Abstract
Chronic use of methamphetamine (METH) leads to long-lasting cognitive dysfunction in humans and in animal models. Modafinil is a wake-promoting compound approved for the treatment of sleeping disorders. It is also prescribed off label to treat METH dependence. In the present study, we investigated whether modafinil could improve cognitive deficits induced by sub-chronic METH treatment in mice by measuring visual retention in a Novel Object Recognition (NOR) task. After sub-chronic METH treatment (1 mg/Kg, once a day for 7 days), mice performed the NOR task, which consisted of habituation to the object recognition arena (5 min a day, 3 consecutive days), training session (2 equal objects, 10 min, day 4), and a retention session (1 novel object, 5 min, day 5). One hour before the training session, mice were given a single dose of modafinil (30 or 90 mg/Kg). METH-treated mice showed impairments in visual memory retention, evidenced by equal preference of familiar and novel objects during the retention session. The lower dose of modafinil (30 mg/Kg) had no effect on visual retention scores in METH-treated mice, while the higher dose (90 mg/Kg) rescued visual memory retention to control values. We also measured ERK phosphorylation in medial prefrontal cortex (mPFC), hippocampus, and nucleus accumbens (NAc) of METH- and vehicle-treated mice that received modafinil 1 hr before exposure to novel objects in the training session, compared to mice placed in the arena without objects. Elevated Extracellular signal-regulated kinase (ERK) phosphorylation was found in the mPFC of vehicle-treated mice, but not in METH-treated mice, exposed to objects (p<0.05). The lower dose of modafinil had no effect on ERK phosphorylation in METH-treated mice, while 90 mg/Kg modafinil treatment restored the ERK phosphorylation induced by novelty in METH-treated mice to values comparable to controls (p<0.05). We found neither a novelty nor treatment effect on ERK phosphorylation in hippocampus or nucleus accumbens (NAc) of vehicle- and METH-treated mice receiving acute 90 mg/Kg modafinil treatment. Our results showed a palliative role of modafinil against METH-induced visual cognitive impairments, possibly by normalizing ERK signaling pathways in mPFC. Modafinil may be a valuable pharmacological tool for the treatment of cognitive deficits observed in human METH abusers as well as in other neuropsychiatric conditions.
Keywords: modafinil, methamphetamine, novelty, ERK, prefrontal cortex
INTRODUCTION
Methamphetamine (METH) is a psychostimulant with a high potential for abuse and addiction. Repeated exposure to METH causes abnormal changes in neurotransmitter activity involved in learning, reward, and executive function (Bamford et al., 2008; Feltenstein and See, 2008). METH also alters neuronal plasticity in brain regions that mediate cognition and motivation (Kauer and Malenka, 2007; Robinson and Kolb, 2004). METH addiction generally begins with recreational use and progresses over time into a compulsive and chronically relapsing disorder, accompanied by psychiatric symptoms including hallucinations and delusions, as well as long-term cognitive deficits (Scott et al., 2007). METH-dependent individuals exhibit high rates of cognitive dysfunction in several neuropsychological domains that include sustained attention, episodic memory, information processing, and impulse control (Monterosso et al., 2005; Nordahl et al., 2003; Simon et al., 2010; Morgan et al., 2012). These cognitive deficits might undermine efforts by METH addicts to stop or reduce METH use and negatively affect the outcome of treatment (Vocci and Appel, 2007). Given the potential links between cognition and treatment outcome in METH dependence, therapeutic approaches that improve cognitive function may be quite promising in the management of METH addiction.
Modafinil (Provigil) is a psychostimulant and cognitive enhancer drug, approved by the U.S. Food and Drug Administration for treating narcolepsy and other sleep disorders. Different studies showed that modafinil cognitive-enhancing properties improved outcome in the treatment of pathologic gamblers (Zack and Poulos, 2009), alcoholics (Schmaal et al., 2013), and patients suffering from other neuropsychiatric conditions (Scoriels et al., 2013). The use of modafinil as a treatment for cocaine and METH dependence remains inconclusive, with studies showing positive outcomes (Dackis et al., 2005; McGough et al., 2009) and studies showing promising but yet non-significant results in reducing drug use (Dackis et al, 2012; Shearer et al., 2009; Heinzerling et al., 2010). For both cocaine and METH users, modafinil was efficacious in improving several domains of cognitive and executive functions (Kalechstein et al, 2013; Gharemani et al., 2011; Kalechstein et al., 2010; Hester et al., 2010; Finke et al., 2010).
Modafinil’s mechanism of action, although somewhat poorly understood, appears to involve multiple neurotransmitter systems. For example, modafinil can act as a weak DA transporter (DAT) inhibitor that increases extracellular DA levels (Mereu et al. 2013). Modafinil influences GABAergic, glutamatergic, noradrenergic, serotoninergic, histaminergic, and orexinergic systems (for a review see Mizenberg and Carter, 2008, Scoriels et al., 2013). In addition, modafinil enhances electrotonic coupling by increasing the effectiveness of gap junctions between neurons (Urbano et al., 2007; Garcia-Rill et al., 2007). We also demonstrated that modafinil can protect against METH toxicity (Raineri et al., 2011; 2012). Specifically, modafinil was able to prevent METH-induced toxic effects that included DA depletion and reductions in tyrosine hydroxylase (TH) and DAT levels in the striatum (Raineri et al., 2011). Furthermore, modafinil also attenuated METH-induced hyperthermia, glial activation, and increased expression of proapoptotic proteins (Raineri et al., 2012).
Compared to classical psychostimulants such as cocaine or amphetamine, the sites of action and behavioral effects of modafinil appear to be different (Mereu et al., 2013). Modafinil showed lower liability to abuse and lower risk of adverse effects on organ systems including the cardiovascular system (Minzenberg and Carter, 2008). Clinically relevant modafinil doses can robustly activate fronto-cortical areas involved in higher cognitive functions and a network of pro-arousing areas, which provide a plausible substrate for the wake-promoting and pro-cognitive effects of the drug (Gozzi et al., 2012). Of relevance to the present study, METH-dependent subjects showed a greater effect of modafinil on brain activation in bilateral insula/ventrolateral prefrontal cortex and anterior cingulate cortices than control participants, suggesting that modafinil improves learning in METH-dependent participants by enhancing neural function in those regions (Ghahremani et al., 2011).
The intracellular signaling pathways that mediate modafinil actions in fronto-cortical areas remain unknown. A potential candidate is the mitogen-activated protein kinase extracellular signal-regulated kinase (MAPK-ERK) cascade. The ERK1/2 pathway plays a critical role in memory function under physiological and pathological conditions (Mizoguchi et al. 2004; Kamei et al. 2006; Nagai et al. 2007, Cammarota et al., 2008). ERK1/2 signaling pathway linked to dopamine D1 receptors (Valjent et al., 2000; Zanassi et al., 2001) is involved in METH-associated contextual memory in rats (Mizoguchi et al., 2004). Additionally, it has been demonstrated that repeated METH treatment in mice induced cognitive impairment in a novel object recognition test, which was associated with deficits of the ERK1/2 pathway in the prefrontal cortex (PFC) (Kamei et al., 2006).
As it was mentioned above, there is only limited information on the mechanisms by which modafinil improves cognition in patients with addictive behaviors in terms of underlying neural substrates. Therefore, in the present study, we designed experiments aimed at testing modafinil’s ability to improve cognitive deficits induced by sub-chronic METH treatment in mice. We used a novel object recognition (NOR) task, which is similar to visual recognition tests widely used in subhuman primates (Ennaceur, 2010), and is sensitive to METH-induced cognitive impairments (Bisagno et al., 2002; Kamei et al., 2006; Reichel et al., 2011). The NOR task evaluates the rodents’ ability to recognize a novel object in the environment, and discrimination and memory performance is obtained upon identification of familiarity and novelty (Antunes and Biala, 2012). So, we also examined how modafinil and METH differentially modulated fronto-cortical phosphorylation of the MAPK isoforms, ERK1/ERK2 following exposure to novelty.
MATERIALS AND METHODS
Animals
C57BL/6 male mice (2–3 month-old) from the School of Exact and Natural Sciences of the University de Buenos Aires (UBA) were housed in a light and temperature-controlled room (12-h light/dark cycle, 22 °C), and were given free access to laboratory chow and tap water. Principles of animal care were followed in accordance with “Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research” (National Research Council, 2003) and approved by Universidad de Buenos Aires authorities (Protocol Number: A5801-01) using OLAW and ARENA directives (NIH, Bethesda, USA).
Pharmacological reagents
Drugs were purchased from either Sigma (St. Louis, MO) or Tocris (Ellisville, MO). Modafinil (racemic mixture of R- and S-enantiomers) was generously donated by Laboratorios Beta S.A. (Argentina).
Drug treatments
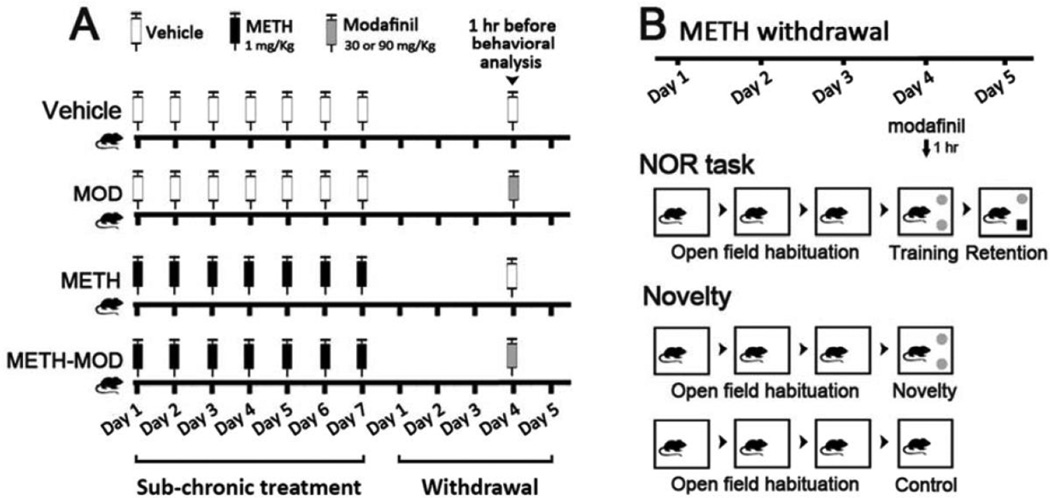
(+)-Methamphetamine hydrochloride (Sigma, St Louis, MO) was administered subcutaneously (sc) once a day for 7 days (1 mg/Kg, calculated as free base, dissolved in sterile saline solution). The METH regimen used in this study was performed according to studies by Kamei et al., (2006). Four days after the last METH injection, modafinil (30 or 90 mg/Kg, dissolved in DMSO-Arabic gum 5% in sterile saline solution) was injected and 1 hour later mice were subjected to behavioral analysis (Novel Object Recognition task or Novelty exposure, Figure1A and 1B). Vehicle groups received the same volume of sterile saline and DMSO/Arabic gum/saline. Drugs were injected at a volume of 10 ml/Kg of body weight.
Figure 1. Schematic representations of the experimental treatments and behavioral analysis.
A) Male C57BL/6 mice were subjected to sub-chronic treatment with saline or METH 1 mg/Kg, sc, once a day for 7 consecutive days. On day 4 after sub-chronic treatment, mice received vehicle or MOD 90 or 30 mg/Kg, ip, 1 hr before behavioral analysis. B) METH withdrawal mice were habituated to the object recognition arena 5 min a day for 3 consecutive days. On withdrawal day 4, mice performed the Novel Object Recognition (NOR) task or were evaluated for the Novelty effect. For the NOR task, mice performed a training session in which they were allowed to freely explore two equal objects for 10 min, and 24 hrs later (day 5) performed a 5 min retention session, where one of the familiar objects was replaced by a novel object. To evaluate the Novelty effect, mice performed a 10 min Novelty session where they were allowed to explore two equal novel objects and euthanized immediately. Control mice were placed in the arena in the absence of objects and the same procedure was performed.
Novel Object Recognition (NOR) task
The NOR task was adapted according to previously reported methods (Kamei et al., 2006). The NOR task evaluates the rodents’ ability to recognize a novel object in the environment. Basically, in the NOR task, there are no positive or negative reinforcers, and this methodology assesses the natural preference for novel objects displayed by rodents (Ennaceur, 2010). The task procedure consists of three phases: habituation, training, and a test phase. In the habituation phase, each animal is allowed to freely explore the arena in the absence of objects. The animal is then removed from the arena and placed in its cage. During the training phase, a single animal is placed in the arena containing two identical sample objects (A + A) for 5 minutes. The experimental context is not drastically different during the training and the test phase. After a retention interval begins the test phase, and the animal is returned to the arena with two objects, one is identical to the sample and the other is novel (A + B). In our experiments, exploration occurred in an open-top arena (40 cm3) made of plexiglass, with the floor covered with clean woodchip bedding. Testing was done during the light phase (8 AM– 8 PM), in a sound-attenuated room, with dimmed illumination (40 W). Mice were individually habituated to the box for 5 min during three consecutive days after the last METH injection, in the absence of objects (Figure 1B: habituation sessions, withdrawal days 1–3). On the fourth day after METH, two identical objects were symmetrically fixed to the floor of the box, 8 cm from one of the walls, and each mouse was allowed to explore the box for 10 min (Figure 1B: training session, withdrawal day 4). Objects were: golf balls, plastic pipes (3 cm diameter, 8 cm high) and plastic cubes (4 cm3), all of which were similar in size but different in color, shape and brightness. Sets of objects were chosen based on preliminary experiments that indicated that they were similarly preferred. Objects were washed with 40% ethanol solution between trials. Following a 24-hour delay, mice were placed back in the box for 5 min where one of the familiar objects was replaced by a novel object (Figure 1B: retention session, withdrawal day 5). The positions of the objects in the test and the objects used as novel or familiar were counterbalanced between the animals in each group and between the control and drug-treated groups. All sessions were recorded and analyzed with Ethovision XT 7.0 tracking software (Noldus, The Netherlands), using nose point-tail base detection. The percentage of exploratory preference (%EP) was calculated as exploration time of the novel object (TN) divided by the total exploration time of both novel (TN) and familiar (TF) objects [%EP=TN/(TN+TF)*100]. Similarly, this calculation can be applied when both objects are identical, in the training session phase, but here the mathematic formula will be exploration time of the right object (TR) divided by the total exploration time of both right (TR) and left (TL) objects [%EP=TR/(TR+TL)*100].
Novelty effect
In a separate set of experiments, mice were individually habituated to object recognition box for 5 min during three consecutive days after the last METH injection (in the absence of objects). On the METH withdrawal day 4, mice were placed in the arena for 10 minutes with objects (Novelty) or without objects (Control) (Figure 1B), and then immediately euthanized. Brains were quickly removed and brain areas were dissected out on an ice-cold plate. Each tissue sample was frozen and stored at −70 °C until processed.
Exploratory activity
We investigated the effect of early METH withdrawal on exploratory activity. Spontaneous locomotor activity was recorded during habituation sessions to the object recognition arena using Ethovision XT 7.0 tracking software (Noldus, The Netherlands). We analyzed locomotor activity (distance travelled) and time spent in the center area of the arena during the 5-min habituation sessions. The center area was defined as one half the total area of the arena.
Western blot
We measured ERK and phosphorylated ERK (pERK) protein expression by Western blot in brain areas of mice subjected to the novelty effect. Brains were removed rapidly after Novelty and Control sessions and brain sections were dissected out and stored at −70 °C. Tissue homogenates were prepared in a solution containing 50 mM Tris-HCl pH 7.5, 150 mM NaCl, 0.1% Triton X100, 0.5% sodium deoxycholate, 0.1% SDS, 1 mM PMSF, 5 µg/ml leupeptin, and 5 µg/ml aprotinin. After removal of cell debris by centrifugation, the protein concentration of the cell lysate was determined. The homogenates were combined with loading buffer containing 4% SDS, 20% glycerol, 10% β-mercaptoethanol, 125 mM Tris, (pH 6.8), and boiled at 100 °C for 5 min. Protein samples (50 µg) were separated by 12.5% SDS-PAGE, and the separated proteins transferred to a PVDF membrane. Immunoblotting was performed using a mouse monoclonal antibody to pERK1/2 (1:500, E-4 sc-7373 Santa Cruz Biotechnology), then membranes were stripped and reprobed with polyclonal rabbit antibody to total ERK2 (1:2000, C-14 sc-154 Santa Cruz Biotechnology). Because there was no change in the levels of total ERK, values of pERK were normalized to the values of total ERK. Immune complexes were detected with anti-mouse and anti-rabbit secondary antibodies and chemiluminescence reagents (Amersham, NJ, USA). Bands were quantified using ImageJ (NIH).
Statistical analysis
InfoStat software (www.infostat.com.ar) was used for statistical comparisons. Statistics were performed using either one-way (treatment) or two-way (novelty and treatment) ANOVA followed by Bonferroni post hoc tests or planned contrasts. Data were transformed when required. Kruskal Wallis ANOVA on Ranks was performed when data did not comply with the assumptions of parametric tests. For the analysis of exploratory activity during habituation sessions, repeated measures two-way ANOVA followed by Bonferroni was performed. Differences were considered significant if p<0.05.
RESULTS
1. Effects of modafinil and methamphetamine on exploratory activity and object recognition memory
1.a Effect of methamphetamine withdrawal on exploratory activity
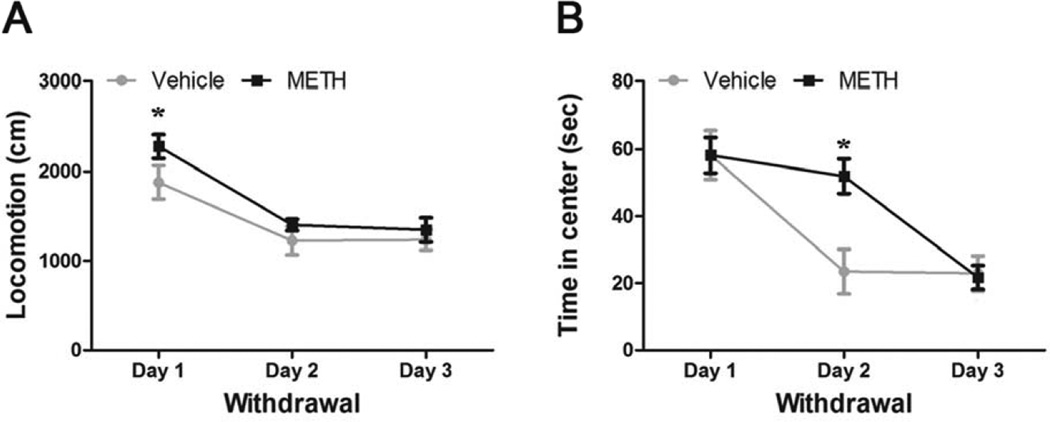
As shown in Figure 1A, mice were treated with a chronic METH or vehicle protocol (METH 1 mg/Kg/day for 7 consecutive days). Next, mice were placed in an object recognition arena 5-min a day for 3 consecutive days, as part of the habituation sessions for the NOR task. We recorded these habituation sessions and analyzed exploratory activity during METH withdrawal, measuring locomotion and time spent in the center of the arena (Figure 2). Repeated measures two-way ANOVA for locomotion showed significant day effect [F(2,50)=21.75, p<0.001], but no treatment or interaction effect (Figure 2A). Bonferroni post hoc test showed that on day 1 of withdrawal both METH- and vehicle-treated mice manifested increased locomotion (p<0.05), compared to day 2 and 3, suggesting habituation to the object recognition arena. Repeated measures two-way ANOVA for time in center showed significant treatment [F(1,50)=5.14, p<0.05], day [F(2,50)=18.81, p<0.001], and interactive [F(2,50)=3.69, p<0.05] effects (Figure 2B). Bonferroni post hoc test showed that on day 2 the Vehicle group diminished the time spent in the center (p<0.05), while the METH group did not. On day 3 both groups spent similar amounts of time exploring the center. These results indicate that, by the time training sessions of the NOR task had started, both METH and control mice showed similar exploratory activity and were similarly habituated to the object recognition arena.
Figure 2. Effect of chronic METH withdrawal on exploratory activity.
Mice were treated with vehicle or METH (1 mg/Kg, sc, once a day for 7 days), and on withdrawal were placed in an open-top arena in a 5 min habituation session for 3 consecutive days. A) Locomotion, *: Day 1 different from Day 2 and Day 3 (p<0.05). B) Time in center, *: vehicle different from METH in Day 2. Exploratory activity during METH withdrawal was evaluated using Ethovision XT 7.0 tracking software (Noldus, Leesburg, Virginia). Repeated measures two-way ANOVA - Bonferroni. Values indicate mean ± SEM (N=8–9),
1.b Acute modafinil effects on methamphetamine withdrawal: object recognition memory
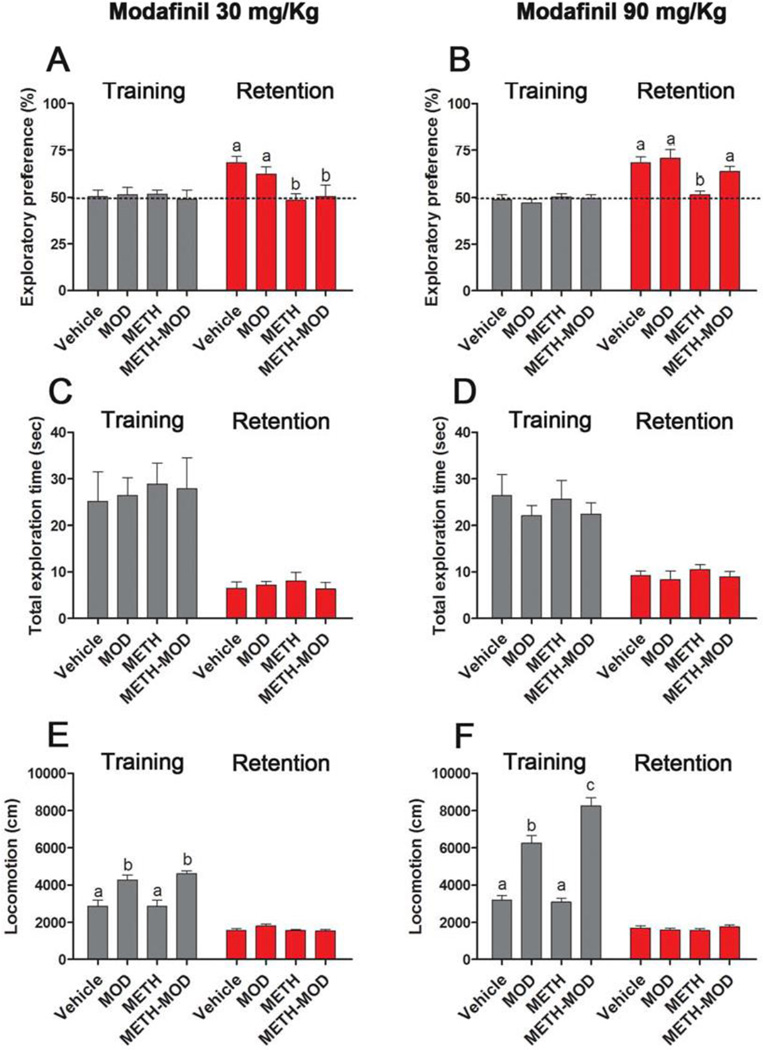
On day 4 of withdrawal, mice received acute vehicle, 30 or 90 mg/Kg modafinil dose and 1 hr later performed the training session of the NOR task. As expected, one way ANOVA found no significant differences in the exploratory preference for the training sessions after either 30 or 90 mg/Kg modafinil (Figure 3A and B). The retention session was performed 24 hrs later, and both 30 mg/Kg and 90 mg/Kg modafinil groups showed significant treatment effect (modafinil 30 mg/Kg: Kruskal Wallis ANOVA on ranks [H=10.26, p<0.05]; modafinil 90 mg/Kg retention session: one way ANOVA-Bonferroni [F(3,70)=8.11, p<0.001]). For both 30 and 90 mg/Kg groups, post hoc tests showed impaired object recognition memory in METH-treated mice, evidenced by similar preference of familiar and novel objects during the retention session of the NOR task (Figure 3A and B). Acute 30 mg/Kg modafinil did not improve object recognition deficits observed in METH-treated mice (Figure 3A). Modafinil at a higher dose, 90 mg/Kg, improved METH-induced object recognition deficits, evidenced by a significant increase in the preference index in the METH-MOD group compared to the METH group (p<0.05), to values comparable to those of the Vehicle group (Figure 3B). Modafinil-treated subjects (for 30 and 90 mg/Kg) showed preference values similar to the Vehicle group, and significantly higher compared to those of the METH group (p<0.05). Total time spent in object exploration during the training and retention sessions did not differ among groups for the two modafinil doses (Figure 3C and D). These results suggest that chronic METH treatment did not have an effect on motivation and exploration, suggesting METH-induced memory impairment rather than a lack of interest in novel stimuli. We also measured locomotor activity during training and retention sessions (Figure 3E and F). Both 30 mg/Kg and 90 mg/Kg modafinil groups showed significant treatment effect in the training session (MOD 30 mg/Kg: one way ANOVA-Bonferroni [F(3,38)=11.45, p<0.001]; MOD 90 mg/Kg: one way ANOVA-Bonferroni [F(3,77)=66.47, p<0.001]). Post hoc tests showed no differences in locomotor activity of Vehicle- and METH-treated mice, while the acutely MOD-treated mice (MOD and METH-MOD groups) showed an increase in locomotor activity compared to Vehicle and METH groups at both modafinil doses (p<0.05). It is noteworthy that the 90 mg/Kg the METH-MOD group exhibited higher locomotor activity compared with modafinil group values (p<0.05) (Figure 3E). One way ANOVA found no significant differences in locomotion for the retention sessions in either 30 or 90 mg/Kg modafinil groups. In addition, modafinil-induced improvement of object recognition memory deficits after METH was replicated in female mice (data not shown). We used the same protocol previously described for male mice using modafinil at a dose that showed positive results, i.e. 90 mg/Kg. Exploratory preferences in retention session (%) were as follows: Vehicle: 65.01±4.20 [a], MOD: 66.34±3.61 [a], METH: 44.91±8.41 [b], METH-MOD: 66.21±7.01 [a], one way ANOVA-Bonferroni [F(3,24)=3.02, p<0.05].
Figure 3. Effect of MOD on METH-induced cognitive impairment in a Novel Object Recognition task.
A and B) Exploratory preference in training and retention sessions of chronic vehicle- and METH-treated mice, injected on withdrawal day 4 with vehicle or MOD 90 and 30 mg/Kg, respectively; the dotted line at 50% indicates equal preference for both familiar and novel object, indicative of visual memory impairment. MOD 90 mg/Kg retention session: ANOVA - Bonferroni. MOD 30 mg/Kg retention session: Kruskal Wallis ANOVA on ranks. C and D) Total exploration time in training and retention sessions of chronic vehicle- and METH-treated mice, injected on withdrawal day 4 with vehicle or MOD 90 and 30 mg/Kg, respectively. E and F) Locomotion in training and retention sessions of chronic vehicle- and METH-treated mice, injected on withdrawal day 4 with vehicle or MOD 90 and 30 mg/Kg, respectively. Time spent exploring objects and locomotion was analyzed with Ethovision XT 7.0 (Leesburg, Virginia). ANOVA - Bonferroni. Values indicate mean ± SEM (N=12–22), different letters: p<0.05.
2. Effects of modafinil and methamphetamine locomotor activity and ERK phosphorylation induced by novelty
The results obtained in the NOR task showed that modafinil, given at a high 90 mg/Kg dose before the first exposure to novel objects, was able to enhance long-term memory retention by restoring the novelty preference in a retention session performed 24 hrs later. As mentioned above, the NOR task evaluates the rodents’ ability to recognize a novel object in the environment and discrimination and memory performance is obtained upon identification of familiarity and novelty (Antunes and Biala, 2012). Novelty is a change from expected likelihood of an event on the basis of both previous information and internal estimates of conditional probabilities (Antunes and Biala, 2012). Animals can be affected by a novel stimulus; i.e. novelty can change the animals’ behavior, induce stress responses, and elicit approach behavior (Bevins et al., 2002). Therefore, we conducted separate experiments in mice subjected to METH withdrawal aimed at investigating novelty-induced changes on mPFC ERK phosphorylation and exploratory activity with or without modafinil administration.
2.a Effect of acute modafinil on mice exposed to novel objects during methamphetamine withdrawal: locomotor activity
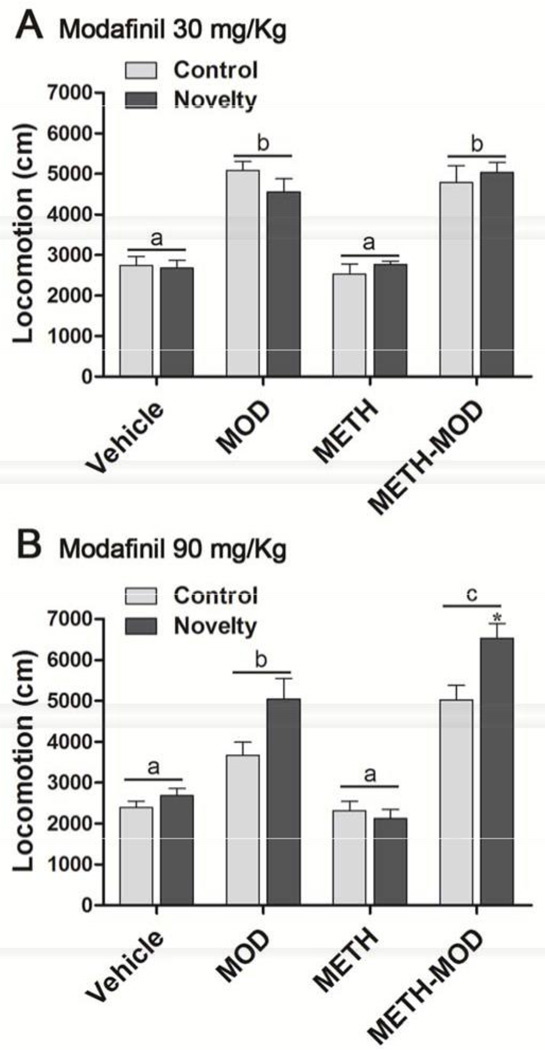
To asses novelty-induced locomotor activity, mice were placed in the arena with or without objects (Novelty and Control groups, respectively) for 10 min, and euthanized immediately (Figure 1B). We recorded Control and Novelty sessions and analyzed locomotor activity. For the 30 mg/Kg modafinil group, two way ANOVA showed significant treatment effect [F(3,33)=56.45, p<0.001], but no novelty or interactive effects. For the 90 mg/Kg modafinil group, two way ANOVA showed significant treatment [F(3,62)=53.30, p<0.001], novelty [F(1,62)=10.43, p<0.01], and interactive [F(3,62)=3.33, p<0.05] effects. Bonferroni post hoc tests demonstrated that Vehicle and METH groups showed neither treatment nor novelty effects on locomotion (Figure 4A and B). Mice given acute 30 mg/Kg modafinil displayed only a main treatment effect, evidenced by MOD and METH-MOD groups showing increased locomotion compared with Vehicle and METH groups (Figure 4A). In agreement with locomotor responses obtained in mice while performing the NOR task (Figure 3E), the MOD and METH-MOD groups receiving 30 mg/Kg modafinil showed similar locomotor effects. In mice receiving acute 90 mg/Kg MOD (Figure 4B), we found elevated locomotion in the MOD and METH-MOD groups compared to Vehicle and METH groups; also, with the 90 mg/Kg dose the METH-MOD group showed higher locomotion than the MOD group. This is in agreement with locomotor responses obtained for the same dose in the NOR task (Figure 3F). We also found within the METH-MOD group increased locomotion in the novelty group when compared to the control group (p<0.05).
Figure 4. Effect of MOD on METH-induced changes in locomotion after novelty exposure.
On withdrawal day 4 after chronic vehicle or METH treatment (1 mg/Kg, sc, once a day for 7 days) mice received acute modafinil and 1 hr later performed a 10 min Novelty session in which they were allowed to explore two equal novel objects and euthanized immediately. Control mice were placed in the arena in the absence of objects and the same procedure was performed. A) Modafinil 30 mg/Kg, B) Modafinil 90 mg/Kg. Locomotion was analyzed with Ethovision XT 7.0 (Leesburg, Virginia). Two way ANOVA - Bonferroni. Values indicate the mean ± SEM (N=4– 9), different letters: p<0.05, *: different from METH-MOD control.
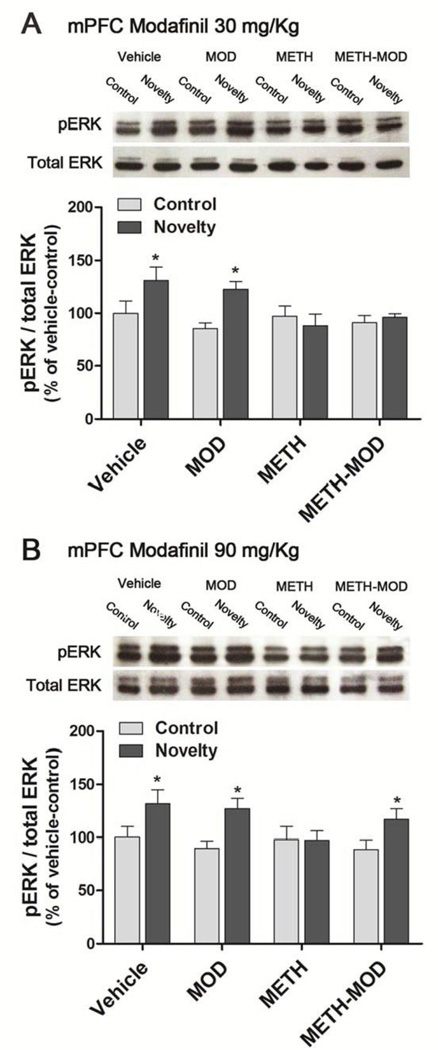
2.b Effect of acute modafinil on mice exposed to novel objects during methamphetamine withdrawal: ERK phosphorylation in mPFC
It was previously shown that one of the molecular mechanisms involved in METH-induced object recognition deficits involve a failure in mPFC ERK phosphorylation after exposure to novel objects (Kamei et al., 2006). Thus, we decided to investigate if modafinil would be able to restore ERK signaling pathway in the mPFC after a Novelty exposure. For both 30 mg/Kg and 90 mg/Kg modafinil experiments, two way ANOVA showed significant novelty effect [modafinil 30 mg/Kg: F(1,35)=5.41, p<0.05; modafinil 90 mg/Kg: F(1,47)=10.33, p<0.01], but no treatment or interactive effects. For both experiments, planned contrasts comparing control vs. novelty within each treatment revealed a significant increase in ERK phosphorylation in the mPFC of the Vehicle and MOD groups immediately after the Novelty session (p<0.05), which was abolished in the METH group. We found that, in agreement with the retention results obtained in the NOR task, METH-MOD group receiving acute 30 mg/Kg modafinil showed no novelty-induced increase in ERK phosphorylation (Figure 5A). In mice that received 90 mg/Kg modafinil we observed that the novelty-induced ERK phosphorylation was restored (p<0.05) (Figure 5B). The levels of total ERK did not differ between experimental groups in any case [modafinil 30 mg/Kg: F(7,35)=1.75, p>0.05; modafinil 90 mg/Kg: F(7,47)=1.00, p>0.05].
Figure 5. Effect of modafinil on METH-induced changes in ERK phosphorylation in the mPFC after novelty exposure.
On withdrawal day 4 after chronic vehicle or METH treatment (1 mg/Kg, sc, once a day for 7 days) mice received acute MOD and 1 hr later performed a 10 min novelty session, then euthanized immediately (novelty group). Control mice were placed in the arena in the absence of objects and the same procedure was performed (control group). A) Modafinil 30 mg/Kg. B) Modafinil 90 mg/Kg. Two way ANOVA followed by planned contrasts between control and novelty within each treatment. Values indicate the mean ± SEM (N=4–9), *: significantly different from control of the respective treatment.
2.c Effect of acute modafinil on mice exposed to novel objects during methamphetamine withdrawal: ERK phosphorylation in the NAc and hippocampus
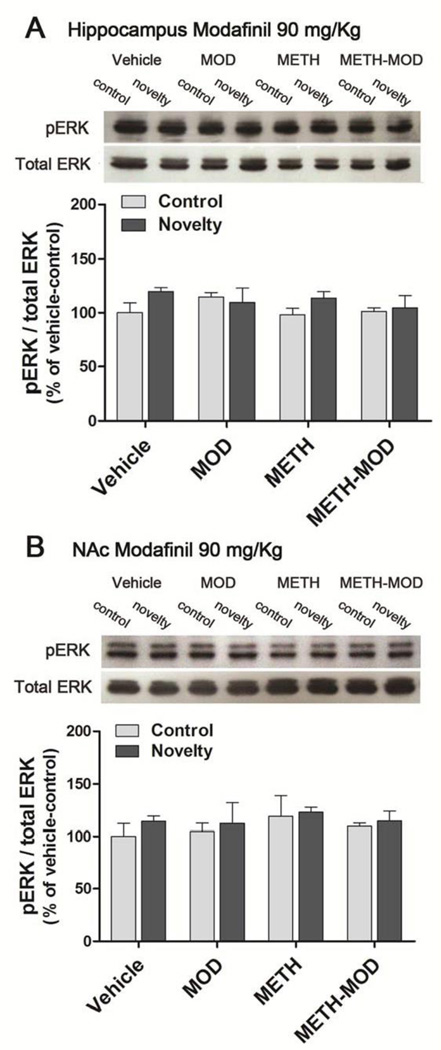
The hippocampus participates in the formation and consolidation of new memories and it was previously shown that MAPK and ERK play important roles (Cammarota et al., 2008). Therefore, we measured novelty-induced ERK phosphorylation in the hippocampus of METH-treated mice, with or without an injection of 90 mg/Kg modafinil (Figure 6A). We found no changes in ERK phosphorylation in the hippocampus in any group subjected to the novelty effect [F(7,31)=1.00, p>0.05] (Figure 6A). The levels of total ERK did not differ between experimental groups in any condition [F(7,31)=1.24, p>0.05].
Figure 6. Effect of MOD on METH-induced changes in ERK phosphorylation in the hippocampus and nucleus accumbens (NAc) after novelty exposure.
On withdrawal day 4 after chronic vehicle or METH treatment (1 mg/Kg, sc, once a day for 7 days) mice received acute modafinil 90 mg/Kg and 1 hr later performed a 10 min novelty session, then were euthanized immediately (novelty group). Control mice were placed in the arena in the absence of objects and the same procedure was performed (control group). A) Hippocampus, B) Nucleus accumbens (NAc). Two way ANOVA. Values indicate the mean ± SEM (N=3–5).
There is evidence suggesting that the NAc is involved in the behavioral response to novelty and other tasks that rely on familiarity discrimination (Sargolini et al., 2003; Nelson et al., 2010). Therefore, we evaluated novelty-induced ERK phosphorylation in the NAc of METH-treated mice, with or without an injection of 90 mg/Kg modafinil (Figure 6B). We found no changes in ERK phosphorylation in NAc in any group subjected to the novelty effect [F(7,26)=0.35, p>0.05]. The levels of total ERK did not differ between experimental groups in any condition [F(7,26)=0.96, p>0.05].
DISCUSSION
The main finding of this study is that acute modafinil administration improved METH-induced object recognition memory deficits. METH-treated mice also showed a failure in ERK phosphorylation in the mPFC, which, in rodents, is detected shortly after a novel object is presented for exploration (Kamei et al., 2006; Nagai et al., 2007). Modafinil administration also restored novelty-induced ERK phosphorylation in the mPFC. To our knowledge, this is the first study addressing modafinil’s beneficial effects on the visual recognition deficits induced by METH in an animal model. Modafinil also showed positive effects in animal models of neurodegeneration (Garcia et al., 2013). Interestingly, modafinil did not modify cognition in naïve rats but was able to reverse memory deficits induced by iron overload, a model of memory impairment related to neurodegenerative disorders (Garcia et al., 2013). Furthermore, these authors used several doses of MOD (0.75, 7.5 or 75 mg/Kg) and found that only the highest dose tested (75 mg/Kg) was able to restore object recognition deficits (Garcia et al., 2013). These data are in agreement with results from the present study since we have observed that modafinil was only effective at a dose of 90 mg/Kg, while 30 mg/Kg modafinil did not show protective effects. Our results are also in agreement with previous studies in humans, where modafinil administration was able to improve cognitive deficits and prefrontal cortical function only in METH-dependent subjects (Gharemani et al., 2011). Additionally, it has been shown that modafinil is more effective in individuals who perform poorly on cognitive tasks relative to those subjects who perform better, indicating that modafinil does not enhance baseline performance but improves cognition in subjects with evident cognitive impairments (Kalechstein et al., 2010; Finke et al., 2010). Recent clinical studies showed that modafinil oral administration in METH users with 7 days of abstinence, at a dose recommended for the treatment of sleeping disorders (200 mg, once daily), although improved several domains of executive function, had no effect on visual memory performance (Hester et al., 2010). Accordingly, Finke and co-workers (2010) showed improved visual processing in participants with low performance baseline when using a higher modafinil dose (400 mg once daily). All together, these results from clinical and pre- clinical studies suggest that a high dose of modafinil might be needed in order to achieve improvements in cognitive functions.
The mPFC mediates executive function and decision making processes, and is therefore a key neuroanatomical region in addictive behaviors (Schoenbaum and Shaham, 2008). Studies using functional magnetic resonance imaging have shown that PFC dysfunction in METH abusers is associated with cognitive impairment (Paulus et al., 2002), probably linked to deficits in DA neurotransmission (Tritsch and Sabatini, 2012). Although the effects of modafinil on midbrain DA neuronal activity remain inconsistent, modafinil acts as a weak DAT inhibitor (Mereu et al., 2013). Consequently, it has been shown that acute modafinil administration increased extracellular DA levels in the mPFC (de Saint Hilaire et al., 2001), the striatum of rodents (Dopheide et al., 2007; Nguyen et al., 2011; Young et al., 2011) and humans (Volkow et al., 2009). Acute modafinil also induced a strong activation in those areas, measured using PET and confirmed by c-Fos immunoreactivity (Gozzi et al., 2012). Modafinil was also found to rescue visual object recognition in a rat model of schizophrenia (Redrobe et al., 2010), which is a condition that presents with diminished dopamine neurotransmission in PFC (Okubo et al., 1997). Thus, modafinil’s ability to enhance cognitive performance in METH abusers might be associated with modafinil’s actions on the DAT (Mereu et al. 2013), stimulating dopaminergic neurotransmission in the mesocorticolimbic system.
It is known that ERK, a member of the MAPK family, is critically linked to dopaminergic neurotransmission and long-term memory retention, but not memory acquisition or short term memory (Valjent et al 2000; Nagai et al., 2007). ERK signaling is activated by stimuli associated with synaptic activity and plasticity, like calcium influx and neurotrophins, and its activation mediates, in part, new mRNA and protein synthesis necessary for long-term memory storage (Cammarota et al., 2008; Kelly et al., 2003). There is also accumulating evidence implicating the ERK pathway in behavioral responses to addictive drugs such as METH and cocaine (Mizoguchi et al 2004; Valjent et al 2000). ERK expression is especially abundant in the mesocorticolimbic DA system, a distribution that underscores the importance of the ERK signaling cascade in mediating DA function (Valjent et al., 2004). ERK signaling is also known to be critically linked to DA D1 receptor activation, which couples with Gs proteins leading to cAMP and PKA cascade activation (Valjent et al., 2000; Nagai et al., 2007). Activated ERK can promote the expression of genes associated with memory and learning, in part, by activating transcription factors such as CREB and Elk-1, which in turn activate gene transcription involved in synaptic and neuronal plasticity such as c-Fos, Zif268, etc. (Cammarota et al., 2008; Zhai et al., 2008). Our results indicate that modafinil modulates ERK phosphorylation in mPFC in a dose-dependent manner, which might be related to modafinil’s ability to enhance DA neurotransmission and stimulation of D1 receptors (Young and Geyer, 2010).
It is well known that repeated exposure to drugs of abuse results in a progressive and long-lasting enhancement of the locomotor response, a phenomenon termed psychomotor (or locomotor) sensitization (Robinson and Berridge, 1993). It is also of interest to discuss our results showing that modafinil induced a cross-sensitization effect with METH. We showed that the same modafinil dose that enhanced visual retention in METH-treated mice, 90 mg/Kg, also elicited a cross-sensitization effect in locomotion. A previous study in mice also found locomotor cross-sensitization between METH and modafinil assessed by challenging modafinil -pretreated mice with 1 mg/Kg METH, and METH-pretreated mice with 50 mg/Kg modafinil (Soeiro et al., 2012). Our results on cross-sensitization between METH and modafinil raise the possibility that neuroadaptations induced by METH and evidenced by acute administration of modafinil might be related to the beneficial effects of modafinil on cognition.
There is some discrepancy in the role of novelty-mediated ERK signaling in the hippocampus, since ERK hyperphosphorylation after training session was observed in rats (Kelly et al., 2003), but not in mice (Kamei et al., 2006). In the present study, we found no novelty-mediated changes in ERK phosphorylation in the hippocampus of the experimental groups, in agreement with the studies performed by Kamei and co-workers (2006) in ICR mice. A possible explanation for the differences observed between species may be that the study performed in rats analyzed specifically the dentate gyrus and CA1 regions of the hippocampus, while the studies in mice where performed on the whole structure, which may have diluted the differences. Nonetheless, our results are in agreement with recent reports showing that the hippocampus, together with the perirhinal and the prefrontal cortices, is involved in the object location and object-in-place tasks, which have a spatial processing component, but not in the novel object recognition task (Forwood et al., 2005; Barker and Warburton, 2011). We also found no novelty-mediated ERK phosphorylation in the NAc of the experimental groups. The role of NAc in novelty processing is controversial, with reports indicating a NAc role only in tasks that involve spatial processing (Mele et al., 2004; Nelson et al., 2010), and reports indicating that the NAc also participates in visual object (non-spatial) processing (Sargolini et al., 2003). Our results indicate that, whether or not the NAc is involved in novelty processing, the ERK pathway in this structure was not affected by either METH or modafinil.
Imaging studies show that drug abusers have marked decreases in DA receptors and DA release. This decrease in DA function is associated with reduced activity in brain areas that mediate inhibitory control and executive function, such as the cingulate gyrus and the dorsolateral PFC. Deficits in DA activity, linked with prefrontal and striatal deregulation, play a central role in the loss of control and compulsive drug intake behavior (Volkow et al., 2009). Although the mechanisms underlying modafinil-induced improvements in prefrontal function and cognitive performance have yet to be delineated, our results further support the notion that modafinil represents an effective therapeutic intervention aimed at restoring brain dopaminergic tone, and therefore restoring intracellular pathways like the MAPK-ERK pathway in the PFC. Moreover, results from the present study further support the hypothesis that drugs that could modulate ERK pathways might in turn be useful for the treatment of the cognitive consequences of drug abuse. Specifically, our results point to modafinil as a good candidate for the treatment of METH dependence, considering modafinil therapy as an agonist-like replacement therapy (Herin et al., 2010).
Highlights.
Methamphetamine produces recognition memory deficits and failures in cortical ERK signaling.
Acute modafinil administration improved methamphetamine-induced memory deficits.
Modafinil restored ERK signaling in medial prefrontal cortex induced by novelty.
We report modafinil protective properties against methamphetamine harmful effects.
Acknowledgments
Dr. Bisagno has been authorized to study drug abuse substances in animal models by A.N.M.A.T. (National Board of Medicine Food and Medical Technology, Ministerio de Salud, Argentina). Dr. Betina Gonzalez is a recipient of a Postdoctoral Award from Fundación Bunge y Born. This work is supported by grants PIP 11420100100072 and PICT 2012-0924, Argentina, and by NIH award P20 GM103425 to the Center for Translational Neuroscience.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Antunes M, Biala G. The novel object recognition memory: neurobiology, test procedure, and its modifications. Cognitive Processing. 2012;13(2):93–110. doi: 10.1007/s10339-011-0430-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamford NS, Zhang H, Joyce JA, Scarlis CA, Hanan W, Wu NP, André VM, Cohen R, Cepeda C, Levine MS, Harleton E, Sulzer D. Repeated exposure to methamphetamine causes long-lasting presynaptic corticostriatal depression that is renormalized with drug readministration. Neuron. 2008;58(1):89–103. doi: 10.1016/j.neuron.2008.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker GR, Warburton EC. When is the hippocampus involved in recognition memory? J Neurosci. 2011;31(29):10721–10731. doi: 10.1523/JNEUROSCI.6413-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevins RA, Besheer J, Palmatier MI, Jensen HC, Pickett KS, Eurek S. Novel-object place conditioning: behavioral and dopaminergic processes in expression of novelty reward. Behav Brain Res. 2002;129(1–2):41–50. doi: 10.1016/s0166-4328(01)00326-6. [DOI] [PubMed] [Google Scholar]
- Bisagno V, Ferguson D, Luine VN. Short toxic methamphetamine schedule impairs object recognition task in male rats. Brain Res. 2002;940(1–2):95–101. doi: 10.1016/s0006-8993(02)02599-4. [DOI] [PubMed] [Google Scholar]
- Cammarota M, Bevilaqua LR, Medina JH, Izquierdo I. ERK1/2 and CaMKII-mediated events in memory formation: is 5HT regulation involved? Behav Brain Res. 2008;195(1):120–128. doi: 10.1016/j.bbr.2007.11.029. [DOI] [PubMed] [Google Scholar]
- Dackis CA, Kampman KM, Lynch KG, Pettinati HM, O'Brien CP. A double-blind, placebo-controlled trial of modafinil for cocaine dependence. Neuropsychopharmacology. 2005;30(1):205–211. doi: 10.1038/sj.npp.1300600. [DOI] [PubMed] [Google Scholar]
- Dackis CA, Kampman KM, Lynch KG, Plebani JG, Pettinati HM, Sparkman T, O'Brien CP. A double-blind, placebo-controlled trial of modafinil for cocaine dependence. J Subst Abuse Treat. 2012;43(3):303–312. doi: 10.1016/j.jsat.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Saint Hilaire Z, Orosco M, Rouch C, Blanc G, Nicolaidis S. Variations in extracellular monoamines in the prefrontal cortex and medial hypothalamus after modafinil administration: a microdialysis study in rats. Neuroreport. 2001;12(16):3533–3537. doi: 10.1097/00001756-200111160-00032. [DOI] [PubMed] [Google Scholar]
- Dopheide MM, et al. Modafinil evokes striatal [(3)H]dopamine release and alters the subjective properties of stimulants. European Journal of Pharmacology. 2007;568(1–3):112–123. doi: 10.1016/j.ejphar.2007.03.044. [DOI] [PubMed] [Google Scholar]
- Ennaceur A. One-trial object recognition in rats and mice: methodological and theoretical issues. Behav Brain Res. 2010;215(2):244–254. doi: 10.1016/j.bbr.2009.12.036. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, See RE. The neurocircuitry of addiction: an overview. Br J Pharmacol. 2008;154:261–274. doi: 10.1038/bjp.2008.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finke K, Dodds CM, Bublak P, Regenthal R, Baumann F, Manly T, Müller U. Effects of modafinil and methylphenidate on visual attention capacity: a TVA-based study. Psychopharmacology (Berl) 2010;210(3):317–329. doi: 10.1007/s00213-010-1823-x. [DOI] [PubMed] [Google Scholar]
- Forwood SE, Winters BD, Bussey TJ. Hippocampal lesions that abolish spatial maze performance spare object recognition memory at delays of up to 48 hours. Hippocampus. 2005;15(3):347–355. doi: 10.1002/hipo.20059. [DOI] [PubMed] [Google Scholar]
- Garcia VA, Souza de Freitas B, Busato SB, D'avila Portal BC, Piazza FC, Schröder N. Differential effects of modafinil on memory in naïve and memory-impaired rats. Neuropharmacology. 2013;75C:304–311. doi: 10.1016/j.neuropharm.2013.07.038. [DOI] [PubMed] [Google Scholar]
- Garcia-Rill E, Heister DS, Ye M, Charlesworth A, Hayar A. Electrical coupling: novel mechanism for sleep-wake control. Sleep. 2007;30(11):1405–1414. doi: 10.1093/sleep/30.11.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghahremani DG, Tabibnia G, Monterosso J, Hellemann G, Poldrack RA, London ED. Effect of modafinil on learning and task-related brain activity in methamphetamine-dependent and healthy individuals. Neuropsychopharmacology. 2011;36(5):950–959. doi: 10.1038/npp.2010.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozzi A, Colavito V, Seke Etet PF, Montanari D, Fiorini S, Tambalo S, Bifone A, Zucconi GG, Bentivoglio M. Modulation of fronto-cortical activity by modafinil: a functional imaging and fos study in the rat. Neuropsychopharmacology. 2012;37(3):822–837. doi: 10.1038/npp.2011.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzerling KG, Swanson AN, Kim S, Cederblom L, Moe A, Ling W, Shoptaw S. Randomized, double-blind, placebo-controlled trial of modafinil for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2010;109(1–3):20–29. doi: 10.1016/j.drugalcdep.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herin DV, Rush CR, Grabowski J. Agonist-like pharmacotherapy for stimulant dependence: preclinical, human laboratory, and clinical studies. Ann N Y Acad Sci. 2010;1187:76–100. doi: 10.1111/j.1749-6632.2009.05145.x. [DOI] [PubMed] [Google Scholar]
- Hester R, Lee N, Pennay A, Nielsen S, Ferris J. The effects of modafinil treatment on neuropsychological and attentional bias performance during 7-day inpatient withdrawal from methamphetamine dependence. Exp Clin Psychopharmacol. 2010;18(6):489–497. doi: 10.1037/a0021791. [DOI] [PubMed] [Google Scholar]
- Kalechstein AD, De La Garza R, 2nd, Newton TF. Modafinil administration improves working memory in methamphetamine-dependent individuals who demonstrate baseline impairment. Am J Addict. 2010;19(4):340–344. doi: 10.1111/j.1521-0391.2010.00052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalechstein AD, Mahoney JJ, 3rd, Yoon JH, Bennett R, De la Garza R., 2nd Modafinil, but not escitalopram, improves working memory and sustained attention in long-term, high-dose cocaine users. Neuropharmacology. 2013;64:472–478. doi: 10.1016/j.neuropharm.2012.06.064. [DOI] [PubMed] [Google Scholar]
- Kamei H, Nagai T, Nakano H, Togan Y, Takayanagi M, Takahashi K, Kobayashi K, Yoshida S, Maeda K, Takuma K, Nabeshima T, Yamada K. Repeated methamphetamine treatment impairs recognition memory through a failure of novelty-induced ERK activation in the prefrontal cortex of mice. Biol Psychiatry. 2006;59(1):75–84. doi: 10.1016/j.biopsych.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- Kelly A, Laroche S, Davis S. Activation of mitogen-activated protein kinase/extracellular signal-regulated kinase in hippocampal circuitry is required for consolidation and reconsolidation of recognition memory. J Neurosci. 2003;23(12):5354–5360. doi: 10.1523/JNEUROSCI.23-12-05354.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnova IN, Cadet JL. Methamphetamine toxicity and messengers of death. Brain Res Rev. 2009;60(2):379–340. doi: 10.1016/j.brainresrev.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh J, Mancino MJ, Feldman Z, Chopra MP, Gentry WB, Cargile C, Oliveto A. Open-label pilot study of modafinil for methamphetamine dependence. J Clin Psychopharmacol. 2009;29(5):488–491. doi: 10.1097/JCP.0b013e3181b591e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mele A, Avena M, Roullet P, De Leonibus E, Mandillo S, Sargolini F, Coccurello R, Oliverio A. Nucleus accumbens dopamine receptors in the consolidation of spatial memory. Behav Pharmacol. 2004;15(5–6):423–431. doi: 10.1097/00008877-200409000-00017. [DOI] [PubMed] [Google Scholar]
- Mereu M, Bonci A, Newman AH, Tanda G. The neurobiology of modafinil as an enhancer of cognitive performance and a potential treatment for substance use disorders. Psychopharmacology. 2013;229(3):415–434. doi: 10.1007/s00213-013-3232-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CA, Campbell SL, Sweatt JD. DNA methylation and histone acetylation work in concert to regulate memory formation and synaptic plasticity. Neurobiol Learn Mem. 2008;89(4):599–603. doi: 10.1016/j.nlm.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minzenberg MJ, Carter CS. Modafinil: a review of neurochemical actions and effects on cognition. Neuropsychopharmacology. 2008 Jun;33(7):1477–1502. doi: 10.1038/sj.npp.1301534. [DOI] [PubMed] [Google Scholar]
- Mizoguchi H, Yamada K, Mizuno M, Mizuno T, Nitta A, Noda Y, Nabeshima T. Regulations of methamphetamine reward by extracellular signal-regulated kinase 1/2/ets-like gene-1 signaling pathway via the activation of dopamine receptors. Mol Pharmacol. 2004;65(5):1293–1301. doi: 10.1124/mol.65.5.1293. [DOI] [PubMed] [Google Scholar]
- Monterosso JR, Aron AR, Cordova X, Xu J, London ED. Deficits in response inhibition associated with chronic methamphetamine abuse. Drug and Alcohol Dependence. 2005;79(2):273–277. doi: 10.1016/j.drugalcdep.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Morgan EE, Woods SP, Poquette AJ, Vigil O, Heaton RK, Grant I. Visual memory in methamphetamine-dependent individuals: deficient strategic control of encoding and retrieval. Aust N Z J Psychiatry. 2012;46(2):141–152. doi: 10.1177/0004867411433212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai T, Takuma K, Kamei H, Ito Y, Nakamichi N, Ibi D, Nakanishi Y, Murai M, Mizoguchi H, Nabeshima T, Yamada K. Dopamine D1 receptors regulate protein synthesis-dependent long-term recognition memory via extracellular signal-regulated kinase 1/2 in the prefrontal cortex. Learn Mem. 2007;14(3):117–125. doi: 10.1101/lm.461407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson AJ, Thur KE, Marsden CA, Cassaday HJ. Dissociable roles of dopamine within the core and medial shell of the nucleus accumbens in memory for objects and place. Behav Neurosci. 2010;124(6):789–799. doi: 10.1037/a0021114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TL, Tian YH, You IJ, Lee SY, Jang CG. Modafinil-induced conditioned place preference via dopaminergic system in mice. Synapse. 2011;65(8):733–741. doi: 10.1002/syn.20892. [DOI] [PubMed] [Google Scholar]
- Nordahl TE, Salo R, Leamon M. Neuropsychological effects of chronic methamphetamine use on neurotransmitters and cognition: A review. J Neuropsychiatry Clin Neurosci. 2003;15(3):317–325. doi: 10.1176/jnp.15.3.317. [DOI] [PubMed] [Google Scholar]
- Okubo Y, Suhara T, Suzuki K, Kobayashi K, Inoue O, Terasaki O, Someya Y, Sassa T, Sudo Y, Matsushima E, Iyo M, Tateno Y, Toru M. Decreased prefrontal dopamine D1 receptors in schizophrenia revealed by PET. Nature. 1997;385(6617):634–636. doi: 10.1038/385634a0. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Hozack NE, Zauscher BE, Frank L, Brown GG, Braff DL, Schuckit MA. Behavioral and functional neuroimaging evidence for prefrontal dysfunction in methamphetamine-dependent subjects. Neuropsychopharmacology. 2002 Jan;26(1):53–63. doi: 10.1016/S0893-133X(01)00334-7. [DOI] [PubMed] [Google Scholar]
- Raineri M, Gonzalez B, Goitia B, Garcia-Rill E, Krasnova IN, Cadet JL, Urbano FJ, Bisagno V. Modafinil abrogates methamphetamine-induced neuroinflammation and apoptotic effects in the mouse striatum. PLoS One. 2012;7(10):e46599. doi: 10.1371/journal.pone.0046599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineri M, Peskin V, Goitia B, Taravini IR, Giorgeri S, Urbano FJ, Bisagno V. Attenuated methamphetamine induced neurotoxicity by modafinil administration in mice. Synapse. 2011;65(10):1087–1098. doi: 10.1002/syn.20943. [DOI] [PubMed] [Google Scholar]
- Redrobe JP, Bull S, Plath N. Translational Aspects of the Novel Object Recognition Task in Rats Abstinent Following Sub-Sub-chronic treatment with Phencyclidine (PCP): Effects of Modafinil and Relevance to Cognitive Deficits in Schizophrenia. Front Psychiatry. 2010;1:146. doi: 10.3389/fpsyt.2010.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, Ramsey LA, Schwendt M, McGinty JF, See RE. Methamphetamine-induced changes in the object recognition memory circuit. Neuropharmacology. 2012;62(2):1119–1126. doi: 10.1016/j.neuropharm.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, Schwendt M, McGinty JF, Olive MF, See RE. Loss of object recognition memory produced by extended access to methamphetamine self-administration is reversed by positive allosteric modulation of metabotropic glutamate receptor 5. Neuropsychopharmacology. 2011;36(4):782–792. doi: 10.1038/npp.2010.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18(3):247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47(Suppl 1):33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Sargolini F, Roullet P, Oliverio A, Mele A. Effects of intra-accumbens focal administrations of glutamate antagonists on object recognition memory in mice. Behav Brain Res. 2003;138(2):153–163. doi: 10.1016/s0166-4328(02)00238-3. [DOI] [PubMed] [Google Scholar]
- Schmaal L, Joos L, Koeleman M, Veltman DJ, van den Brink W, Goudriaan AE. Effects of modafinil on neural correlates of response inhibition in alcohol-dependent patients. Biol Psychiatry. 2013;73(3):211–218. doi: 10.1016/j.biopsych.2012.06.032. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Shaham Y. The role of orbitofrontal cortex in drug addiction: a review of preclinical studies. Biol Psychiatry. 2008;63(3):256–262. doi: 10.1016/j.biopsych.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoriels L, Jones PB, Sahakian BJ. Modafinil effects on cognition and emotion in schizophrenia and its neurochemical modulation in the brain. Neuropharmacology. 2013;64:168–184. doi: 10.1016/j.neuropharm.2012.07.011. [DOI] [PubMed] [Google Scholar]
- Scott JC, Woods SP, Matt GE, Meyer RA, Heaton RK, Atkinson JH, Grant I. Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychol Rev. 2007;17(3):275–297. doi: 10.1007/s11065-007-9031-0. [DOI] [PubMed] [Google Scholar]
- Shearer J, Darke S, Rodgers C, Slade T, van Beek I, Lewis J, Brady D, McKetin R, Mattick RP, Wodak A. A double-blind, placebo-controlled trial of modafinil (200 mg/day) for methamphetamine dependence. Addiction. 2009;104(2):224–233. doi: 10.1111/j.1360-0443.2008.02437.x. [DOI] [PubMed] [Google Scholar]
- Simon SL, Dean AC, Cordova X, Monterosso JR, London ED. Methamphetamine dependence and neuropsychological functioning: evaluating change during early abstinence. J Stud Alcohol Drugs. 2010;71(3):335–344. doi: 10.15288/jsad.2010.71.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeiro Ada C, Moreira KD, Abrahao KP, Quadros IM, Oliveira MG. Individual differences are critical in determining modafinil-induced behavioral sensitization and cross-sensitization with methamphetamine in mice. Behav Brain Res. 2012;233(2):367–374. doi: 10.1016/j.bbr.2012.05.023. [DOI] [PubMed] [Google Scholar]
- Tritsch NX, Sabatini BL. Dopaminergic modulation of synaptic transmission in cortex and striatum. Neuron. 2012;76(1):33–50. doi: 10.1016/j.neuron.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbano FJ, Leznik E, Llinás RR. Modafinil enhances thalamocortical activity by increasing neuronal electrotonic coupling. Proc Natl Acad Sci U S A. 2007;104(30):12554–12559. doi: 10.1073/pnas.0705087104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Corvol JC, Pages C, Besson MJ, Maldonado R, Caboche J. Involvement of the extracellular signal-regulated kinase cascade for cocaine-rewarding properties. J Neurosci. 2000;20(23):8701–8709. doi: 10.1523/JNEUROSCI.20-23-08701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Pagès C, Hervé D, Girault JA, Caboche J. Addictive and non-addictive drugs induce distinct and specific patterns of ERK activation in mouse brain. Eur J Neurosci. 2004;19(7):1826–1836. doi: 10.1111/j.1460-9568.2004.03278.x. [DOI] [PubMed] [Google Scholar]
- Vocci FJ, Appel NM. Approaches to the development of medications for the treatment of methamphetamine dependence. Addiction. 2007;102(Suppl 1):96–106. doi: 10.1111/j.1360-0443.2007.01772.x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler M, et al. Effects of modafinil on dopamine and dopamine transporters in the male human brain: clinical implications. JAMA: the Journal of the American Medical Association. 2009;301(11):1148–1154. doi: 10.1001/jama.2009.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Geyer MA. Action of modafinil-increased motivation via the dopamine transporter inhibition and D1 receptors? Biol Psychiatry. 2010;67(8):784–787. doi: 10.1016/j.biopsych.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Kooistra K, Geyer MA. Dopamine receptor mediation of the exploratory/hyperactivity effects of modafinil. Neuropsychopharmacology. 2011;36(7):1385–1396. doi: 10.1038/npp.2011.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zack M, Poulos CX. Effects of the atypical stimulant modafinil on a brief gambling episode in pathological gamblers with high vs. low impulsivity. J Psychopharmacol. 2009;23(6):660–671. doi: 10.1177/0269881108091072. [DOI] [PubMed] [Google Scholar]
- Zanassi P, Paolillo M, Feliciello A, Avvedimento EV, Gallo V, Schinelli S. cAMP-dependent protein kinase induces cAMP-response element-binding protein phosphorylation via an intracellular calcium release/ERK-dependent pathway in striatal neurons. J Biol Chem. 2001;276(15):11487–11495. doi: 10.1074/jbc.M007631200. [DOI] [PubMed] [Google Scholar]
- Zhai H, Li Y, Wang X, Lu L. Drug-induced alterations in the extracellular signal-regulated kinase (ERK) signalling pathway: implications for reinforcement and reinstatement. Cell Mol Neurobiol. 2008;28(2):157–172. doi: 10.1007/s10571-007-9240-3. [DOI] [PMC free article] [PubMed] [Google Scholar]