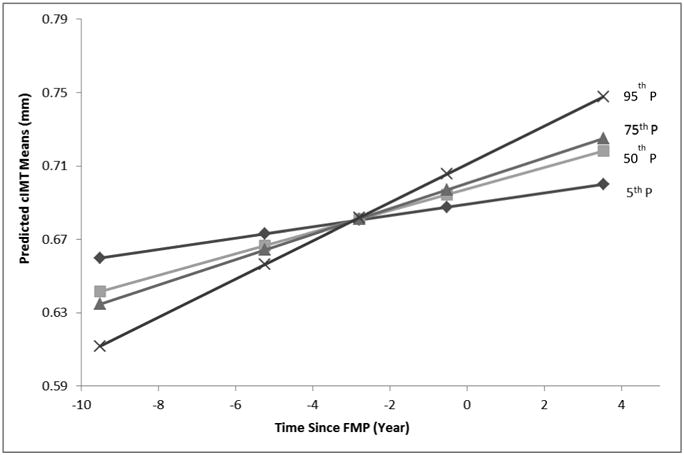
a Predicted cIMT by percentiles of change in HDL-C at selected time points relative to FMP: 5th: -9.51 Years ; 25th: -5.23 Years ; 50th: -2.79 Years; 75th: -0.51 Years; and 95th: 3.53 Years. Adjusted for age at baseline, time since FMP, BMI, SBP, LDL-C and log transformed hs-CRP
No significant associations were found between baseline or changes since baseline of HDL-C or apoA and progression of other subclinical measures of atherosclerosis (AD or cPlaque presence), Supplemental Tables 2 and 3.
Discussion
The current study suggests that higher level of HDL-C is not necessarily cardioprotective over the menopausal transition. Increasing HDL-C before the FMP was associated with less progression in cIMT, while increasing HDL-C after the FMP was associated with greater cIMT progression. The modification in the cardioprotective effects of HDL-C on cIMT by time since FMP was independent of multiple covariates, including age, race/ethnicity, education, BMI, SBP, LDL-C, use of CVD medications and hs-CRP. Similar patterns, although not significant, were seen for apoA, the main protein carried on HDL particles.
Clinical studies have used HDL-C as the metric for assessing the cardioprotective effects of HDL. However, emerging evidence has questioned the paradigm that elevating HDL-C is necessarily beneficial. Clinical trials evaluating drugs that elevate levels of HDL-C were terminated early because of an apparent lack of clinical benefit in subjects with established CVD (13, 14). A meta-regression analysis of 108 randomized controlled trials that tested lipid modifying interventions to reduce CVD risk in 299, 310 participants at risk of cardiovascular events, concluded that increasing levels of circulating HDL-C does not reduce the risk of CHD events, CHD death, or all-cause mortality (15). Similar inconsistencies were reported in experimental animals; transgenic-induced elevation in HDL-C increases atherosclerosis, while a major reduction in HDL-C levels by gene transfer, leads to overexpression of HDL receptors, reduces atherosclerosis (16). These observations highlight the complexity of the relationship between HDL-C and CVD and suggest that levels of HDL-C may not fully reflect the actual cardioprotective function of HDL.
In women, evidence not only suggests that levels of HDL-C may not be the best metric to reveal the cardioprotective capacity of HDL, but also proposes a potential change in the quality of HDL as women get older. Earlier studies reported increases in non-fatal stroke, cerebral infarction, carotid and coronary atherosclerosis with higher HDL-C, mainly in older or postmenopausal women (4-7). However none of these studies evaluated the longitudinal impact of time since FMP and the changes in HDL over menopause on progression of atherosclerosis. Findings from the current study were consistent with these previous reports and specifically suggest, for the first time, a critical impact of time elapsed since the FMP on HDL cardioprotective capacity in women. We hypothesize that HDL becomes dysfunctional over the menopausal transition, and that HDL-C as a static measure of the cholesterol contents of HDL particles does not accurately reflect this dysfunction.
Menopause-related hormonal alterations, especially estradiol reduction, stimulate the buildup of CVD risk factors (3). Estradiol is considered a potent antioxidant (17, 18). As women lose this benefit over the menopausal transition, an increase in lipid peroxidation and formation of reactive oxygen species would be expected. This in turn may impact the protein composition of HDL in a way that depletes anti-inflammatory/anti-oxidant proteins and enriches proinflammatory proteins. In a small sample of premenopausal and postmenopausal women (≤ 30 in each group), an ex vivo biochemical analysis of lipoproteins suggested that postmenopausal HDL particles exhibited impairment in its anti-oxidant ability to limit LDL oxidation independent of HDL-C (19). These results suggest that HDL particles may lose certain anti-atherogenic properties over the menopausal transition. One protein component that appears to be involved in the loss of anti-oxidative and anti-inflammatory activities of HDL is complement C3 protein, which was found to be enriched in HDL3 among CAD subjects (20). Interestingly, we reported higher levels of serum C3 in postmenopausal women compared to premenopausal women (21).
The menopause transition coincides with the accumulation of several adverse alterations in sex hormones (22, 23), body weight and fat distribution (24, 25), and lipid/lipoprotein profile (26), may place women at higher risk of chronic inflammation. Whether this inflammation could impair the quality of HDL particles as women transition through menopause will require further research. Adjusting for hs-CRP in our study did not explain the unexpected association between increase in HDL-C and the greater progression of cIMT. This could be because HDL-C is just a crude measure of the cholesterol content of HDL particles and thus does not reflect any changes in other aspects of HDL. HDL is a family of particles that range in diameter from 8 nm to 12 nm and vary 4-fold in cholesterol content (27). Importantly, the protein composition of large and small HDL particles is not the same (20, 28), and therefore HDL particles may have distinct functions based on their size and protein composition. In the Framingham Offspring Study, although levels of HDL-C were inversely associated with CHD, levels of pre-α and α-3 HDL particles (small HDL) were positively correlated with CHD risk (29). HDL has numerous antiatherogenic functions that are not captured by HDL-C. The best recognized antiatherogenic function of HDL particles is their ability to stimulate the reverse cholesterol transport from peripheral cells to the liver (28). Interestingly, lower HDL capacity to induce cholesterol efflux from macrophages predicts future cardiovascular events in apparently healthy subjects, independent of HDL-C levels (30). Taken together, subclasses and functionality of HDL particles may be more important than the cholesterol content and can reflect better any changes in the cardioprotective capacity of HDL. Future studies should assess associations between levels of inflammatory markers in women at midlife and better metrics of HDL quality such as concentrations of HDL subclasses or HDL function measures.
The main limitations of the current study were: 1) the unavailability of an exact FMP date for 45% of the eligible participants. We developed an algorithm (supplemental materials) that utilized all available data from SWAN parent study and were able to impute an FMP date for 75% of those without an FMP date. Sensitivity analyses including only those with known FMP showed exactly similar results, which strongly suggests a low possibility of misclassification bias due to imputing FMP dates, 2) the unavailability of HDL subclasses and function measures over time which limit our ability to assess whether HDL becomes dysfunctional as we have hypothesized, 3) the current study was limited to black and white midlife women, and therefore our findings may not generalize to other racial/ethnic groups, and 4) the low prevalence of plaque presence at the baseline scan which may reduce our power to detect meaningful associations. On the other hand, the main strength of this analysis is being the first to assess role of time elapsed since FMP on associations of changes in HDL-C and apoA and progression of subclinical atherosclerosis over menopause. Our findings clearly suggest a potential role of menopause to explain the paradoxical associations of higher HDL-C with greater CVD risk reported in older women.
In conclusion, as women transition through menopause, increases in HDL-C levels are independently associated with greater cIMT progression. Thus, the quality of HDL may be altered over the menopausal transition rendering HDL dysfunctional and not providing the expected cardioprotective effect. Future studies should evaluate other metrics of HDL over menopause that could better reflect the cardioprotective capacity of HDL particles and clearly determine the source of dysfunctionality. Examining the quality of HDL is an evolving research area with the potential to facilitate the detection of populations at risk for targeting novel therapy.
Supplementary Material
Supplemental Table 1. Beta Coefficients for the Interactions of Baseline and Change in Lipid Measures and time Relative to FMP on Carotid Intima-Media Thickness among Participants with Known Date of Final menstrual Period, N=131 women / 398 observations
Supplemental Table 2. Beta Coefficients for the Interactions of Baseline HDL-C and Change in HDL-C and time Relative to FMP on Carotid Adventitial diameter and Plaque Presence
Supplemental Table 3. Beta Coefficients for the Interactions of Baseline apoA and Change in apoA and time Relative to FMP on Carotid Adventitial diameter and Plaque Presence
Acknowledgments
We would like to thank Dr. Elaine Waetjen, Professor of Obstetrics and Gynecology for her significant contribution to impute the final menstrual period among women who did not report a date.
Clinical Centers: University of Michigan, Ann Arbor – Siobán Harlow, PI 2011 – present, MaryFran Sowers, PI 1994-2011; Massachusetts General Hospital, Boston, MA – Joel Finkelstein, PI 1999 – present; Robert Neer, PI 1994 – 1999; Rush University, Rush University Medical Center, Chicago, IL – Howard Kravitz, PI 2009 – present; Lynda H. Powell, PI 1994 – 2009; University of California, Davis/Kaiser – Ellen Gold, PI; University of California, Los Angeles – Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY – Carol Derby, PI 2011 – present, Rachel Wildman, PI 2010 – 2011; Nanette Santoro, PI 2004 – 2010; University of Medicine and Dentistry – New Jersey Medical School, Newark – Gerson Weiss, PI 1994 – 2004; and the University of Pittsburgh, Pittsburgh, PA – Karen Matthews, PI.
NIH Program Office: National Institute on Aging, Bethesda, MD – Winifred Rossi 2012 - present; Sherry Sherman 1994 – 2012; Marcia Ory 1994 – 2001; National Institute of Nursing Research, Bethesda, MD – Program Officers.
Central Laboratory: University of Michigan, Ann Arbor – Daniel McConnell (Central Ligand Assay Satellite Services).
Coordinating Center: University of Pittsburgh, Pittsburgh, PA – Maria Mori Brooks, PI 2012 - present; Kim Sutton-Tyrrell, PI 2001 – 2012; New England Research Institutes, Watertown, MA - Sonja McKinlay, PI 1995 – 2001.
Steering Committee: Susan Johnson, Current Chair Chris Gallagher, Former Chair
We thank the study staff at each site and all the women who participated in SWAN
Financial support: The Study of Women's Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women's Health (ORWH) (Grants U01NR004061; U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495). SWAN Heart was supported by the National Heart, Lung, and Blood Institute (NHLBI) (Grants HL065581, HL065591). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH.
Footnotes
Conflict of interest/Financial disclosure: Dr. El Khoudary reports grants from AHA, during the conduct of the study; Ms. Wang has nothing to disclose; Drs. Brooks, Thurston, Derby, and Matthews reports grants from NIH, during the conduct of the study.
References
- 1.Ansell BJ, Fonarow GC, Fogelman AM. High-density lipoprotein: is it always atheroprotective? Curr Atheroscler Rep. 2006;8:405–411. doi: 10.1007/s11883-006-0038-4. [DOI] [PubMed] [Google Scholar]
- 2.Barter PJ, Nicholls S, Rye KA, Anantharamaiah GM, Navab M, Fogelman AM. Antiinflammatory properties of HDL. Circ Res. 2004;95:764–772. doi: 10.1161/01.RES.0000146094.59640.13. [DOI] [PubMed] [Google Scholar]
- 3.Shaw LJ, Bugiardini R, Merz CN. Women and ischemic heart disease: evolving knowledge. J Am Coll Cardiol. 2009;54:1561–1575. doi: 10.1016/j.jacc.2009.04.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bots ML, Elwood PC, Nikitin Y, Salonen JT, Freire de Concalves A, Inzitari D, Sivenius J, Benetou V, Tuomilehto J, Koudstaal PJ, Grobbee DE. Total and HDL cholesterol and risk of stroke. EUROSTROKE: a collaborative study among research centres in Europe. J Epidemiol Community Health. 2002;56:i19–i24. doi: 10.1136/jech.56.suppl_1.i19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keidar S, Bogner I, Gamliel-Lazarovich A, Leiba R, Fuhrman B, Kouperberg E. High plasma high-density lipoprotein levels, very low cardiovascular risk profile, and subclinical carotid atherosclerosis in postmenopausal women. J Clin Lipidol. 2009;3:345–350. doi: 10.1016/j.jacl.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Woodard GA, Brooks MM, Barinas-Mitchell E, Mackey RH, Matthews KA, Sutton-Tyrrell K. Lipids, menopause, and early atherosclerosis in Study of Women's Health Across the Nation Heart women. Menopause. 2011;18:376–384. doi: 10.1097/gme.0b013e3181f6480e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fan AZ, Dwyer JH. Sex differences in the relation of HDL cholesterol to progression of carotid intima-media thickness: the Los Angeles Atherosclerosis Study. Atherosclerosis. 2007;195:e191–e196. doi: 10.1016/j.atherosclerosis.2007.03.045. [DOI] [PubMed] [Google Scholar]
- 8.Sowers M, Crawford S, Sternfeld B, Morganstein D, Gold EB, Greendale GA, Evans D, Neer R, Matthews K, Sherman S, Lo A, Weiss G, Kelsey J. SWAN: a multicenter, multiethnic, community-based cohort study of women and the menopausal transition. In: Lobo RA, Kelsey J, Marcus R, editors. Menopause: Biology and Pathology. New York, NY: Academic Press; 2000. pp. 175–188. [Google Scholar]
- 9.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 10.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentration in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 11.Hedeker D, Gibbons R. Longitudinal data analysis. New Jersey: A John Wiley and Son INC; 2006. pp. 69–76. [Google Scholar]
- 12.Longitudinal data analysis with discrete and continuous responses course notes. Cary, NC, USA: SAS Institute Inc; 2009. [Google Scholar]
- 13.Rader DJ, Tall AR. The not-so-simple HDL story: Is it time to revise the HDL cholesterol hypothesis? Nature Medicine. 2012;18:1344–1346. doi: 10.1038/nm.2937. [DOI] [PubMed] [Google Scholar]
- 14.Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJ, Komajda M, Lopez-Sendon J, Mosca L, Tardif JC, Waters DD, Shear CL, Revkin JH, Buhr KA, Fisher MR, Tall AR, Brewer B ILLUMINATE Investigators. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357:2109–2122. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- 15.Briel M, Ferreira-Gonzalez I, You JJ, Karanicolas PJ, Akl EA, Wu P, Blechacz B, Bassler D, Wei X, Sharman A, Whitt I, Alves da Silva S, Khalid Z, Nordmann AJ, Zhou Q, Walter SD, Vale N, Bhatnagar N, O'Regan C, Mills EJ, Bucher HC, Montori VM, Guyatt GH. Association between change in high density lipoprotein cholesterol and cardiovascular disease morbidity and mortality: systematic review and meta-regression analysis. BMJ. 2009;338:b92. doi: 10.1136/bmj.b92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bérard AM, Föger B, Remaley A, Shamburek R, Vaisman BL, Talley G, Paigen B, Hoyt RF, Jr, Marcovina S, Brewer HB, Jr, Santamarina-Fojo S. High plasma HDL concentrations associated with enhanced atherosclerosis in transgenic mice overexpressing lecithin-cholesteryl acyltransferase. Nat Med. 1997;3:744–749. doi: 10.1038/nm0797-744. [DOI] [PubMed] [Google Scholar]
- 17.Ahmad S, Scott JE. Estradiol enhances cell-associated paraoxonase 1 (PON1) activity in vitro without altering PON1 expression. Biochem Biophys Res Commun. 2010;397:441–446. doi: 10.1016/j.bbrc.2010.05.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wakatsuki A, Ikenoue N, Sagara Y. Effects of estrogen on susceptibility to oxidation of low-density and high-density lipoprotein in postmenopausal women. Maturitas. 1998;28:229–234. doi: 10.1016/s0378-5122(97)00072-8. [DOI] [PubMed] [Google Scholar]
- 19.Zago V, Sanguinetti S, Brites F, Berg G, Verona J, Basilio F, Wikinski R, Schreier L. Impaired high density lipoprotein antioxidant activity in healthy postmenopausal women. Atherosclerosis. 2004;177:203–210. doi: 10.1016/j.atherosclerosis.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 20.Vaisar T, Pennathur S, Green PS, Gharib SA, Hoofnagle AN, Cheung MC, Byun J, Vuletic S, Kassim S, Singh P, Chea H, Knopp RH, Brunzell J, Geary R, Chait A, Zhao XQ, Elkon K, Marcovina S, Ridker P, Oram JF, Heinecke JW. Shotgun proteomics implicates protease inhibition and complement activation in the antiinflammatory properties of HDL. The Journal of Clinical Investigation. J Clin Invest. 2007;117:746–56. doi: 10.1172/JCI26206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.El Khoudary SR, Shields KJ, Chen HY, Matthews KA. Menopause, complement, and hemostatic markers in women at midlife: the Study of Women's Health Across the Nation. Atherosclerosis. 2013;231:54–58. doi: 10.1016/j.atherosclerosis.2013.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guthrie JR, Dennerstein L, Taffe JR, Ebeling PR, Randolph JF, Burger HG, Wark JD. Central abdominal fat and endogenous hormones during the menopausal transition. Fertility and Sterility. 2003;79:1335–1340. doi: 10.1016/s0015-0282(03)00361-3. [DOI] [PubMed] [Google Scholar]
- 23.Janssen I, Powell LH, Kazlauskaite R, Dugan SA. Testosterone and visceral fat in midlife women: the Study of Women's Health Across the Nation (SWAN) fat patterning study. Obesity (Silver Spring) 2010;18:604–610. doi: 10.1038/oby.2009.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lovejoy JC, Champagne CM, de Jonge L, Xie H, Smith SR. Increased visceral fat and decreased energy expenditure during the menopausal transition. International Journal of Obesity (Lond) 2008;32:949–958. doi: 10.1038/ijo.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.El Khoudary SR, Shields KJ, Janssen I, Hanley C, Budoff MJ, Barinas-Mitchell E, Everson-Rose SA, Powell LH, Matthews KA. Cardiovascular Fat, Menopause and Sex Hormones in Women: The SWAN Cardiovascular Fat Ancillary Study. J Clin Endocrinol Metab. 2015;100:3304–3312. doi: 10.1210/JC.2015-2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matthews KA, Crawford SL, Chae CU, Everson-Rose SA, Sowers MF, Sternfeld B, Sutton-Tyrrell K. Are changes in cardiovascular disease risk factors in midlife women due to chronological aging or to the menopausal transition? J Am Coll Cardiol. 2009;54:2366–2373. doi: 10.1016/j.jacc.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rothblat GH, Phillips MC. High-density lipoprotein heterogeneity and function in reverse cholesterol transport. Current Opinion in Lipidology. 2010;21:229–238. doi: 10.1097/mol.0b013e328338472d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenson RS, Brewer HB, Jr, Chapman MJ, Fazio S, Hussain MM, Kontush A, Krauss RM, Otvos JD, Remaley AT, Schaefer EJ. HDL measures, particle heterogeneity, proposed nomenclature, and relation to atherosclerotic cardiovascular events. Clin Chem. 2011;57:392–410. doi: 10.1373/clinchem.2010.155333. [DOI] [PubMed] [Google Scholar]
- 29.Asztalos BF, Cupples LA, Demissie S, Horvath KV, Cox CE, Batista MC, Schaefer EJ. High-density lipoprotein subpopulation profile and coronary heart disease prevalence in male participants of the Framingham Offspring Study. Arterioscler Thromb Vasc Biol. 2004;24:2181–2187. doi: 10.1161/01.ATV.0000146325.93749.a8. [DOI] [PubMed] [Google Scholar]
- 30.Rohatgi A, Khera A, Berry JD, Givens EG, Ayers CR, Wedin KE, Neeland IJ, Yuhanna IS, Rader DR, de Lemos JA, Shaul PW. HDL Cholesterol Efflux Capacity and Incident Cardiovascular Events. N Engl J Med. 2014;371:2383–2393. doi: 10.1056/NEJMoa1409065. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Beta Coefficients for the Interactions of Baseline and Change in Lipid Measures and time Relative to FMP on Carotid Intima-Media Thickness among Participants with Known Date of Final menstrual Period, N=131 women / 398 observations
Supplemental Table 2. Beta Coefficients for the Interactions of Baseline HDL-C and Change in HDL-C and time Relative to FMP on Carotid Adventitial diameter and Plaque Presence
Supplemental Table 3. Beta Coefficients for the Interactions of Baseline apoA and Change in apoA and time Relative to FMP on Carotid Adventitial diameter and Plaque Presence


