Abstract
Background
This study assessed the echocardiographic predictors of sudden cardiac death (SCD) within two population-based cohorts.
Methods and Results
Echocardiograms were obtained on 2383 participants (1993–95) from the Atherosclerosis Risk in Communities (ARIC) Study (100% African-American) and 5366 participants (1987–89 and 1994–95) from the Cardiovascular Health Study (CHS). The main outcome was physician-adjudicated SCD. We used Cox proportional hazards models with incident coronary heart disease (CHD) and heart failure as time-dependent covariates to assess the association between echocardiographic variables and SCD, adjusting for Framingham risk score (FRS) variables, CHD, and renal function. Cohort-specific results were meta-analyzed. During a median follow-up of 7.3 years and 13.1 years, 44 ARIC Study and 275 CHS participants had SCD, respectively. In the meta-analyzed results, the adjusted hazard ratios (95% confidence intervals) for predictors of SCD were 3.07 (2.29–4.11) for reduced left ventricular ejection fraction (LVEF); 1.85 (1.36–2.52) for mitral annular calcification; 1.64 (1.07–2.51) for mitral E/A >1.5 and 1.52 (1.14–2.02) for mitral E/A <0.7 (vs mitral E/A 0.7–1.5); 1.30 (1.15–1.48) per one standard deviation (SD) increase in left ventricular mass; and 1.15 (1.02–1.30) per one SD increase in left atrial diameter. A receiver-operating characteristic model for prediction of SCD using FRS variables had a c-statistic of 0.61 for ARIC and 0.67 for CHS; the full multivariable model including all echocardiographic variables had a c-statistic of 0.76 for ARIC and 0.74 for CHS.
Conclusions
In addition to reduced LVEF, we identified other echocardiographic-derived variables predictive for SCD that provided incremental value over clinical risk factors.
Keywords: sudden cardiac death, echocardiography, African American, prospective cohort study
Sudden cardiac death (SCD) is a major health concern that accounts for an estimated 300,000 to 375,000 deaths in the United States annually.1,2 SCD constitutes 50% of deaths due to cardiovascular disease.2 Survival from arrest remains poor despite advances in resuscitation. Identification of predisposing factors may be essential in the early intervention and prevention of SCD. A number of methods to predict SCD exist, dating back several decades to include simple, validated tools such as the Framingham risk index.3 While many SCD predictors continue to emerge from the literature, including genetic variants, anatomic assessments using imaging modalities, and various clinical and laboratory data, the cornerstone of SCD risk stratification in patients without a prior cardiac arrest is reduced left ventricular (LV) ejection fraction (LVEF).4 Reduced LVEF of any cause is the strongest known predictor of SCD. Despite this, SCD frequently occurs in the absence of reduced LVEF. For example, in the community-based Oregon Sudden Unexpected Death Study (Ore-SUDS), of the 39% with echocardiographic data available, a known reduction in LVEF was present in just 51% of women and 65% of men with SCD.5 Information on reduced LVEF alone is therefore not enough to characterize individuals at a higher risk of SCD. The identification of additional SCD risk factors by echocardiogram could help better find those at higher risk of SCD. Given the widespread availability of echocardiography, this could have a substantial impact on the general population.
The objective of this study was to assess the association between echocardiographic variables associated with structural heart changes and SCD among individuals who participated in the ARIC Study and CHS.
Methods
Study population
The ARIC Study has been described elsewhere.6 Briefly, the ARIC Study is a prospective population-based investigation of risk factors for atherosclerosis, coronary heart disease (CHD), and stroke. The study includes 15,792 persons aged 45–64 years at baseline (1987–89), randomly chosen from four United States communities: Forsyth County, NC; Jackson, MS; Minneapolis suburbs, MN; and Washington County, MD. In the Jackson, MS cohort, a representative sample was exclusively selected from African-American residents of the city. The ARIC Study cohort members have completed five clinic examinations. Data were first collected in 1987–89, and then again in 1990–92, 1993–95, 1996–98, and 2011–13. Echocardiographic studies for this analysis were performed at the Jackson, MS field site during the third or fourth study visit (1993–96).7
The CHS cohort design and recruitment methods have been previously reported.8 The CHS consists of a prospectively designed cohort of men and women ≥ 65 years, designed to evaluate cardiovascular risk factors and outcomes in free-living elderly subjects. The original cohort of 5201 patients was selected randomly from Medicare beneficiaries in four US locations: Washington County, MD; Forsyth County, NC; Allegheny County, PA; and Sacramento County, CA. An additional 687 African-Americans were recruited three years later, in 1992 and 1993, to enhance minority representation. A total of 5888 participants (2495 men and 3393 women) were included from 1989–1993. A total of 5683 (5176 from the original cohort and an additional 507 from the African-American cohort) underwent echocardiography. Baseline echocardiographic assessment was performed during the 1989–1990 clinic visit for the initial cohort and during the 1994–1995 clinic visit for the African-American cohort.
A total of 2445 persons in the ARIC Study and the 5683 persons in the CHS underwent echocardiography. We chose echocardiographic predictor variables that are associated with structural changes, were defined similarly in both cohorts, and have known prognostic value. We included participants who had any of the following measurements or assessments available: mitral annular calcification (MAC)(yes/no), aortic sclerosis (yes/no), LVEF (reduced if ejection fraction was <50% in the ARIC Study and <55% in the CHS), left atrium diameter, aortic root diameter, LV mass, LV mass index, interventricular septal thickness (IVST), posterior wall thickness (PWT), relative wall thickness (RWT), LV internal dimensions in end diastole (LVIDd) and systole (LVIDs), and transmitral peak E-wave and A-wave velocities. In the CHS, roughly a third of patients were missing M-mode measurements, and therefore we could only calculate LV mass in 63.5% of the CHS cohort. Older age, chronic obstructive pulmonary disease, and obesity were associated with missing M-mode data. The sample size for our primary analysis was 2383 ARIC Study and 5366 CHS participants, which included 95.3% of those with echocardiography.
All study participants provided informed consent at baseline and follow-up exams. The ARIC Study and CHS were approved by Institutional Review Boards at participating institutions.
Echocardiographic measurements
The designs of the echocardiographic protocols for the ARIC Study7 and CHS9,10 were described previously. The IVST, PWT, LVIDd, LVIDs, left atrium diameter, and aortic root diameter were obtained using M mode according to the conventions established by the American Society of Echocardiography at the time of the index examination. LV mass was derived using the formula described by Devereaux and associates using the M mode data.11 The ratio of PWT and IVST versus LVIDd determined relative wall thickness. The criterion for MAC was similar to previous studies.12–14 MAC was defined as an echo-dense structure located at the atrioventricular groove and posterior mitral leaflet, visualized in multiple views in both cohorts. Aortic valve sclerosis was similarly identified as focal or diffuse aortic cusp thickening, stiffness, and/or increased echogenicity (calcification) with normal aortic cusp excursion and a peak trans-aortic valve flow velocity <2.0 m/s in both cohorts.
Mitral inflow velocity was measured by pulsed-wave Doppler from the apical 4-chamber view with the sample volume positioned at the tips of the mitral leaflets. The inflow variables analyzed were 1) peak Doppler E wave velocity and 2) early (E wave) to late (A wave) ratio (E/A). Additional specific information about the echocardiography studies performed in the 2 cohorts is outlined below.
Echocardiogram Performance and Reading Protocol
Briefly, for the ARIC Study participants, images were acquired using the Acuson XP128/10c echocardiography machine (GPS medical). A single cardiologist performed all echocardiographic readings. LV systolic function was considered reduced if LVEF was <50%. LVEF was derived semi-quantitatively using a modified Quinones technique and visual assessment of the LV apex.
In the CHS cohort, 2-dimensional images were recorded on super VHS tape following a standard protocol with the use of a cardiac ultrasound machine (model SSH-160A, Toshiba, Tustin, California). Two trained independent readers, both unaware of the participants’ clinical information, read images at a core laboratory. Global LV systolic function was qualitatively assessed from the 2 dimensional echocardiogram images. Left ventricular systolic function was subjectively categorized as normal (EF≥55%), borderline (EF<55 and ≥45), or abnormal (EF<45%), with 94% inter-reader agreement and 98% intrareader agreement of paired studies (eMethods). In this study, left ventricular systolic function was considered reduced if LVEF was <55%. Representative images from the CHS are shown in Supplemental Figures 1–4.
Outcomes ascertainment
In the ARIC Study and CHS cohorts, respective ARIC Study and CHS events committees classified cardiovascular deaths. A separate review of the CHD deaths was conducted to identify SCD events, and included review of death certificates, and when available, autopsy reports, next-of-kin interviews, and questionnaires to decedents’ physicians. The reviewers were blinded to the echocardiographic data. The primary outcome, SCD, was similarly defined in both the ARIC Study and CHS: a sudden pulseless condition presumed to be due to a ventricular tachyarrhythmia in a previously stable individual without evidence of a noncardiac cause of cardiac arrest. All SCD cases occurred outside the hospital or in the emergency department, and the individuals could not have a life-threatening noncardiac comorbidity or be under hospice or nursing home care. For unwitnessed SCDs, the participant must have been seen within 24 hours of the arrest in a stable condition and without evidence of a noncardiac cause of cardiac arrest.
In the ARIC Study, all CHD deaths that occurred by December 31, 2001, were reviewed by a panel of 5 physicians to identify SCD events. Each event was independently classified by 2 physicians. If there was disagreement, a third investigator reviewed the event to provide a final classification. After review of available data, CHD deaths were classified as definite sudden arrhythmic death, possible sudden arrhythmic death, not sudden arrhythmic death, or unclassifiable.15 For the present analysis, SCD was defined as definite or possible sudden arrhythmic death in the ARIC Study.
In the CHS, all CHD deaths through December 31, 2006, were reviewed by a cardiologist to classify SCD cases. A blinded second physician review of a random sample of 70 of these death records showed an 88% inter-reviewer agreement and κ = 0.74 for SCD. Both of these physicians also participated on the ARIC Study SCD review panel. After review of available data in the CHS, CHD deaths were classified as definite, possible, or not SCD.16 For the present analysis, the CHS definition of SCD included definite and possible SCD.
In both cohorts, the secondary outcome, non-sudden cardiovascular (CVD) deaths (NSCD), was defined as CVD death not meeting SCD criteria. For the ARIC Study cohort, International Classification of Diseases codes were used from patient death certificates to identify CVD deaths.
Assessment of other covariates
For this analysis we used covariates measured at the time of echocardiogram. Definitions of the covariates are detailed in the Supplemental Methods.
Statistical analysis
Means (standard deviation [SD]) for continuous variables and counts with percentages for categorical variables were calculated. Person-years at risk were calculated from the date of the echocardiographic exam until the date of SCD or NSCD, other death, loss to follow-up, or end of follow-up, whichever occurred first.
Cohort-specific analyses were conducted. Cox proportional-hazards models were used to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) of SCD by echocardiographic variables. An initial model adjusted for age, sex, and in the CHS, race. A second model additionally adjusted for Framingham risk score and prevalent CHD. A third model also adjusted for renal function (estimated glomerular filtration rate [eGFR]). Finally, a fourth model also adjusted for incident CHD and heart failure as time-dependent covariates and potential mediators of the association of echocardiographic variables with SCD. Cohort-specific analyses were combined using fixed-effects meta-analysis. Between-cohort heterogeneity was determined calculating the I-squared statistic and the Cochrane’s Q statistic for heterogeneity. Low levels of the I-squared statistic and non-significant Q statistic indicate lack of evidence of heterogeneity between both cohorts. The combined associations were considered the primary results. A similar set of analyses was conducted for NSCD.
Receiver-operating characteristic curve analysis was constructed to determine the incremental value of incorporating echocardiographic variables to Framingham risk score variables for the prediction of SCD. A new value was built for each individual echocardiographic variable added to the Framingham risk score. Also, a new value was built for all echocardiographic variables added to the Framingham risk score.
Commercial software was used for statistical analysis of ARIC Study data (SAS, version 9.2; SAS Institute Inc.) and CHS data (SPSS, version 16; SPSS, Inc; and Stata, version 11.2; StataCorp). All P values reported were 2-sided, and were considered statistically significant if <0.05. However, the focus of our primary analysis was in estimation of HRs and their 95%CIs, rather than statistical significance.
Results
The incidence of SCD in the ARIC Study was 2.59 (1.91–3.44) per 1,000 person-years and in the CHS was 4.38 (3.89–4.93) per 1,000 person-years over a median (interquartile range) follow-up of 7.3 (1.4) and 13.1 (8.3) years, respectively. Table 1 lists the baseline characteristics of both cohorts. The CHS cohort was older with a mean baseline age of 72.9 years versus 58.8 years in the ARIC Study. All participants in the ARIC Study were African-American, compared to 12.3% in the CHS. The prevalence of anti-arrhythmic drug therapy was low in either cohort.
Table 1.
Baseline characteristics in the ARIC Jackson field center, 1993–1995, and CHS, 1989–1995, stratified by later SCD occurrence
| Variable | ARIC | CHS | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Total sample (n=2383) | No SCD (n=2339) | SCD (n=44) | Total sample (n=5366) | No SCD (n=5091) | SCD (n=275) | |
| Age, years | 58.8 (5.7) | 58.8 (5.7) | 61.6 (5.6) | 72.9 (5.6) | 72.9 (5.5) | 73.3 (5.4) |
| Female, % | 64 | 65 | 55 | 58 | 59 | 40 |
| African-American, % | 100 | 100 | 100 | 12 | 12 | 15 |
| Current smoker, % | 20 | 20 | 30 | 12 | 12 | 15 |
| Hypertension, % | 60 | 60 | 77 | 58 | 57 | 67 |
| Diabetes, % | 24 | 24 | 30 | 15 | 15 | 25 |
| Total cholesterol ≥ 240 mg/dL, % | 18 | 19 | 14 | 21 | 22 | 20 |
| HDL cholesterol < 60 mg/dL, % | 65 | 65 | 80 | 69 | 68 | 81 |
| Prevalent CHD, % | 5 | 4 | 23 | 19 | 18 | 35 |
| eGFR mL/min/1.73 m2 | 101 (19) | 102 (19) | 91 (18) | 79 (23) | 79 (23) | 77 (23) |
| Beta-blockers, % | 9 | 9 | 11 | 13 | 13 | 16 |
| Anti-arrhythmics, % | 0.6 | 0.5 | 2 | 3 | 3 | 6 |
| Calcium channel blockers, % | 16 | 16 | 27 | 13 | 13 | 16 |
ARIC=Atherosclerosis Risk in Communities Study; CHD=coronary heart disease; CHS=Cardiovascular Health Study; eGFR=estimated glomerular filtration rate; HDL=high density lipoprotein; SCD=sudden cardiac death
Values correspond to either means (standard deviation) or percentages
Table 2 shows the baseline echocardiographic variables in both cohorts. Participants in the ARIC Study had a greater mean LV mass and a slightly greater mitral peak E velocity; the CHS had more with reduced LVEF, presence MAC, aortic sclerosis, and a mitral E/A less than 0.7 m/s.
Table 2.
Echocardiographic variables at baseline, ARIC, 1993–1998, and CHS 1989–1995, stratified by later SCD occurrence
| Variable | ARIC | CHS | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| N | Total Sample | No SCD (n=2339) | SCD (n=44) | N | Total Sample | No SCD (n=5091) | SCD (n=275) | |
| Mitral annular calcification (%) | 2368 | 5 | 5 | 14 | 3757† | 42 | 41 | 58 |
| Reduced LV ejection fraction (%) | 2373 | 3 | 2 | 16 | 5091† | 9 | 8 | 24 |
| Aortic sclerosis (%) | 2368 | 5 | 5 | 13 | 3657†,* | 45 | 45 | 49 |
| Left atrium diameter, cm | 1931 | 4 (0.6) | 4 (0.5) | 4 (0.8) | 5192 | 4 (0.7) | 4 (0.7) | 4 (0.7) |
| Aortic root diameter, cm | 2149 | 3 (0.4) | 3 (0.4) | 3 (0.5) | 5203 | 3 (0.5) | 3 (0.5) | 3 (0.5) |
| LV mass index, g/m^2.7 | 1815 | 50 (16) | 50 (16) | 60 (26) | 3611† | 39 (13) | 39 (12) | 44 (14) |
| Mitral E/A | ||||||||
| < 0.7 | 184 | 8% | 8% | 13% | 1033 | 20% | 20% | 27% |
| 0.7 to 1.5 | 1847 | 85% | 85% | 79% | 3854 | 75% | 75% | 64% |
| > 1.5 | 150 | 7% | 7% | 8% | 303 | 6% | 6% | 9% |
| Mitral Peak E, m/s | 2187 | 0.77 (0.2) | 0.77 (0.2) | 0.84 (0.3) | 5201 | 0.72 (0.2) | 0.72 (0.2) | 0.72 (0.2) |
ARIC=Atherosclerosis Risk in Communities Study; CHD=coronary heart disease; CHS=Cardiovascular Health Study; SCD=sudden cardiac death
Values correspond to mean (standard deviation) or percentage
Reduced ‘n’ to between 3550 to 3612
No data available for second African American cohort
Table 3 shows the cohort specific data. In a multivariable model adjusted for potential confounders (Table 3, Model 3), reduced LVEF, presence of MAC, and increased LV mass were associated with SCD in both cohorts. In the ARIC Study, increased aortic root diameter and mitral peak E velocity were also associated with SCD. In the CHS, increased left atrial diameter, and mitral E/A <0.7 or >1.5 were also associated with SCD. With the addition of CHD and heart failure as time-dependent covariates (Table 3, Model 4), only increased LV mass and mitral peak E velocity remained associated with SCD in the ARIC Study, but there was little effect on the associations in the CHS.
Table 3.
SCD incidence rate and hazard ratio (95% confidence interval) by echocardiographic variables, ARIC, 1993–2002, and CHS 1989–2002
| Mitral annular calcification | ||||||
|---|---|---|---|---|---|---|
| ARIC | CHS | |||||
|
| ||||||
| No | Yes | p-value | No | Yes | p-value | |
| SCD cases | 37 | 6 | 67 | 91 | ||
| Person-years | 16118 | 796 | 29696 | 20088 | ||
| SCD Incidence rate (95% CI)* | 2.30 (1.64 – 3.13) | 7.54 (3.13 – 15.5) | 2.26 (1.78 – 2.87) | 4.53 (3.69 – 5.56) | ||
| Model 1: HR (95% CI) | 1(REF) | 2.78 (1.16 – 6.63) | 0.02 | 1(REF) | 2.07 (1.50 – 2.85) | <0.001 |
| Model 2: HR (95% CI) | 1(REF) | 2.71 (1.14 – 6.45) | 0.02 | 1(REF) | 2.07 (1.51 – 2.84) | <0.001 |
| Model 3: HR (95% CI) | 1(REF) | 2.56 (1.08 – 6.09) | 0.03 | 1(REF) | 2.07 (1.51 – 2.85) | <0.001 |
| Model 4: HR (95% CI) | 1(REF) | 1.78 (0.73 – 4.35) | 0.20 | 1(REF) | 1.86 (1.34 – 2.58) | <0.001 |
|
| ||||||
| Aortic Sclerosis | ||||||
| ARIC | CHS | |||||
|
| ||||||
| No | Yes | p-value | No | Yes | p-value | |
|
| ||||||
| SCD cases | 37 | 6 | 78 | 74 | ||
| Person-years | 16041 | 879 | 27318 | 21277 | ||
| SCD Incidence rate (95% CI)* | 2.31 (1.65 – 3.14) | 6.83 (2.84 – 14.07) | 2.86 (2.29 – 3.57) | 3.48 (2.77 – 4.37) | ||
| Model 1: HR (95% CI) | 1(REF) | 2.29 (0.95 – 5.51) | 0.06 | 1(REF) | 1.21 (0.88 – 1.67) | 0.25 |
| Model 2: HR (95% CI) | 1(REF) | 2.40 (1.01 – 5.72) | 0.05 | 1(REF) | 1.27 (0.93 – 1.75) | 0.14 |
| Model 3: HR (95% CI) | 1(REF) | 2.16 (0.90 – 5.16) | 0.08 | 1(REF) | 1.28 (0.93 – 1.76) | 0.13 |
| Model 4: HR (95% CI) | 1(REF) | 1.88 (0.78 – 4.53) | 0.16 | 1(REF) | 1.09 (0.79 – 1.53) | 0.59 |
|
| ||||||
| Reduced LV ejection fraction | ||||||
| ARIC | CHS | |||||
|
| ||||||
| No | Yes | p-value | No | Yes | p-value | |
|
| ||||||
| SCD cases | 36 | 7 | 208 | 67 | ||
| Person-years | 16629 | 341 | 58667 | 4143 | ||
| SCD Incidence rate (95% CI)* | 2.16 (1.54 – 2.96) | 20.53 (9.15 – 40.31) | 3.55 (3.10 – 4.06) | 16.17 (12.7 – 20.6) | ||
| Model 1: HR (95% CI) | 1(REF) | 8.13 (3.56 – 18.56) | <0.0001 | 1(REF) | 3.89 (2.93 – 5.16) | <0.001 |
| Model 2: HR (95% CI) | 1(REF) | 5.06 (2.06 – 12.45) | 0.0004 | 1(REF) | 3.26 (2.44 – 4.37) | <0.001 |
| Model 3: HR (95% CI) | 1(REF) | 4.25 (1.70 – 10.63) | 0.002 | 1(REF) | 3.24 (2.42 – 4.33) | <0.001 |
| Model 4: HR (95% CI) | 1(REF) | 1.95 (0.76 – 4.98) | 0.16 | 1(REF) | 3.22 (2.37 – 4.37) | <0.001 |
| Left atrium diameter | ||||||
|---|---|---|---|---|---|---|
| ARIC tertiles (cm) | CHS tertiles (cm) | |||||
|
| ||||||
| <3.62 | 3.62 – 4.05 | >4.05 | <3.60 | 3.60 – 4.13 | >4.13 | |
| SCD cases | 9 | 9 | 17 | 71 | 80 | 112 |
| Person-years | 4580 | 4572 | 4634 | 20881 | 20997 | 19032 |
| SCD Incidence rate (95% CI)* | 1.97 (0.97 – 3.59) | 1.97 (0.97 – 3.59) | 3.67 (2.22 – 5.74) | 3.40 (2.70 – 4.29) | 3.81 (3.06 – 4.74) | 5.89 (4.89 – 7.08) |
| Model 1: HR (95% CI) | 1 (ref.) | 1.00 (0.40 – 2.52) | 1.81 (0.81 – 4.07) | 1 (ref.) | 1.05 (0.77 – 1.45) | 1.46 (1.08 – 1.98) |
| Model 2: HR (95% CI) | 1 (ref.) | 0.97 (0.38 – 2.44) | 1.50 (0.69 – 3.54) | 1 (ref.) | 1.03 (0.75 – 1.42) | 1.34 (0.99 – 1.81) |
| Model 3: HR (95% CI) | 1 (ref.) | 0.93 (0.65 – 3.37) | 1.48 (0.65 – 3.37) | 1 (ref.) | 1.03 (0.75 – 1.41) | 1.33 (0.98 – 1.80) |
| Model 4: HR (95% CI) | 1 (ref.) | 0.91 (0.36 – 2.41) | 1.40 (0.61 – 3.21) | 1 (ref.) | 1.06 (0.76 – 1.48) | 1.27 (0.92 – 1.74) |
| Aortic root diameter | ||||||
|---|---|---|---|---|---|---|
| Aric tertiles (cm) | CHS tertiles (cm) | |||||
|
| ||||||
| <2.92 | 2.92 – 3.29 | >3.29 | <2.96 | 2.96 – 3.35 | >3.35 | |
| SCD cases | 8 | 12 | 22 | 73 | 81 | 110 |
| Person-years | 5195 | 4984 | 5146 | 21487 | 20747 | 18791 |
| SCD Incidence rate (95% CI)* | 1.54 (0.73 – 2.91) | 2.41 (1.31 – 4.08) | 4.28 (2.76 – 6.36) | 3.40 (2.70 – 4.27) | 3.90 (3.14 – 4.85) | 5.85 (4.86 – 7.06) |
| Model 1: HR (95% CI) | 1 (ref.) | 1.43 (0.58 – 3.53) | 2.34 (0.97 – 5.62) | 1 (ref.) | 0.94 (0.68 – 1.30) | 1.01 (0.72 – 1.42) |
| Model 2: HR (95% CI) | 1 (ref.) | 1.42 (0.58 – 3.49) | 2.59 (1.15 – 5.84) | 1 (ref.) | 0.98 (0.72 – 1.35) | 1.23 (0.90 – 1.68) |
| Model 3: HR (95% CI) | 1 (ref.) | 1.38 (0.56 – 3.38) | 2.41 (1.06 – 5.45) | 1 (ref.) | 0.98 (0.71 – 1.35) | 1.21 (0.89 – 1.66) |
| Model 4: HR (95% CI) | 1 (ref.) | 1.34 (0.55 – 3.29) | 2.12 (0.92 – 4.85) | 1 (ref.) | 0.99 (0.71 – 1.37) | 1.13 (0.82 – 1.57) |
| LV mass index | ||||||
|---|---|---|---|---|---|---|
| ARIC tertiles (g/m^2.7) | CHS tertiles (g/m^2.7) | |||||
|
| ||||||
| <41.4 | 41.4 – 53.1 | >53.1 | <33.07 | 33.07 – 42.20 | >42.20 | |
| SCD cases | 7 | 9 | 16 | 36 | 51 | 79 |
| Person-years | 4278 | 4318 | 4327 | 15391 | 15002 | 13197 |
| SCD Incidence rate (95% CI)* | 1.64 (0.73 – 3.21) | 2.08 (1.03 – 3.80) | 3.70 (2.20 – 5.86) | 2.34 (1.69 – 3.24) | 3.40 (2.58 – 4.47) | 5.99 (4.80 – 7.46) |
| Model 1: HR (95% CI) | 1 (ref.) | 1.24 (0.46 – 3.34) | 2.09 (0.86 – 5.09) | 1 (ref.) | 1.48 (0.96 – 2.26) | 2.41 (1.62 – 3.58) |
| Model 2: HR (95% CI) | 1 (ref.) | 1.12 (0.41 – 3.01) | 1.58 (0.62 – 4.01) | 1 (ref.) | 1.38 (0.90 – 2.12) | 2.02 (1.35 – 3.01) |
| Model 3: HR (95% CI) | 1 (ref.) | 1.11 (0.41 – 2.99) | 1.49 (0.58 – 3.79) | 1 (ref.) | 1.39 (0.91 – 2.14) | 2.03 (1.36 – 3.02) |
| Model 4: HR (95% CI) | 1 (ref.) | 1.21 (0.45 – 3.26) | 1.40 (0.56 – 3.54) | 1 (ref.) | 1.26 (0.81 – 1.96) | 1.69 (1.12 – 2.58) |
| Mitral E to A | ||||||
|---|---|---|---|---|---|---|
| ARIC (m/s) | CHS (m/s) | |||||
|
| ||||||
| <0.70 | 0.70 – 1.5 | >1.5 | <0.70 | 0.70 – 1.5 | >1.5 | |
| SCD cases | 5 | 30 | 3 | 71 | 170 | 23 |
| Person-years | 1270 | 13294 | 1030 | 10106 | 47698 | 3170 |
| SCD Incidence rate (95% CI)* | 3.94 (1.49 – 8.63) | 2.26 (1.55 – 3.18) | 2.91 (0.81 – 7.77) | 7.03 (5.57 – 8.87) | 3.56 (3.07 – 4.14) | 7.26 (4.82 – 10.92) |
| Model 1: HR (95% CI) | 1.40 (0.43 – 4.71) | 1 (ref.) | 1.43 (0.43 – 4.71) | 1.84 (1.39 – 2.46) | 1 (ref.) | 1.94 (1.25 – 3.00) |
| Model 2: HR (95% CI) | 1.15 (0.42 – 3.11) | 1 (ref.) | 1.35 (0.41 – 4.49) | 1.84 (1.39 – 2.44) | 1 (ref.) | 1.82 (1.18 – 2.83) |
| Model 3: HR (95% CI) | 0.97 (0.36 – 2.66) | 1 (ref.) | 1.42 (0.43 – 4.69) | 1.81 (1.37 – 2.40) | 1 (ref.) | 1.78 (1.15 – 2.77) |
| Model 4: HR (95% CI) | 0.24 (0.08 – 0.73) | 1 (ref.) | 0.91 (0.33 – 2.56) | 1.73 (1.29 – 2.32) | 1 (ref.) | 1.85 (1.16 – 2.96) |
| Mitral Peak E | ||||
|---|---|---|---|---|
| ARIC (m/s) | CHS (m/s) | |||
|
| ||||
| Continuous, per 1 SD | p-value+ | Continuous, per 1 SD | p-value+ | |
| Model 1: HR (95% CI) | 1.49 (1.14 – 1.96) | 0.004 | 1.11 (0.98 – 1.26) | 0.09 |
| Model 2: HR (95% CI) | 1.37 (1.06 – 1.77) | 0.02 | 1.04 (0.92 – 1.17) | 0.55 |
| Model 3: HR (95% CI) | 1.39 (1.08 – 1.79) | 0.01 | 1.04 (0.92 – 1.18) | 0.52 |
| Model 4: HR (95% CI) | 1.29 (1.01 – 1.66) | 0.04 | 1.04 (0.92 – 1.18) | 0.51 |
ARIC=Atherosclerosis Risk in Communities Study; CHD=coronary heart disease; CHS=Cardiovascular Health Study; CVD=cardiovascular disease; eGFR=estimated glomerular filtration rate; HDLc=high density lipoprotein cholesterol; LV=left ventricular; SCD=sudden cardiac death
Crude incidence rates per 1000 person-years
Refer to Supplemental Table 2 for continuous variable data.
Model 1: Cox proportional hazards model adjusted for age, sex, **race (in CHS only)
Model 2: Cox proportional hazards model adjusting for Framingham risk score as one variable: (sex, age, total cholesterol, HDLc, blood pressure, diabetes and smoking), and also prevalent CHD
Model 3: Model 2 + eGFR
Model 4: Model 3 + time-dependent heart failure, time-dependent CHD
The association of echocardiographic variables with NSCD was also assessed (Supplemental Table 1).
A receiver-operating characteristic model for prediction of SCD using FRS variables had a c-statistic of 0.61 (95%CI 0.53–0.69) for ARIC and 0.67 (0.64–0.70) for CHS; the full multivariable model including all echocardiographic variables had a c-statistic of 0.76 for ARIC (0.66–0.87) and 0.74 for CHS (0.69–0.75) (Table 4).
Table 4.
C-statistic of sudden cardiac death with echocardiographic variables added to the Framingham risk score
| ARIC C-statistic (95% CI) | CHS C-statistic (95% CI) | |
|---|---|---|
| Framingham risk score | 0.608 (0.526–0.690) | 0.669 (0.638–0.700) |
|
| ||
| FRS + Univariate: (each variable added to the score separately) | ||
| Mitral annular calcification | 0.648 (0.567–0.729) | 0.677 (0.633–0.721) |
| Reduced LV ejection fraction | 0.659 (0.573–0.744) | 0.719 (0.688–0.750) |
| Aortic sclerosis | 0.620 (0.535–0.705) | 0.671 (0.628–0.714) |
| Left atrium diameter, continuous | 0.608 (0.522–0.694) | 0.669 (0.637–0.702) |
| Left atrium diameter, tertiles | 0.619 (0.537–0.702) | 0.669 (0.637–0.701) |
| Aortic root diameter, continuous | 0.682 (0.609–0.756) | 0.666 (0.635–0.697) |
| Aortic root diameter, tertiles | 0.637 (0.564–0.710) | 0.666 (0.635–0.697) |
| LV mass index, continuous | 0.648 (0.542–0.755) | 0.687 (0.645–0.728) |
| LV mass index, tertiles | 0.623 (0.538–0.707) | 0.670 (0.628–0.712) |
| Mitral E/A, continuous | 0.629 (0.542–0.715) | 0.666 (0.634–0.698) |
| Mitral E/A, categories | 0.633 (0.553–0.713) | 0.671 (0.639–0.703) |
| Mitral Peak E, continuous | 0.644 (0.552–0.735) | 0.668 (0.635–0.700) |
|
| ||
| FRS + Multivariate: (all variables added to the score) | 0.764 (0.657–0.870) | 0.741 (0.687–0.749) |
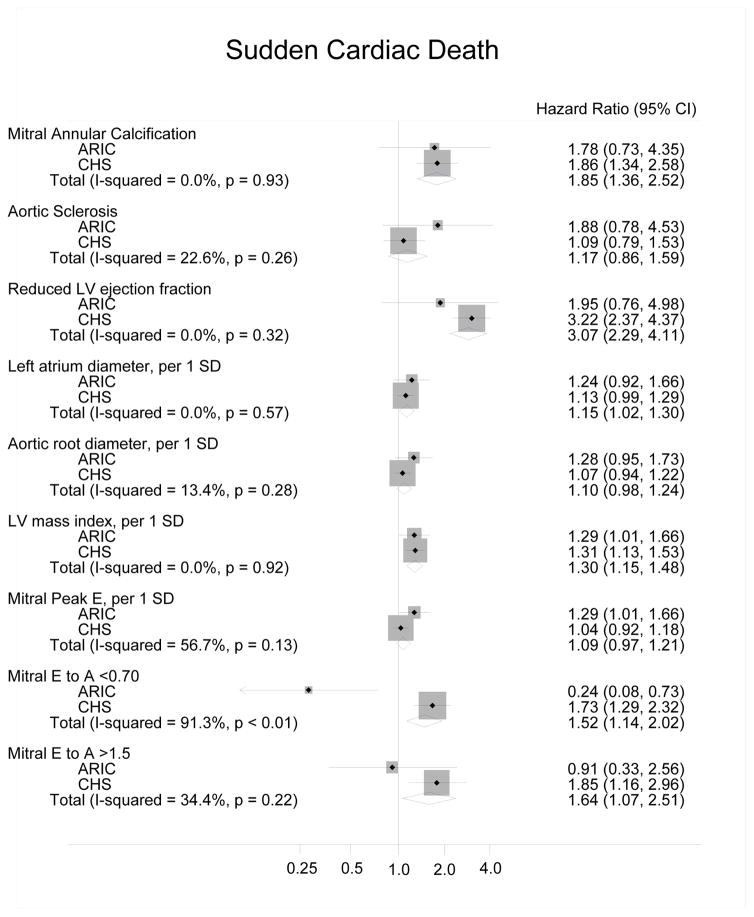
The meta-analyzed data for the ARIC Study and CHS, using Model 4 adjustments, found several echocardiographic variables to have significant associations with SCD. Reduced LVEF showed a threefold risk of SCD, presence of MAC a twofold risk of SCD, and mitral E/A >1.5 or <0.7 a one-and-a-half fold risk of SCD. Both LV mass and increased left atrial diameter were also statistically significant (Figure).
Figure.
Forest plot of the meta-analyzed and risk-adjusted hazard ratios for SCD in the ARIC Study and CHS, using CHD and heart failure as time-dependent variables (Model 4)
For NSCD, the meta-analyzed results using Model 4 adjustments had HRs (95%CI) of 1.77 (1.46–2.13) for reduced LVEF; 1.70 (1.47–1.97) for mitral E/A <0.7; 1.53 (1.20–1.96) for mitral E/A>1.5; 1.45 (1.23–1.71) for MAC; and 1.44 (1.22–1.71) for aortic sclerosis. LV mass and LAD had smaller, albeit significant, associations with NSCD (Supplemental Figure 5).
In addition, a receiver-operating characteristic model for prediction of SCD using FRS variables had a c-statistic of 0.61 for ARIC and 0.67 for CHS; the full multivariable model including all echocardiographic variables had a c-statistic of 0.76 for ARIC and 0.74 for CHS.
To assure the validity of the qualitative EF assessment in the CHS, we performed a sensitivity analysis in which we substituted EF with fractional shortening, a quantitative assessment of left ventricular performance. This resulted in only a minimal change in the cumulative c-statistic to 0.73 (0.68–0.79) (Supplemental Table 3).
Discussion
This large population-based study evaluated the association of multiple echocardiographic variables with SCD, with findings having several important clinical implications. Based on the meta-analyzed results from the ARIC Study and CHS cohorts, recognition should be given to MAC, mitral E/A >1.5 or <0.7, increased LV mass, and increased left atrial diameter, in addition to reduced LVEF, as potential markers of SCD in the general population. Of the previously accepted echocardiographic predictors of SCD (reduced LVEF and LV hypertrophy),4,17 only reduced LVEF has been studied in randomized controlled trials investigating the use of implantable cardioverter defibrillators (ICDs), which reduced all-cause mortality in persons having ischemic18–20 or non-ischemic20 LV dysfunction. Yet many SCDs occur in patients not having recognized LV dysfunction. In a population-based study in the Netherlands, among the 41% with prior echocardiographic data available, just 50% had an LVEF ≤50%.21 In the community-based Ore-SUDS study, of the 39% with previous echocardiographic data, a reduction in LVEF was present in 51% of women and 65% of men. In these studies, it is possible the overall prevalence of LV dysfunction would have been different had the entire population been screened with echocardiograms. In our study, just 16.3% of ARIC Study and 24.4% of CHS participants with SCD had reduced LVEF at the time of their baseline echocardiogram. Based on current guidelines for ICD placement, which is largely based on reduced LVEF, many of the subjects in these studies would not have had an ICD indication. These findings suggest a need to identify additional predictors of SCD besides reduced LVEF.
In this analysis, MAC was associated with increased SCD incidence after adjusting for risk factors. MAC has been associated with increased mortality, CHD, congestive heart failure, and ischemic stroke.12–14,22,23 Although the mechanism is not well understood, MAC has been considered to be a marker of atherosclerosis13 and perhaps even a form of atherosclerosis.24,25 It is believed that MAC and atherosclerosis are related through a number of coronary risk factors.13,14 Certain conduction abnormalities are higher in patients with MAC and conduction delay is a risk factor for SCD.2 Previous reports have implicated MAC as a cause of SCD occurring in the absence of acute ischemia.26 This is the first population based cohort study, to our knowledge, showing a positive association between MAC and SCD.
Several studies support an association between LVH and CVD,2,17,23,27–29 and in this study we found that LVH, as measured by increased LV mass, predicted greater incidence of SCD after adjustments for risk factors and CHD. While electrocardiographic (ECG) detection of LVH is also associated with increased SCD risk,30 echocardiographic measurement of LV mass is a more accurate and reliable tool than ECG for measuring LVH.31–33 A plausible explanation for SCD in those with increased LV mass is that it can precede LV dysfunction.34 Other postulated mechanisms include myocardial fibrosis, a component in the development of LVH, known to facilitate reentrant ventricular arrhythmias.35,36 Fibrosis and LVH may lead to neurohormonal activation and further fibrosis due to angiotensin II.37 Neurohormonal activation and autonomic dysregulation may also directly predispose to arrhythmias. Finally, prolonged ventricular repolarization may occur in LVH, increasing the susceptibility to ventricular fibrillation.38
A prior study from the CHS (age ≥65 years) showed that increasing aortic root diameter was a modest predictor of increased CVD mortality,39 but a Chinese cohort did not show a significant association between greater aortic root diameter and CVD mortality after age adjustment.40 Investigations on aortic root diameter and CVD mortality are otherwise sparse in the literature, and there are no studies examining SCD. A potential explanation for SCD in those with aortic root diameter is its association with both increased LV mass and LV dysfunction.41,42 While a previous CHS analysis showed aortic valve sclerosis to confer a 50% increased risk of cardiovascular morbidity and mortality, it was not associated with increased SCD in either the ARIC Study or CHS cohorts.
This is the first study to find an association between left atrium diameter and SCD, while a prior study found it to predict first clinical cardiovascular events.23,43,44 Given the well-known association between left atrium diameter and atrial fibrillation, and the latter shown to be associated with SCD,45 it is possible that atrial fibrillation could explain this association between SCD and left atrium diameter. However, left atrial diameter increases with increasing diastolic load, and is therefore also a marker of diastolic heart failure.
SCD is common in diastolic heart failure (i.e. heart failure with preserved ejection fraction). SCD accounted for 28% of all deaths in the CHARM-Preserved study46 (candesartan versus placebo, LVEF>40%) and 26% of all deaths in the I-PRESERVE study47 (irbesartan versus placebo, LVEF ≥45%). Investigators have therefore sought ways of predicting SCD in this group.48 Previous CHS data showed peak E velocity to predict incident CHF and the mitral E/A ratio to predict incident CHF in a U-shaped manner.49 The meta-analyzed ARIC Study and CHS data found mitral E/A <0.7 or >1.5 to be associated with increased SCD incidence. However, the individual ARIC Study and CHS results were discrepant, with the ARIC Study cohort demonstrating a lower SCD risk with a mitral E/A <0.7. Without other important diastolic parameters such as left atrial volume and mitral annular tissue velocities, along with the low number of SCD in the ARIC Study, it may be difficult to interpret the significance of this finding.
Finally, we should also acknowledge that traditional risk factors help identify SCD, and that clinical trials have shown that the treatment of risk factors will reduce CHD mortality, much of which is SCD.50 A population-based, preventive strategy aimed at avoiding or reducing risk factors would thus reduce the public health burden of SCD.
Strengths
The ARIC Study and CHS are two large population-based cohorts addressing CVD in the United States with prospective information on echocardiographic risk markers and SCD. In addition, there was a long follow-up, inclusion of non-white participants, extensive measurement of covariates, and physician adjudication of all SCD cases. Finally, the identified echocardiographic risk markers are easily obtainable, and our results may be generalizable to daily practice.
Limitations
Our study has several limitations. The meta-analysis weighs more heavily towards CHS given the ratio of SCD of about 6:1 between CHS and ARIC. In addition, the power to detect heterogeneity between only two studies is low. The power to discriminate between different echocardiographic covariates was limited by relatively low numbers of SCD. We did not adjust for possible intercorrelated echocardiographic covariates to determine if some were superfluous. For example, when calculating the risk of SCD for reduced LVEF, we did not adjust for LV mass, which is a predictor of reduced LVEF.34 Changes in echocardiographic variables might have occurred during follow-up, which would have led to misclassification and potential bias in the hazard ratios. The same might be true for CHD and HF status, though we adjusted for these as time-dependent covariates. Variables such as dyssynchrony, wall motion abnormalities, left atrial volume, and other diastolic parameters were not available. Importantly, the SCD variable was an epidemiological classification and, in the event of unwitnessed SCD, it was impossible to determine whether there was underlying ischemia. In most cases, we did not know the degree of underlying coronary artery disease at the time of death or whether the SCD was solely arrhythmic.
Conclusions
This study suggests that in a middle-aged to elderly population, reduced LVEF, MAC, mitral E/A >1.5 or <0.7, increased LV mass, and left atrial diameter are associated with an increased risk of SCD. In addition, these echocardiographic variables provided incremental prognostic value over clinical risk factors. These readily measurable variables could help identify patients who would benefit from preventive or therapeutic interventions and, in turn, reduce death rates. However, the clinical utility of their measurement and any interventions would need to be assessed in randomized clinical trials.
Supplementary Material
Clinical Perspective.
Sudden cardiac death (SCD) in the community continues to be a major public health problem. In this study we assessed baseline echocardiographic variables from 2383 participants from the Atherosclerosis Risk in Communities Study and 5366 participants from the Cardiovascular Health Study. We found several echocardiographic predictors of SCD. These included reduced left ventricular ejection fraction, mitral annular calcification, mitral E/A >1.5 or <0.7, increased left ventricular mass, and increased left atrial diameter. Furthermore, these echocardiographic predictors provided incremental value over clinical risk factors in predicting SCD. We believe clinicians can use this information to better understand who may be at risk of SCD.
Acknowledgments
The authors thank the staff and participants of the ARIC Study and CHS for their important contributions. We also thank Dr. Sanjiv Shah for providing us with images from the Cardiovascular Health Study.
Sources of Funding
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). SCD adjudication in ARIC was supported by the Donald W. Reynolds Foundation. The authors thank the staff and participants of the ARIC study for their important contributions.
This research was supported by contracts HHSN268201200036C, HHSN268200800007C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and grant U01HL080295 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by R01AG023629 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at CHS-NHLBI.org. Support for Dr. Sotoodehnia was provided by HL111089, HL116747, and HL092111.
Footnotes
Disclosures
None.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart Disease and Stroke Statistics--2012 Update: A Report From the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zipes DP, Wellens HJJ. Sudden Cardiac Death. Circulation. 1998;98:2334–2351. doi: 10.1161/01.cir.98.21.2334. [DOI] [PubMed] [Google Scholar]
- 3.Wilson PWF, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of Coronary Heart Disease Using Risk Factor Categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 4.Epstein AE, DiMarco JP, Ellenbogen KA, Estes NAM, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL, Hlatky MA, Newby LK, Page RL, Schoenfeld MH, Silka MJ, Stevenson LW, Sweeney MO. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: Executive Summary. Circulation. 2008;117:2820–2840. [Google Scholar]
- 5.Chugh SS, Uy-Evanado A, Teodorescu C, Reinier K, Mariani R, Gunson K, Jui J. Women have a lower prevalence of structural heart disease as a precursor to sudden cardiac arrest: The Ore-SUDS (Oregon Sudden Unexpected Death Study) J Am Coll Cardiol. 2009;54:2006–2011. doi: 10.1016/j.jacc.2009.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 7.Skelton TN, Andrew ME, Arnett DK, Burchfiel CM, Garrison RJ, Samdarshi TE, Taylor HA, Hutchinson RG. Echocardiographic left ventricular mass in African-Americans: the Jackson cohort of the Atherosclerosis Risk in Communities Study. Echocardiography. 2003;20:111–120. doi: 10.1046/j.1540-8175.2003.03000.x. [DOI] [PubMed] [Google Scholar]
- 8.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 9.Gardin JM, Wong ND, Bommer W, Klopfenstein HS, Smith VE, Tabatznik B, Siscovick D, Lobodzinski S, Anton-Culver H, Manolio TA. Echocardiographic design of a multicenter investigation of free-living elderly subjects: the Cardiovascular Health Study. J Am Soc Echocardiogr Off Publ Am Soc Echocardiogr. 1992;5:63–72. doi: 10.1016/s0894-7317(14)80105-3. [DOI] [PubMed] [Google Scholar]
- 10.Gardin JM, Siscovick D, Anton-Culver H, Lynch JC, Smith VE, Klopfenstein HS, Bommer WJ, Fried L, O’Leary D, Manolio TA. Sex, age, and disease affect echocardiographic left ventricular mass and systolic function in the free-living elderly. The Cardiovascular Health Study. Circulation. 1995;91:1739–1748. doi: 10.1161/01.cir.91.6.1739. [DOI] [PubMed] [Google Scholar]
- 11.Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–458. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 12.Benjamin EJ, Plehn JF, D’Agostino RB, Belanger AJ, Comai K, Fuller DL, Wolf PA, Levy D. Mitral Annular Calcification and the Risk of Stroke in an Elderly Cohort. N Engl J Med. 1992;327:374–379. doi: 10.1056/NEJM199208063270602. [DOI] [PubMed] [Google Scholar]
- 13.Fox E, Harkins D, Taylor H, McMullan M, Han H, Samdarshi T, Garrison R, Skelton T. Epidemiology of mitral annular calcification and its predictive value for coronary events in African Americans: the Jackson Cohort of the Atherosclerotic Risk in Communities Study. Am Heart J. 2004;148:979–984. doi: 10.1016/j.ahj.2004.05.048. [DOI] [PubMed] [Google Scholar]
- 14.Fox CS, Vasan RS, Parise H, Levy D, O’Donnell CJ, D’Agostino RB, Benjamin EJ. Mitral annular calcification predicts cardiovascular morbidity and mortality: the Framingham Heart Study. Circulation. 2003;107:1492–1496. doi: 10.1161/01.cir.0000058168.26163.bc. [DOI] [PubMed] [Google Scholar]
- 15.Peacock JM, Ohira T, Post W, Sotoodehnia N, Rosamond W, Folsom AR. Serum magnesium and risk of sudden cardiac death in the Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J. 2010;160:464–470. doi: 10.1016/j.ahj.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siscovick DS, Raghunathan TE, King I, Weinmann S, Wicklund KG, Albright J, Bovbjerg V, Arbogast P, Smith H, Kushi LH. Dietary intake and cell membrane levels of long-chain n-3 polyunsaturated fatty acids and the risk of primary cardiac arrest. JAMA J Am Med Assoc. 1995;274:1363–1367. doi: 10.1001/jama.1995.03530170043030. [DOI] [PubMed] [Google Scholar]
- 17.Haider AW, Larson MG, Benjamin EJ, Levy D. Increased left ventricular mass and hypertrophy are associated with increased risk for sudden death. J Am Coll Cardiol. 1998;32:1454–1459. doi: 10.1016/s0735-1097(98)00407-0. [DOI] [PubMed] [Google Scholar]
- 18.Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML. Prophylactic Implantation of a Defibrillator in Patients with Myocardial Infarction and Reduced Ejection Fraction. N Engl J Med. 2002;346:877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 19.Desai As FJC. Implantable defibrillators for the prevention of mortality in patients with nonischemic cardiomyopathy: A meta-analysis of randomized controlled trials. JAMA J Am Med Assoc. 2004;292:2874–2879. doi: 10.1001/jama.292.23.2874. [DOI] [PubMed] [Google Scholar]
- 20.Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, McNulty SE, Clapp-Channing N, Davidson-Ray LD, Fraulo ES, Fishbein DP, Luceri RM, Ip JH. Amiodarone or an Implantable Cardioverter‚ÄìDefibrillator for Congestive Heart Failure. N Engl J Med. 2005;352:225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 21.Gorgels APM, Gijsbers C, de Vreede-Swagemakers J, Lousberg A, Wellens HJJ. Out-of-hospital cardiac arrest--the relevance of heart failure. The Maastricht Circulatory Arrest Registry. Eur Heart J. 2003;24:1204–1209. doi: 10.1016/s0195-668x(03)00191-x. [DOI] [PubMed] [Google Scholar]
- 22.Barasch E, Gottdiener JS, Marino Larsen EK, Chaves PHM, Newman AB. Cardiovascular morbidity and mortality in community-dwelling elderly individuals with calcification of the fibrous skeleton of the base of the heart and aortosclerosis (The Cardiovascular Health Study) Am J Cardiol. 2006;97:1281–1286. doi: 10.1016/j.amjcard.2005.11.065. [DOI] [PubMed] [Google Scholar]
- 23.Gardin JM, McClelland R, Kitzman D, Lima JA, Bommer W, Klopfenstein HS, Wong ND, Smith VE, Gottdiener J. M-mode echocardiographic predictors of six- to seven-year incidence of coronary heart disease, stroke, congestive heart failure, and mortality in an elderly cohort (the Cardiovascular Health Study) Am J Cardiol. 2001;87:1051–1057. doi: 10.1016/s0002-9149(01)01460-6. [DOI] [PubMed] [Google Scholar]
- 24.Thubrikar MJ, Deck JD, Aouad J, Chen JM. Intramural stress as a causative factor in atherosclerotic lesions of the aortic valve. Atherosclerosis. 1985;55:299–311. doi: 10.1016/0021-9150(85)90108-x. [DOI] [PubMed] [Google Scholar]
- 25.Adler Y, Koren A, Fink N, Tanne D, Fusman R, Assali A, Yahav J, Zelikovski A, Sagie A. Association between mitral annulus calcification and carotid atherosclerotic disease. Stroke. 1998;29:1833–1837. doi: 10.1161/01.str.29.9.1833. [DOI] [PubMed] [Google Scholar]
- 26.Quick E, Byard RW. Mitral annulus calcification and sudden death. J Forensic Leg Med. 2013;20:204–206. doi: 10.1016/j.jflm.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 27.Frohlich ED. Left ventricular hypertrophy and sudden death. J Am Coll Cardiol. 1998;32:1460–1462. doi: 10.1016/s0735-1097(98)00414-8. [DOI] [PubMed] [Google Scholar]
- 28.Turakhia MP, Schiller NB, Whooley MA. Prognostic significance of increased left ventricular mass index to mortality and sudden death in patients with stable coronary heart disease (from the Heart and Soul Study) Am J Cardiol. 2008;102:1131–1135. doi: 10.1016/j.amjcard.2008.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taylor HA, Penman AD, Han H, Dele-Michael A, Skelton TN, Fox ER, Benjamin EJ, Arnett DK, Mosley TH., Jr Left ventricular architecture and survival in African-Americans free of coronary heart disease (from the Atherosclerosis Risk in Communities [ARIC] study) Am J Cardiol. 2007;99:1413–1420. doi: 10.1016/j.amjcard.2006.12.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cupples LA, Gagnon DR, Kannel WB. Long- and short-term risk of sudden coronary death. Circulation. 1992;85:I11–18. [PubMed] [Google Scholar]
- 31.Levy D, Labib SB, Anderson KM, Christiansen JC, Kannel WB, Castelli WP. Determinants of sensitivity and specificity of electrocardiographic criteria for left ventricular hypertrophy. Circulation. 1990;81:815–820. doi: 10.1161/01.cir.81.3.815. [DOI] [PubMed] [Google Scholar]
- 32.Woythaler JN, Singer SL, Kwan OL, Meltzer RS, Reubner B, Bommer W, DeMaria A. Accuracy of echocardiography versus electrocardiography in detecting left ventricular hypertrophy: comparison with postmortem mass measurements. J Am Coll Cardiol. 1983;2:305–311. doi: 10.1016/s0735-1097(83)80167-3. [DOI] [PubMed] [Google Scholar]
- 33.Reichek N, Helak J, Plappert T, Sutton MS, Weber KT. Anatomic validation of left ventricular mass estimates from clinical two-dimensional echocardiography: initial results. Circulation. 1983;67:348–352. doi: 10.1161/01.cir.67.2.348. [DOI] [PubMed] [Google Scholar]
- 34.Drazner MH, Rame JE, Marino EK, Gottdiener JS, Kitzman DW, Gardin JM, Manolio TA, Dries DL, Siscovick DS. Increased left ventricular mass is a risk factor for the development of a depressed left ventricular ejection fraction within five years: the Cardiovascular Health Study. J Am Coll Cardiol. 2004;43:2207–2215. doi: 10.1016/j.jacc.2003.11.064. [DOI] [PubMed] [Google Scholar]
- 35.Choudhury L, Mahrholdt H, Wagner A, Choi KM, Elliott MD, Klocke FJ, Bonow RO, Judd RM, Kim RJ. Myocardial scarring in asymptomatic or mildly symptomatic patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2002;40:2156–2164. doi: 10.1016/s0735-1097(02)02602-5. [DOI] [PubMed] [Google Scholar]
- 36.Weber KT, Janicki JS, Pick R, Capasso J, Anversa P. Myocardial fibrosis and pathologic hypertrophy in the rat with renovascular hypertension. Am J Cardiol. 1990;65:1G–7G. doi: 10.1016/0002-9149(90)90952-w. [DOI] [PubMed] [Google Scholar]
- 37.Gonzalez A, Lopez B, Querejeta R, Diez J. Regulation of myocardial fibrillar collagen by angiotensin II. A role in hypertensive heart disease? J Mol Cell Cardiol. 2002;34:1585–1593. doi: 10.1006/jmcc.2002.2081. [DOI] [PubMed] [Google Scholar]
- 38.Gillis AM, Mathison HJ, Kulisz E, Lester WM. Dispersion of ventricular repolarization in left ventricular hypertrophy: influence of afterload and dofetilide. J Cardiovasc Electrophysiol. 1998;9:988–997. doi: 10.1111/j.1540-8167.1998.tb00140.x. [DOI] [PubMed] [Google Scholar]
- 39.Gardin JM, Arnold AM, Polak J, Jackson S, Smith V, Gottdiener J. Usefulness of aortic root dimension in persons > or = 65 years of age in predicting heart failure, stroke, cardiovascular mortality, all-cause mortality and acute myocardial infarction (from the Cardiovascular Health Study) Am J Cardiol. 2006;97:270–275. doi: 10.1016/j.amjcard.2005.08.039. [DOI] [PubMed] [Google Scholar]
- 40.Lai C-L, Chien K-L, Hsu H-C, Su T-C, Chen M-F, Lee Y-T. Aortic root dimension as an independent predictor for all-cause death in adults <65 years of age (from the Chin-Shan Community Cardiovascular Cohort Study) Echocardiography. 2010;27:487–495. doi: 10.1111/j.1540-8175.2009.01072.x. [DOI] [PubMed] [Google Scholar]
- 41.Palmieri V, Bella JN, Arnett DK, Roman MJ, Oberman A, Kitzman DW, Hopkins PN, Paranicas M, Rao DC, Devereux RB. Aortic root dilatation at sinuses of valsalva and aortic regurgitation in hypertensive and normotensive subjects: The Hypertension Genetic Epidemiology Network Study. Hypertension. 2001;37:1229–1235. doi: 10.1161/01.hyp.37.5.1229. [DOI] [PubMed] [Google Scholar]
- 42.Palmieri V, de Simone G, Roman MJ, Schwartz JE, Pickering TG, Devereux RB. Ambulatory blood pressure and metabolic abnormalities in hypertensive subjects with inappropriately high left ventricular mass. Hypertension. 1999;34:1032–1040. doi: 10.1161/01.hyp.34.5.1032. [DOI] [PubMed] [Google Scholar]
- 43.Nagarajarao HS, Penman AD, Taylor HA, Mosley TH, Butler K, Skelton TN, Samdarshi TE, Aru G, Fox ER. The predictive value of left atrial size for incident ischemic stroke and all-cause mortality in African Americans: the Atherosclerosis Risk in Communities (ARIC) Study. Stroke. 2008;39:2701–2706. doi: 10.1161/STROKEAHA.108.515221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kizer JR, Bella JN, Palmieri V, Liu JE, Best LG, Lee ET, Roman MJ, Devereux RB. Left atrial diameter as an independent predictor of first clinical cardiovascular events in middle-aged and elderly adults: The Strong Heart Study (SHS) Am Heart J. 2006;151:412–418. doi: 10.1016/j.ahj.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 45.Chen LY, Sotoodehnia N, Bůžková P, Lopez FL, Yee LM, Heckbert SR, Prineas R, Soliman EZ, Adabag S, Konety S, Folsom AR, Siscovick D, Alonso A. Atrial fibrillation and the risk of sudden cardiac death: the atherosclerosis risk in communities study and cardiovascular health study. JAMA Intern Med. 2013;173:29–35. doi: 10.1001/2013.jamainternmed.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Solomon SD, Wang D, Finn P, Skali H, Zornoff L, McMurray JJV, Swedberg K, Yusuf S, Granger CB, Michelson EL, Pocock S, Pfeffer MA. Effect of candesartan on cause-specific mortality in heart failure patients: the Candesartan in Heart failure Assessment of Reduction in Mortality and morbidity (CHARM) program. Circulation. 2004;110:2180–3. doi: 10.1161/01.CIR.0000144474.65922.AA. [DOI] [PubMed] [Google Scholar]
- 47.Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, Anderson S, Donovan M, Iverson E, Staiger C, Ptaszynska A. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359:2456–67. doi: 10.1056/NEJMoa0805450. [DOI] [PubMed] [Google Scholar]
- 48.Adabag S, Rector TS, Anand IS, McMurray JJ, Zile M, Komajda M, McKelvie RS, Massie B, Carson PE. A prediction model for sudden cardiac death in patients with heart failure and preserved ejection fraction. Eur J Heart Fail. 2014;16:1175–82. doi: 10.1002/ejhf.172. [DOI] [PubMed] [Google Scholar]
- 49.Aurigemma GP, Gottdiener JS, Shemanski L, Gardin J, Kitzman D. Predictive value of systolic and diastolic function for incident congestive heart failure in the elderly: the cardiovascular health study. J Am Coll Cardiol. 2001;37:1042–1048. doi: 10.1016/s0735-1097(01)01110-x. [DOI] [PubMed] [Google Scholar]
- 50.Grundy SM. Primary Prevention of Coronary Heart Disease: Integrating Risk Assessment With Intervention. Circulation. 1999;100:988–998. doi: 10.1161/01.cir.100.9.988. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.