Abstract
The experience of early life stress can trigger complex neurochemical cascades that influence emotional and addictive behaviors later in life in both adolescents and adults. Recent evidence suggests that excessive alcohol drinking, and drug-seeking behavior in general, is co-morbid with depressive-like behavior. Both behaviors are reported in humans exposed to early life adversity, and are prominent features recapitulated in animal models of early life stress (ELS) exposure. Currently, little is known about whether or how ELS modulates reward system nuclei. In this study we use operant conditioning of rats to show that the maternal separation stress (MS) model of ELS consumes up to 3-fold greater quantities of 10% vol/vol EtOH in 1-hour, consistently over a 3-week period. This was correlated with a significant 22% reduction in the number of neurons in the VTA of naïve MS rats, similar to genetically alcohol-preferring (P) rats which show a 35% reduction in tyrosine hydroxylase (TH)-positive dopaminergic neurons in the VTA. MS rats had a significantly higher 2-fold immobility time in the forced swim test (FST) and reduced sucrose drinking compared to controls, indicative of depressive-like symptomology and anhedonia. Consistent with this finding, stereological analysis revealed that amygdala neurons were 25% greater in number at P70 following MS exposure. Examination of the dentate gyrus of the hippocampus, a region involved in encoding emotional memory, reveals fewer dentate gyrus neurons, without effect on the number of astrocytes or length of astrocytic fibers. These data indicate that MS animals exhibit neuroanatomical changes in reward centers similar to those reported for high alcohol drinking rats, but aspects of astrocyte morphometry remained unchanged. These data are of high relevance to understand the breadth of neuronal pathology that ensues in reward loci following ELS.
Keywords: Stereology, dentate gyrus, amygdala, VTA, early life stress, alcohol-preferring rats, P rats, maternal separation, drug addiction
1.0 Introduction
The brain’s reward system governs natural eating and drinking behaviors that are essential to survival, but this system can be hijacked to mediate the development of substance abuse and addiction (Wise, 2013). The connectivity of and activity within reward loci can be dramatically altered by environmental stimuli during all stages of development; however, its functionality is particularly susceptible to robust alterations during early developmental stages (Dayan et al., 2010) which can lead to maladaptive behaviors that persist through adulthood. Adverse early life experiences (ELS), especially early exposure to chronic stress, was shown to disrupt normal reward seeking mechanisms and confer susceptibility of individuals to neuropsychiatric conditions such as depressive disorders (Enoch, 2011), (Pryce et al., 2005, Ruedi-Bettschen et al., 2006), but especially excessive drug and alcohol use. Increasingly more pre-clinical data is emerging to support the hypothesis that ELS is a risk factor for excessive alcohol use or abuse (Huot et al., 2001, Garcia-Gutierrez et al., 2015). Even in rodent models where MS does not seem to directly induce increased alcohol preference, evidence suggests that the neuroanatomical or neurochemical disruptions can be dormant until the experience of a second stressor which serves as a trigger for excessive alcohol consumption (Penasco et al., 2015). Thus, the effects of MS are insidious and possibly involve multiple overlapping, sometimes subtle mechanisms.
The basis for this increased predisposition to addictive behavior is not well understood, but ELS can influence the microcircuitry of loci that regulate affect, reward, and addiction. In the absence of any drug exposure in ELS animals, corticolimbic structures like the frontal cortex, hippocampus and nucleus accumbens show reduced dendritic length and dendritic spine number as a result of postnatal stress, or MS, (Huot et al., 2002, Monroy et al., 2010, Romano-Lopez et al., 2015) and results in atrophy of dendrites in the PFC (Murmu et al., 2006), and hippocampus (Jia et al., 2010) of prenatally stressed animals.
In the absence of MS or other ELS, the normal transition from drug overuse to dependency is also characterized by similar progressive changes in microstructure and circuitry of the same regions of the brain’s reward system (He et al., 2005, Zhou et al., 2007), influencing connectivity, cerebrovascular structure (Cadet et al., 2014), neurochemical substrate levels (Raftogianni et al., 2014), as well as glial cell morphology (Fattore et al., 2002).
The reward system includes the limbic system (hypothalamus, amygdala, hippocampus, septal nuclei and the anterior cingulate gyrus), nucleus accumbens (NAc) and ventral tegmental area (VTA). The VTA, located in the midbrain, contains dopaminergic neurons that project to the NAc and PFC (Aransay et al., 2015) to mediate compulsive drug seeking (Brake et al., 2004), and promotes behaviors associated with the reinforcing effects of ethanol exposure (Gessa et al., 1985, Gatto et al., 1994, Rodd et al., 2005a) and other drugs of abuse. In the NAc of MS rats, there is a reduction in the dopamine transporter DAT presumably as a result of a reduction in afferents from midbrain-localized dopaminergic neurons (Ciliax et al., 1995, Brake et al., 2004), however specific effects of MS on the VTA which has capacity to respond to GABA, glutamate, opioids, cannabinoids, and stress hormones is not known. The VTA can be powerfully regulated by the stress hormone, corticotropin releasing factor (CRF), which when injected into the VTA reinstates drug seeking behavior following extinction of such behavior (Blacktop et al., 2016). Thus stress can directly modify reward and the motivation for drug seeking.
In the limbic system, the amygdala is key for processing emotionally motivated behaviors that are salient in major depressive disorder and addiction (LeDoux, 2007). The amygdala is tightly linked to the dentate gyrus of the hippocampus for processing emotional memory (Abe, 2001, Abe et al., 2008), and indeed, many studies of early life stress, including maternal deprivation/separation stress report extensive alterations in amygdala circuitry (Callaghan and Richardson, 2011, Danielewicz and Hess, 2014, Toda et al., 2014). Likewise, alcohol-drinking rats also exhibit depression symptomatology and anxiety, presumably involving amygdala circuits, in the absence of any classic external inducers of these symptoms (Ciccocioppo et al., 2006).
Both the amygdala and the VTA send projections to NAc, where input regarding mood, motivation, and reward converge (LeDoux, 2007, Koob, 2009). The hypothalamic-pituitary-adrenal (HPA) axis, which regulates circulating hormone, is activated in MS to result in heightened expression and release of hormones (ACTH, corticosterone, CRF, other metabolites) from each of its components (Liu et al., 2000, Plotsky et al., 2005, Aisa et al., 2008). These elevated circulating hormones contact receptors throughout the brain but especially on mesocorticolimbic neurons of the VTA, amygdala, and hippocampus (Aisa et al., 2008, Wise and Morales, 2010, Jawahar et al., 2015). In addition, indirect inhibition or activation of receptor systems such as the endocannabinoids and noradrenergic system add additional complexity to the stress response, and could act in parallel, additively or synergistically to amplify a CRF-mediated stress response that facilitates drug consumption and depressive symptoms (Romano-Lopez et al., 2015).
In this study, we hypothesized that adverse events such as early life stress, may alter mesocorticolimbic neuron development and maturation to induce depressive-like behaviors, lack of pleasure seeking and alcohol drinking. We used a rat model of chronic maternal separation to emulate ELS, in order to investigate the neuroanatomical alterations within reward system nuclei associated with MS-induced alcohol drinking behavior. Because ELS reportedly hijacks the HPA axis, the reward circuits, and other neurocognitive features, understanding the altered anatomical correlates to alcohol drinking and other progressive pre-addictive behaviors is essential for the development of effective strategies to combat and prevent addiction.
2.0 Materials and Methods
Animals
There were a total of 62 animals used in this study. Pregnant Sprague Dawley rats were obtained from Harlan Laboratories [Frederick, MD, USA] and offspring used in this study were born onsite at the veterinary facility. At postnatal day 2, pups were subjected to the MS paradigm described below, and were later behaviorally tested for excessive drinking behaviors as adults. Alcohol preferring (P) and non-alcohol preferring (NP) adult rats were obtained from the Alcohol Research Center, Indiana University School of Medicine. P rats are well characterized as performers of an operant response for access to ethanol that is not performed by the NP rats. Since P rats are genetically predisposed to alcohol drinking and have high CRF levels, they were used in this study to compare the naïve state of their VTA to that of the MS animals to determine if there are similar disruptions in the reward loci of both models. Animals were housed 2 per cage, with a reverse 12 h light/dark cycle and provided with food and water, ad libitum, until operant training after which they were housed 1 per cage. All studies were approved by the Howard University Institutional Animal Care and Use Committee (IACUC), and conducted with strict adherence to the Guide for the Care and Use of Laboratory Animals (Committee for the Update of the Guide for the Care and Use of Laboratory Animals et al., 2011).
Maternal Separation Regimen
The maternal separation paradigm was conducted as described previously (Wang and Gondre-Lewis, 2013). Briefly, beginning at P2 until weaning at P21, pups were removed daily from their mothers’ home cage, moved to a different room altogether and exposed to maternal separation in individual chambers for 3 hours from 11 am to 2 pm each day. The MS room temperature was monitored and maintained at 29° C with a heater to simulate the warmth of the mother’s body. Pups were returned to their mothers’ home cage after 3 hours. For experimental comparisons, whole litters were assigned to one of two groups: controls (CTL) and maternally deprived/separated (MS). CTL rat pups were kept with their mothers during the entire postnatal period. After MS, rats were weaned at P22 and allowed to mature in the animal facility. They were re-entered into the study in late adolescence (~P42) for the FST, or as adults (>P70) for alcohol responding.
Operant Apparatus and Alcohol Drinking Training Protocol
Adult MS rats (n=10) were trained to lever press for ethanol starting at P60–P70. The training described below takes approximately 4 weeks, followed by 21 days of testing. Standard operant chambers [Coulbourn Instruments, Inc., Lehigh Valley, PA] were enclosed in an isolated box. The operant apparatus contained two levers, two dipper manipulanda, triple cue lights over each lever, and a house light. The dipper cup size was 0.1 mL. The Coulbourn Graphic State “3” operant software was used to capture lever presses for the alcohol. Rats were trained to orally self-administer EtOH daily for 1h under a fixed ratio [FR] 1 schedule employing the sucrose fading technique (Harvey et al., 2002). After a period of stabilization on the FR1 schedule, the response requirement was then increased to an FR4 schedule, i.e., the rat had to press levers 4 times in order to receive the reward. For each schedule, responding was considered stable when responses were within ± 20% of the average responses for five consecutive days. Once responding was stabilized at FR4, the rats were allowed access every day over a 3-week period using a 5-day access and 2-day resting modifications of well-established procedures (June and Eiler, 2007, Liu et al., 2011).
Forced Swim Test
To conduct swimming sessions, rats during late adolescence (n=17) at P40–P43 were placed individually for 15 min in a Pyrex cylinder (21×46 cm) filled with water 23–25°C, to a depth of 30cm so that rat limbs could not reach the bottom of the cylinder. 24 h after their first exposure, a second test was conducted for 5 min in the cylinder and behaviors recorded with a video camera placed above the cylinder for subsequent analysis. Behavior sampling of the frequency of immobility in each 5-s period of the 300-s test was recorded and analyzed. The data shown represent the summation of the time spent immobile or floating as assessed using the ForcedSwimScan System, (CleverSys, Inc., Reston VA, USA).
Sucrose Home Cage Drinking
Rats (N = 10/treatment group) were separated into single cages and a 10 mL sipper tube containing a 3% (w/v) sucrose solution in tap water was placed in each cage. Animals were given access to the solution with simultaneous free access to food for 2 hours. Solution intake was calculated based on bottle volumes before and after the 2 h period and measured for 3 consecutive days.
Perfusion and Histology
There were n=28 animals used for histological or immunohistochemical analysis of amygdala, DG, and VTA: 6 MS, 6 Sprague Dawley controls, 8 P, and 8 NP controls. At P70 or P90 for MS and P groups, respectively, animals were perfused as previously described (Wang and Gondre-Lewis, 2013), with some modifications. Adult animals were deeply anesthetized with Isothesia (Isoflurane) (Butler Animal Health Supply, Dublin, OH) and when insensate, underwent a thoracotomy for transcardial perfusion with 0.9% NaCl to clear the blood in the circulatory system. This was followed with a slow perfusion with chilled 4% paraformaldehyde (PFA) in 0.1 M phosphate/4% sucrose buffer, pH 7.4. Brains were post-fixed overnight in the 4%PFA/4% sucrose/0.1 M phosphate buffer, and then transferred to and stored in phosphate buffered saline (PBS), pH 7.4, at 4° C until ready for processing. MS and CTL brains were arranged and embedded in a gelatin array using Neuroscience Associates, Inc. BrainArray technology, and P/NP rat brains were processed individually. Brains were freeze-sectioned on a sliding microtome to generate free-floating 50 μm coronal sections, and every 9th section mounted onto gelatin-coated slides and processed for thionine-Nissl staining and GFAP immunohistochemistry to detect astrocytes. P/NP rats were sectioned in a similar manner, except every 4th 20 μm section was used for TH immunohistochemistry to detect dopaminergic neurons.
Immunohistochemistry
Immunocytochemistry was performed as previously described with some modifications (Wang and Gondre-Lewis, 2013, Young et al., 2013). Free floating sections were washed in PBS and incubated in blocking buffer containing 0.3% TX-100, 3% NGS/1%BSA for 1 h. Sections were then incubated with either anti-GFAP or anti-TH antibody (Abcam, Inc., Cambridge, MA) at 4°C washed, and incubated with biotinylated anti-rabbit IgG. This was followed by exposure to ABC Elite reagent for 1 h and subsequent incubation with DAB (Vector Laboratories, Burlingame, CA). Sections were mounted on gelatin-coated slides. To facilitate identification of GFAP+ cell bodies, GFAP slides were counterstained with Nissl, and all slides were serially dehydrated with increasing concentrations of alcohol (70%, 90%, 95%, 100%) followed by Histosol. Slides were coverslipped with DPX mounting reagent. Images were taken with a Zeiss Axio Oberver Z1 (Zeiss, Gottingen, Germany) equipped for phase contrast light microscopy.
Stereological Analysis
Unbiased stereology was used to estimate mean total number of neurons in the amygdala and VTA, as well as astrocyte number and length in the DG via the optical fractionator method (Pakkenberg and Gundersen, 1989, Manaye et al., 2013, Wang and Gondre-Lewis, 2013). Data collection was carried out using both the StereoInvestigator program (MicroBright Field, Inc.) and the Stereologer software (Stereology Resource Center, Inc. FL). Each system used an X–Y–Z motorized stage. The Stereologer system was integrated with a Leica DM2500 microscope and a DCAM-compliant 1394 camera. The Stereoinvestigator was attached to a Nikon E800 microscope and Optromics high-resolution video camera. The region of interest was outlined at low power (4X or 5X) over the entire rostral to caudal length of the right hemisphere. Cells were counted or fiber lengths were marked on a thin focal plane scanning with a 63X objective in the z-axis. The appropriate grid size and counting frames were determined based on results of pilot studies for each region (Pakkenberg and Gundersen, 1989). Sampling grid sizes (amygdala and VTA, 200 μm × 200 μm; DG astrocytes, 600 μm × 600 μm) and counting frames (VTA and amygdala, 30 μm × 30 μm; DG astrocytes, 80 μm × 80 μm) were optimized to achieve a mean Gundersen coefficient of error (CE) of < 0.1 (Pakkenberg and Gundersen, 1989). A guard volume 2.0 μm deep on both sides of the section was used to avoid introduction of errors due to sectioning artifacts, including uneven section surfaces. This unbiased counting method was repeated at approximately 200 systematic-random locations across all sections for each case to achieve a high stringency level, as evidenced by a coefficient of error less than 10% (CE < 0.10) for both CTL and MS groups. Neurons within the VTA or amygdala were identified as Nissl positive cell bodies containing a nucleolus clearly in focus within the counting frame, with lightly stained surrounding cytoplasm. Neurons were further distinguished by size and their morphology (Domesick et al., 1983). Therefore, although rare, smaller glial-shaped cells were not counted. Astrocytes of all sizes were included in the count if they met the inclusion criteria: cell bodies containing both Nissl and GFAP –positive astrocytic processes clearly associated with a Nissl stained cell body.
Estimates of astrocytic process length were made at random locations through each reference space and the mean of these used to estimate the total length (Mouton et al., 2002). Briefly, at each sample location, virtual spheres were focused through the Z-axis. The stage was moved through the tissue in the Z-axis in 10 steps of 1 μm each. At each focal plane concentric circles of increasing then decreasing diameter were superimposed over the magnified image of fibers (Fig 5). The total length of glial fibers was estimated from the sum of intersections through the entire reference space, and the known fractions at each sampling level, according to the fractionator method (Manaye et al., 2013). 6 animals per group were analyzed for stereology (3 males and 3 females). Table 1 shows a stereology parameters table for the amygdala, VTA, and DG with values generated by the Stereologer or Stereoinvestigator. Figure 1 shows representative sections spanning the anterior-posterior extent of the brain regions analyzed with the regions analyzed encircled in red.
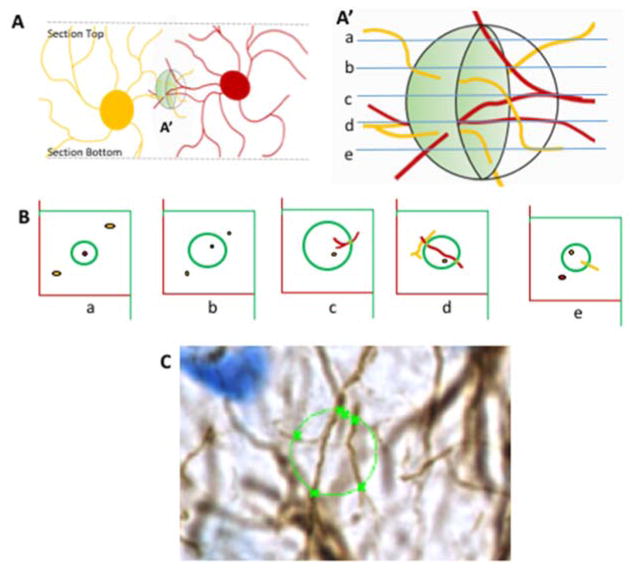
Figure 5. Schematic representing the use of Isotropic sphere (spaceball) probe for fiberlength analysis.
A) View through the Z axis of a section with two astrocytes represented. The virtual sphere (spaceball) is in the shaded region A′. A′) Enlargement of the virtual spaceball sphere with optical sections (a–e) to analyze the fibers as they cross the perimeter of the sphere at that level. B) Images of each optical plane along the z-axis of the sphere. At any given plane, fibers are only counted if they are in focus as they traverse the perimeter of an optical Z plane. At plane d, the red astrocyte fiber is long enough to traverse the perimeter of the sphere twice, whereas the yellow astrocyte does not cross at that plane; therefore 2 instances are counted. C) Image of one of the sections of the specimen used in this study. The x represents where in-focus fibers cross the perimeter of the optical plane (circle) of the space ball probe which was 10μm in diameter.
Table 1. Stereological Parameters.
This table reflects the conditions under which neurons in the VTA and amygdala, and astrocytes of the DG were counted. SSF, section sampling fraction, is the number of sections analyzed divided by the total number of sections through the region. ASF, area sampling fraction, is the area sampled divided by the total area of the region. ΣQ-, the sum of markers counted, the total number of neurons or astrocytes counted within the regions of interest. Vref, reference volume, is the total volume measured for the region of interest. CE, the coefficient of error, a second-order stereological parameter for total observed variation, should be maintained at <0.1
| SSF | ASF | ΣQ- | Vref (mm3) | CE | |
|---|---|---|---|---|---|
| CTL VTA | 0.11 | 0.08 | 504 | 1.950 | 0.05 |
| MD VTA | 0.11 | 0.08 | 388 | 2.034 | 0.06 |
| CTL Amygdala | 0.11 | 0.01 | 416 | 6.673 | 0.05 |
| MD Amygdala | 0.11 | 0.01 | 536 | 7.101 | 0.04 |
| CTL DG | 0.11 | 0.02 | 341 | 8.836 | 0.04 |
| MD DG | 0.11 | 0.02 | 295 | 8.035 | 0.04 |
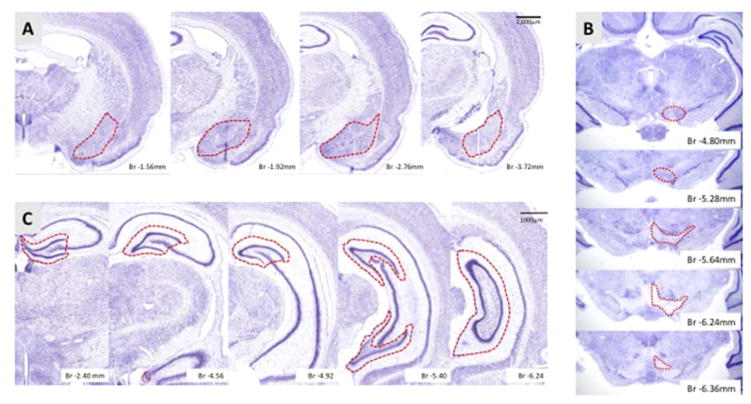
Figure 1. Anterior-posterior extent of anatomical regions used for stereological measurements.
A) Sections of amygdala, includes the basal, central, medial, lateral nuclei from bregma −1.3 to −4.2 mm. B) Sections of the VTA includes the rostral VTA, parainterfascicular, paranigral, and parabrachial pigmented nuclei from bregma −4.56 to −6.84 mm. C) Sections of the DG, includes rostral most DG with all three layers included for astrocyte analysis from bregma −1.72 mm to −6.84 mm. The red outline is a representative contour to demarcate the region of interest to be counted as was outlined during stereological analysis. Nissl sections for A and C were from the brainmaps.org interactive atlas, and the bregma levels and regional borders were identified with Paxinos Atlas.
Cell counting in the VTA
Non-stereological methods were used to count tyrosine hydroxylase (TH)-positive neurons in the VTA of P and NP rats, n=8 per group. For each rat, TH neurons in 17–25 tissue sections, encompassing the entire length of the VTA, were manually counted at 40X, averaged per section and divided by the periodicity (in mm). Data are expressed as cells/mm2.
Statistical Analysis
ANOVA was used to analyze and obtain statistics of behavior data, and neuron or astrocyte numbers generated by stereological methods. Where the F-test showed a difference, a Newman-Keuls multiple comparison post-hoc test was applied for further comparisons. The descriptive statistics are displayed in graphs as mean +/− standard error of the mean (SEM). A p value <0.05 was considered significant.
3.0 Results
Excessive alcohol drinking is associated with a reduction of neurons in the VTA of MS rats
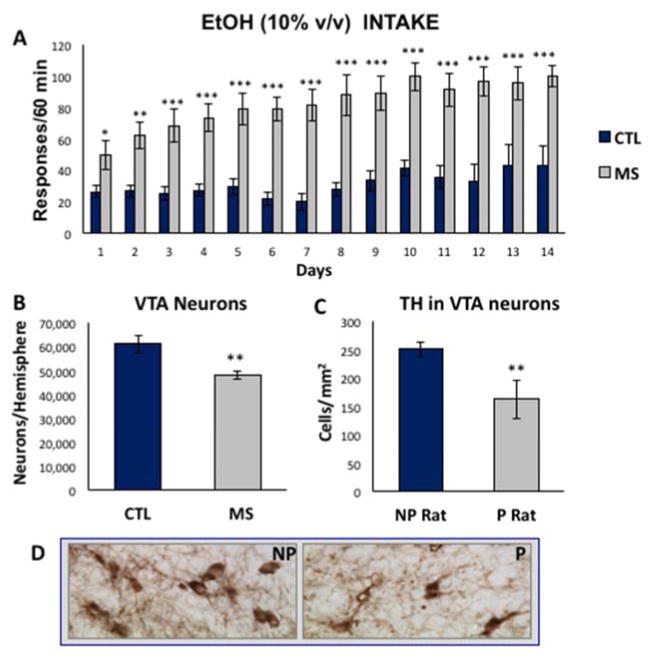
Adult rats were trained to lever press for alcohol in an operant chamber, following exposure to the maternal separation paradigm during the neonatal period. Figure 2A shows the mean number of lever presses during 1 hour in the operant chamber each day for 14 days. There was a significant and sustained increase in the amount of responding for alcohol in MS rats compared to control with a significant main effect of group (F (1,234) =60.24, p<0.0001, n=10 per group). Post hoc analyses confirmed the increased responding of MS rats compared to controls for each day of testing, p≤0.05. Because the VTA is a primary mesolimbic locus mediating the rewarding/reinforcing properties of alcohol, we investigated if MS exposure alters VTA neurons in the adult brain. Using unbiased stereological principles and the optical fractionator method, we found a marked 25% reduction of neurons in the VTA of naïve (never exposed to alcohol) MS rats, 48 ± 1.7 × 103 vs. 61 ± 3.7 × 103 in CTL, with a significant main effect of group (F (1,5) =22.05, p=0.005, n=6 per group). In fact, reduced dopamine neuron numbers in the VTA of MS animals was consistent with reduced dopaminergic TH+ neurons in the VTA of naïve P rats, 164 ± 34 cells/mm2 compared to non-preferring (NP) rats, 252 ± 13 cells/mm2, as illustrated in Figure 2C, p<0.01, n=8 per group. The P rat is a well-characterized model of specially bred, genetically alcohol-preferring rats that consume high levels of alcohol (Morzorati, 1998, Rodd et al., 2005a). Figure 2D shows representative tyrosine hydroxylase staining of the VTA of P and NP rats. The entire anterior posterior extent of the VTA was used for analysis, and this is shown in figure 1 in Nissl sections where the perimeter of the region is demarcated in red.
Figure 2. Increased alcohol drinking and reduction of VTA neurons in MS adult rats, similar to P rats.
A) Compared to control SD rats (blue), MS rats (gray) trained to lever press for 10% alcohol on an FR4 schedule exhibit sustained increased levels of alcohol drinking during 14 days of access, n=10 per group. B) Stereological analysis of P70 adult rat VTA showed a reduction in the number of dopamine-like neurons in animals exposed to early postnatal (P2–P21) MS, n=6 per group. C) TH-immunoreactivity in the VTA of alcohol-preferring (P) rats revealed a reduction of large TH+ dopaminergic neurons, n=8 per group. D) Representative images of TH+ immunoreactivity in the VTA of P and NP animals. Data are expressed as mean ± SEM. *, p<0.05; **, p<0.01, p<0.001.
Depressive-like behavior and anhedonia in MS rats is correlated with increased amygdala neurons
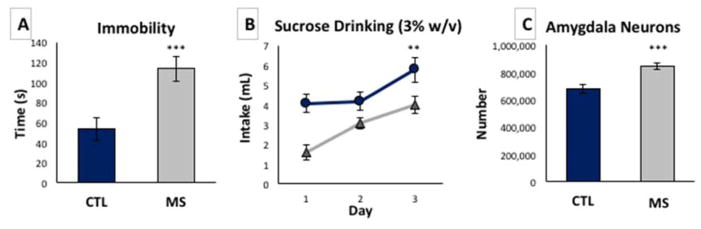
Figure 3A shows that when subjected to the forced swim test, a behavior used to examine defeat and despair as indicators of depression symptoms, MS rats showed greater immobility times (113.6 ± 12.3 s) compared to control rats (53.5 ± 11.2 s), averaging about 2X as much time in the immobile state as controls with a significant main effect of group (F (1,15) =13.6, p=0.002, n=16 per group). Because depression and anhedonia are often co-occurring, we tested pleasure-seeking behavior by giving animals access to 3% sucrose for 1 hour over 3 consecutive days. MS rats consistently drank significantly less of the sweet cocktail than controls during each access period (Fig 3B), p<0.05, for days 1, 2, and 3. Because the amygdala is a key transducer of negative affect, including depression and anxiety and can regulate escalation of alcohol use, in figure 3C we used unbiased stereological methods to evaluate if the number of resident neurons of the amygdala are changed by exposure to maternal separation. There was a significant 25% increase in the number of neurons in the amygdala after exposure to MS, 84 ± 2.4 × 105 compared to 68 ± 2.9 × 104 in controls, with a significant main effect of group F (1,5) =23.0, p=0.005, n=6 per group.
Figure 3. Depression symptomatology, anhedonia, and increased amygdala neurons in MS adult rats.
A) Immobility in the forced swim test of control SD rats (blue) compared to MS rats (gray) show increased immobility in MS, n=16 per group. B) Sucrose (3% w/v) intake via bottle drinking of MS over 3 consecutive days is reduced compared to control, n=10 per group. C) Stereological analysis of naïve P70 adult rat amygdala (n=6 per group) showed increased neuron number in animals exposed to MS during the pre-weaning period. Data are expressed as mean ± SEM. **, p<0.01 ***, p < 0.001.
MS causes a reduction in granule neurons of the Dentate Gyrus but has no effect on astrocytes
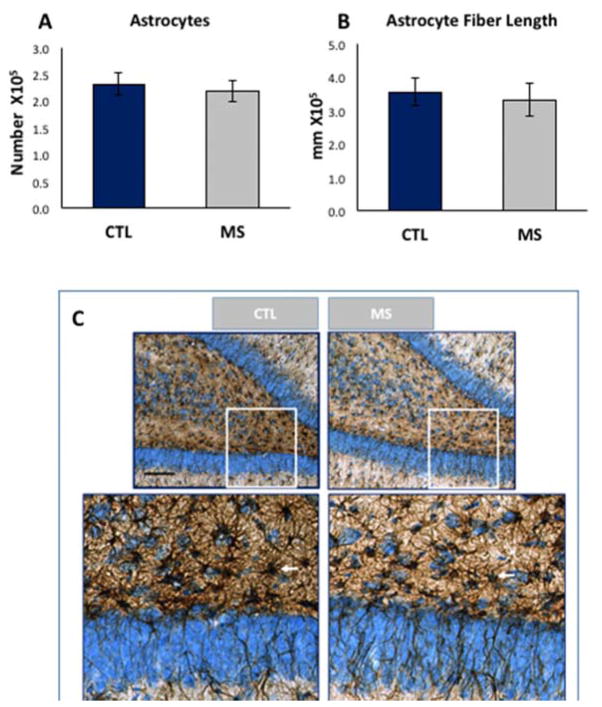
In previous studies, it was established that the DG of the hippocampus was significantly reduced due to MS, an event that began in infancy and lasts through adulthood (Oomen et al., 2011, Wang and Gondre-Lewis, 2013). Because astrocytes are important for modulating synaptic transmission, we tested if their numbers were reduced similar to granule neurons as an extension of the pathology due to MS. Astrocytes were assayed by immunocytochemistry with antibodies against an astrocyte-specific intermediate filament protein, glial fibrillary acidic protein (GFAP), to investigate morphological features. Unlike the reduction of DG neurons induced by MS, when GFAP+ astrocytes were quantitatively evaluated for total cell number (Fig 4A), MS rats revealed no statistical difference compared to controls, F (1,5) =0.39, p>0.05, n=6 per group. To determine if there were greater or fewer astrocytic fibers as an indicator of astrocyte complexity and perhaps synaptic function, we estimated the total fiber lengths of astrocytes in the brain of MS and CTL rats. Using a 10μm isotropic sphere probe to measure fiber crossings, it was evident that astrocytic fiber lengths did not change as a result of MS (Fig 4B, p>0.05, n=6). We then performed a qualitative assessment of morphological features and tissue distribution of astrocytes and found similar variations in astrocyte morphologies across the different animals in MS and control, although MS groups trended toward less dense staining. Fig. 4C shows the distribution of astrocytes, and a typical area used with the spaceball probe. The spaceball technique uses a virtual sphere lodged within the z-axis of the tissue section (Fig 5A) to estimate process length based on the number of times the fibers enter and exit at a specific focal plane (Fig 5A′). Fibers that terminate within the sphere are not counted, nor are those that bypass the perimeter of the sphere (Fig 5B). In the software, the optical fractionator method is combined with the counting of fiber crossings in the equation (total L = 2 • ΣI • F1 • F2 • F3• F4), where, total L = total length of linear feature (units = μm, mm, cm, etc.). ΣI = sum of intersections on all sections. F1 = 1/section sampling fraction= 1/number sections sampled/total sections. F2 = 1/area sampling fraction = 1/area of section sampled/total area. F3 = 1/thickness sampling fraction = 1/h/t. A fourth fraction, F4, is a grid constant that describes the ratio between the volume of one sphere (Vsphere) to the volume of the sampling box (Vbox). In figure 5C, a section through a typical 10μm probe is shown, with marked areas of the perimeter where axons crossed.
Figure 4. Stereological analysis of astrocyte number and astrocyte fiber length in hippocampal dentate gyrus of MS and control rats.
A) Mean astrocyte counts or B) total astrocyte fiber length did not differ between MS or control groups, n=6 per group. D) Representative example of GFAP+ astrocytes in MS and CTL animals processed adjacently on the same embedding sheet, and imaged at the same illumination levels. Arrows show Astrocyte cell bodies counted in the infra- and supra-granular DG space. Bottom panels are enlarged region of insets (white square); red circles represent the spaceball probe intersected with either thin or thick fibers. Data are expressed as mean ± SEM. *, p<0.05 by ANOVA.
4.0 Discussion
In the current study, we used unbiased stereology to analyze the effect of MS on mesolimbic neurons and we report that MS caused a reduction in the number of adult VTA neurons and an increase in amygdala neurons. The DG of the hippocampus, important for emotional/motivational processing of memory was also shown to be reduced following MS (Oomen et al., 2011, Wang and Gondre-Lewis, 2013). These MS-induced morphometric abnormalities were consistent with a predisposition of the rat to excessive alcohol drinking, depressive behavior, and anhedonia as assessed with operant conditioning, the forced swim test, and sucrose drinking, respectively. Preliminary studies indicate that mesolimbic effects may not extend to influence astrocyte function in the DG. This study extends the findings that stressful early life experiences increase tendency toward depressive-like behaviors and alcohol drinking in adult animals (Vazquez et al., 2006, Garcia-Gutierrez et al., 2015) to examine potential associations between neuroanatomical adaptations in brain regions of the reward system and the MS-induced alcohol drinking and depressive behaviors.
Dopaminergic cells of the VTA are critical for reinforcing behaviors related to motivation and incentive. In non-alcohol-dependent individuals, VTA dopaminergic neurons show high sensitivity to alcohol exposure by increasing dopamine release into its efferent targets, most notably nucleus accumbens (Smith and Weiss, 1999, Rodd et al., 2005b, Franklin et al., 2009). In our study, we found that MS-induced predisposition for alcohol intake is associated with decreased numbers of neurons in the VTA of alcohol naïve MS rats. VTA volume remained unchanged, thus increasing VTA neuronal density. A similar study found no changes in total neurons in the VTA in juveniles even as Ki67, a marker of neuronal proliferation was reduced (Chocyk et al., 2011). Recently, this same group used BrdU dating and Nissl stain to demonstrate that total neurons of the VTA were increased in adults, but that these increases were due to non-dopamine neuron proliferation (Monroy et al., 2010, Chocyk et al., 2015). Together, these studies indicate that MS could change the balance of cell types in the VTA. A strong component of the VTA circuitry, are GABA neurons that help modulate the excitatory input to dopamine neurons (Michaeli et al., 2012); therefore, It is likely that MS influences the stoichiometry of neurons in the VTA so as to reduce the Dopamine:GABA neuron ratio. Enhancing GABA neurons in this circuitry would provide additional inhibitory input to already reduced VTA dopaminergic neurons as shown in this study. Indeed, our finding of decreased VTA dopaminergic neurons in alcohol preferring rats, and in naïve MS rats supports the idea that the regulation of VTA-mediated rewarding effects are impaired in both such that more alcohol is required to elicit rewarding properties.
Furthermore, the naïve brain of alcohol preferring rats has 20–25% decreased density of dopamine D2 receptors at the onset, in several areas of the CNS including the VTA (McBride et al., 1993). Therefore, the observed reduction in VTA neurons in P and MS rats are consistent with the notion that mechanisms in the VTA could be similar for both P rats and MS rats. Although, we did not examine D2 receptor levels in our study, it is possible that the decreased dopamine receptor levels in VTA and of dopamine transporter at VTA nerve terminals (Brake et al., 2004) result from decreased neurons in the VTA, likely an important anatomical adaptation for the reinforcement of alcohol drinking behavior. Further studies may reveal that in addition to the lower density that these neurons have alternate connectivity and receptor characteristics. Therefore the drinking behavior reported here is an example of stress-induced drug seeking mediated through the VTA possibly by actions of elevated stress hormone, CRF, as was recently proposed as a possible mechanism for CRF in the reinstatement of cocaine use (Borgland et al., 2010, Blacktop et al., 2016) as CRF modulates the sensitivity of dopaminergic neurons in the VTA to respond to glutamate excitatory input as well as GABA inhibitory input (Wise and Morales, 2010, Blacktop et al., 2016).
The amygdala is most prominently associated with fear and anxiety processing and its altered function is implicated in a large series of neuropsychiatric disorders including major depressive disorder (LeDoux, 2007). It is also heavily implicated in the processing of rewards and each of its nuclear groups is implicated in some aspect of reward learning and motivation and drug addiction (LeDoux, 2007, Koob, 2009). In animals, depressive-like behavior is tested with the forced swim test, where animals that have a short latency to immobility and remain immobile longer are evaluated as exhibiting behavioral despair; a behavior that is reversible with multiple antidepressant drugs (Castagne et al., 2011). Anhedonia is the lack of pleasure seeking, and is tested in rats by their preference for a sweet drink, usually a 3% sucrose solution. In general, non-depressed animals drink more sucrose than those with depression (Strekalova and Steinbusch, 2010). Maternally deprived rats in this study showed a two-fold increase in immobility and an average 45% decrease in sucrose drinking behavior. Because amygdala function is strongly implicated in depressive behaviors in both human clinical and animal basic research (Dowd and Barch, 2012), we evaluated neurons in the amygdala. Associated with these behaviors was a significant increase in numbers of neurons in the amygdala of adult rats that were maternally deprived during the early postnatal period. One study on depressed humans, showed reduced amygdala responsiveness to positive stimuli with increased responsiveness to negative stimuli (Stuhrmann et al., 2013) and a larger amygdala as a predictor of anxiety and depression in young individuals (Qin et al., 2014). In a rat model of ELS, it was found that a temporary inactivation of the amygdala rescued the depressive behavior in forced swim test (Raineki et al., 2012). Our finding shows strong support for altered structural formation of the amygdala during postnatal periods with ELS. The increased neuronal number may signify the potential of increased amygdala activity. Since our measurements were in naïve rats prior to ethanol exposure, we do not know how the amygdala in MS individuals might change after chronic exposure to ethanol leading to alcohol dependence. After the transition to the addictive state, the amygdala of dependent individuals is actually smaller in volume than in the non-addicted state (Koob, 2009).
The dentate gyrus of the rat hippocampus, important for memory-related processing of limbic responses, is highly susceptible to MS-induced stress. The DG is one of a few neuroanatomical sites permissive to adult neurogenesis and works reciprocally with the amygdala to encode emotionally relevant memories. Granule neurons in the principle layer of the DG are reduced (Oomen et al., 2011, Wang and Gondre-Lewis, 2013) as early as postnatal day 15, a period during which the region has high plasticity (Wang and Gondre-Lewis, 2013). This could mean that the emotional stress induced by MS led to a decrease in neurogenesis or an increase in apoptotic processes, a process which is extensively documented in adults exposed to MS (Lee et al., 2001, Fabricius et al., 2008, Aisa et al., 2009, Hulshof et al., 2011). Astrocytes are neuroglia which modulate the efficacy of synaptic transmission and provide nutrients and support for synaptic communication, and their morphology and number could vary depending on the efficacy of neurotransmission (Kimelberg and Nedergaard, 2010, Young et al., 2013). To test if astrocytes are also reduced as a result of MS, we used the optical fractionator method together with the isotropic sphere probe to measure cell number and fiber length. Our findings in the current study indicate no statistical difference in DG astrocytes and their fiber length, even as DG neurons of MS rats are reduced. It is possible that other features of astrocytes are changed. For example, in our stereological approach, the space ball (isotropic) probe we used did not differentiate between thick or thin processes, and thus although total fiber length were unchanged, functional morphology studies could reveal previously unreported astroglia regulation. In addition, the literature contains conflicting data that astrocytes are both reduced or unchanged in the hippocampus, wildly dependent on the MS/MD paradigm used (Leventopoulos et al., 2007, Llorente et al., 2009). More extensive examination of astrocyte morphology is necessary to determine changes in activation state as a result of exposure to ELS.
The effects of ELS via postnatal MS or prenatal stress induces global epigenetic changes which may initially begin by permanently altering the organism’s responsiveness to stress within the HPA axis by re-setting of temporal development of central CRF systems, thus influencing neuroendocrine function (Plotsky et al., 2005). This may be subsequently followed by epigenetic modifications involving histone acetylation or DNA methylation to control gene expression in various brain loci as they are challenged to respond to the external environment during development and in adulthood (Wang et al., 2014, Jawahar et al., 2015, Vaiserman, 2015). Whereas genes downstream of glucocorticoid signaling are hypomethylated to enhance stress system responsiveness, other genes regulating reward could be downregulated as was shown in the NAc of MS, which induced increased methyl transferases (DNMTs) in the NAc (Anier et al., 2014). However, epigenetic regulation in models of ELS are complex and require gene specific examination of widespread epigenetic regulations (Gudsnuk and Champagne, 2012, Anacker et al., 2014, Anier et al., 2014, Wang et al., 2014, Jawahar et al., 2015).
Finally, an important consideration in these studies is sex-based differences in the response to stress and to ethanol exposure. The literature supports many nuances in responding where one sex might be more sensitive than the other or have a more prolonged effect. However, for the concepts reported here, the change in dendritic alterations at anatomical regions like the PFC, VTA or NAc were reported for both sexes (Murmu et al., 2006, Jia et al., 2010, Monroy et al., 2010), and certainly excessive drinking and depression symptomatology as a result of MS are widely found in males and females, although nuances in aspects of the response may vary depending on the animal strain, experimental drinking paradigm and method of inducing maternal separation or deprivation (Caldji et al., 2000, Roman et al., 2004, Roman et al., 2005).
5.0 Conclusions
Cumulatively, these findings extend the current knowledge on neuroanatomical correlates of depression and reward seeking behavior induced by early life stress. The predisposition to transition from alcohol use to alcohol dependence during adulthood is strongly linked to early childhood experiences. This study implies that the propensity to initiate drinking and for depressive symptoms is pre-wired as a result of MS, and is likely associated with changes in neuronal survival and proliferation during critical periods of early development due to a re-setting of CRF systems in the brain. This early developmental insult with stressful experiences changes the trajectory of brain development leading to alterations in neuronal numbers in brain areas regulating the reward system and induces pathological behaviors in adulthood. Future studies should examine the circuits involving endocannabinoids, norepinephrine, GABA and serotonin, and how stress-mediated epigenetic factors could influence their signaling to mediate psychoaffective and addiction symptoms.
Highlights.
Early life stress is associated with a reduction of VTA neurons and enhanced alcohol seeking behavior.
Reduction of VTA neurons after MS parallels that of genetically high alcohol drinking rats.
Early life stress is associated with elevated amygdala neurons and depressive and anhedonia-like behaviors
Unlike granule neurons of the dentate gyrus, astrocyte number and may not be altered by ELS.
Acknowledgments
This research is supported by NIH/NIAAA grant R01AA021262 to MGL. The authors would like to thank the late Dr. Harry June for discussions and contributions, and Ms. Kaitlin Warnock for technical assistance. We offer a special thanks to Dr. Timothy A. Gondré-Lewis for critical reading of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6.0 References
- Abe K. Modulation of hippocampal long-term potentiation by the amygdala: a synaptic mechanism linking emotion and memory. Jpn J Pharmacol. 2001;86:18–22. doi: 10.1254/jjp.86.18. [DOI] [PubMed] [Google Scholar]
- Abe K, Niikura Y, Fujimoto T, Akaishi T, Misawa M. Involvement of dopamine D2 receptors in the induction of long-term potentiation in the basolateral amygdala-dentate gyrus pathway of anesthetized rats. Neuropharmacology. 2008;55:1419–1424. doi: 10.1016/j.neuropharm.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Aisa B, Elizalde N, Tordera R, Lasheras B, Del Rio J, Ramirez MJ. Effects of neonatal stress on markers of synaptic plasticity in the hippocampus: implications for spatial memory. Hippocampus. 2009;19:1222–1231. doi: 10.1002/hipo.20586. [DOI] [PubMed] [Google Scholar]
- Aisa B, Tordera R, Lasheras B, Del Rio J, Ramirez MJ. Effects of maternal separation on hypothalamic-pituitary-adrenal responses, cognition and vulnerability to stress in adult female rats. Neuroscience. 2008;154:1218–1226. doi: 10.1016/j.neuroscience.2008.05.011. [DOI] [PubMed] [Google Scholar]
- Anacker C, O’Donnell KJ, Meaney MJ. Early life adversity and the epigenetic programming of hypothalamic-pituitary-adrenal function. Dialogues Clin Neurosci. 2014;16:321–333. doi: 10.31887/DCNS.2014.16.3/canacker. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anier K, Malinovskaja K, Pruus K, Aonurm-Helm A, Zharkovsky A, Kalda A. Maternal separation is associated with DNA methylation and behavioural changes in adult rats. Eur Neuropsychopharmacol. 2014;24:459–468. doi: 10.1016/j.euroneuro.2013.07.012. [DOI] [PubMed] [Google Scholar]
- Aransay A, Rodriguez-Lopez C, Garcia-Amado M, Clasca F, Prensa L. Long-range projection neurons of the mouse ventral tegmental area: a single-cell axon tracing analysis. Front Neuroanat. 2015;9:59. doi: 10.3389/fnana.2015.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blacktop JM, Vranjkovic O, Mayer M, Van Hoof M, Baker DA, Mantsch JR. Antagonism of GABA-B but not GABA-A receptors in the VTA prevents stress- and intra-VTA CRF-induced reinstatement of extinguished cocaine seeking in rats. Neuropharmacology. 2016;102:197–206. doi: 10.1016/j.neuropharm.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Ungless MA, Bonci A. Convergent actions of orexin/hypocretin and CRF on dopamine neurons: Emerging players in addiction. Brain Res. 2010;1314:139–144. doi: 10.1016/j.brainres.2009.10.068. [DOI] [PubMed] [Google Scholar]
- Brake WG, Zhang TY, Diorio J, Meaney MJ, Gratton A. Influence of early postnatal rearing conditions on mesocorticolimbic dopamine and behavioural responses to psychostimulants and stressors in adult rats. Eur J Neurosci. 2004;19:1863–1874. doi: 10.1111/j.1460-9568.2004.03286.x. [DOI] [PubMed] [Google Scholar]
- Cadet JL, Bisagno V, Milroy CM. Neuropathology of substance use disorders. Acta Neuropathol. 2014;127:91–107. doi: 10.1007/s00401-013-1221-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldji C, Francis D, Sharma S, Plotsky PM, Meaney MJ. The effects of early rearing environment on the development of GABAA and central benzodiazepine receptor levels and novelty-induced fearfulness in the rat. Neuropsychopharmacology. 2000;22:219–229. doi: 10.1016/S0893-133X(99)00110-4. [DOI] [PubMed] [Google Scholar]
- Callaghan BL, Richardson R. Maternal separation results in early emergence of adult-like fear and extinction learning in infant rats. Behav Neurosci. 2011;125:20–28. doi: 10.1037/a0022008. [DOI] [PubMed] [Google Scholar]
- Castagne V, Moser P, Roux S, Porsolt RD. Rodent models of depression: forced swim and tail suspension behavioral despair tests in rats and mice. Curr Protoc Neurosci. 2011;Chapter 8(Unit 8):10A. doi: 10.1002/0471142301.ns0810as55. [DOI] [PubMed] [Google Scholar]
- Chocyk A, Dudys D, Przyborowska A, Majcher I, Mackowiak M, Wedzony K. Maternal separation affects the number, proliferation and apoptosis of glia cells in the substantia nigra and ventral tegmental area of juvenile rats. Neuroscience. 2011;173:1–18. doi: 10.1016/j.neuroscience.2010.11.037. [DOI] [PubMed] [Google Scholar]
- Chocyk A, Majcher-Maslanka I, Przyborowska A, Mackowiak M, Wedzony K. Early-life stress increases the survival of midbrain neurons during postnatal development and enhances reward-related and anxiolytic-like behaviors in a sex-dependent fashion. Int J Dev Neurosci. 2015;44:33–47. doi: 10.1016/j.ijdevneu.2015.05.002. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Economidou D, Cippitelli A, Cucculelli M, Ubaldi M, Soverchia L, Lourdusamy A, Massi M. Genetically selected Marchigian Sardinian alcohol-preferring (msP) rats: an animal model to study the neurobiology of alcoholism. Addict Biol. 2006;11:339–355. doi: 10.1111/j.1369-1600.2006.00032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciliax BJ, Heilman C, Demchyshyn LL, Pristupa ZB, Ince E, Hersch SM, Niznik HB, Levey AI. The dopamine transporter: immunochemical characterization and localization in brain. J Neurosci. 1995;15:1714–1723. doi: 10.1523/JNEUROSCI.15-03-01714.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Committee for the Update of the Guide for the Care and Use of Laboratory Animals, Institute for Laboratory Animal Research, Division on Earth and Life Studies, National Research Council. Guide for the Care and Use of Laboratory Animals: Eighth Edition (2011) National Academies; 2011. [Google Scholar]
- Danielewicz J, Hess G. Early life stress alters synaptic modification range in the rat lateral amygdala. Behav Brain Res. 2014;265:32–37. doi: 10.1016/j.bbr.2014.02.012. [DOI] [PubMed] [Google Scholar]
- Dayan J, Bernard A, Olliac B, Mailhes AS, Kermarrec S. Adolescent brain development, risk-taking and vulnerability to addiction. J Physiol Paris. 2010;104:279–286. doi: 10.1016/j.jphysparis.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Domesick VB, Stinus L, Paskevich PA. The cytology of dopaminergic and nondopaminergic neurons in the substantia nigra and ventral tegmental area of the rat: a light- and electron-microscopic study. Neuroscience. 1983;8:743–765. doi: 10.1016/0306-4522(83)90007-6. [DOI] [PubMed] [Google Scholar]
- Dowd EC, Barch DM. Pavlovian reward prediction and receipt in schizophrenia: relationship to anhedonia. PLoS One. 2012;7:e35622. doi: 10.1371/journal.pone.0035622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch MA. The role of early life stress as a predictor for alcohol and drug dependence. Psychopharmacology (Berl) 2011;214:17–31. doi: 10.1007/s00213-010-1916-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabricius K, Wortwein G, Pakkenberg B. The impact of maternal separation on adult mouse behaviour and on the total neuron number in the mouse hippocampus. Brain Struct Funct. 2008;212:403–416. doi: 10.1007/s00429-007-0169-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattore L, Puddu MC, Picciau S, Cappai A, Fratta W, Serra GP, Spiga S. Astroglial in vivo response to cocaine in mouse dentate gyrus: a quantitative and qualitative analysis by confocal microscopy. Neuroscience. 2002;110:1–6. doi: 10.1016/s0306-4522(01)00598-x. [DOI] [PubMed] [Google Scholar]
- Franklin KM, Engleman EA, Ingraham CM, McClaren JA, Keith CM, McBride WJ, Murphy JM. A single, moderate ethanol exposure alters extracellular dopamine levels and dopamine d receptor function in the nucleus accumbens of wistar rats. Alcohol Clin Exp Res. 2009;33:1721–1730. doi: 10.1111/j.1530-0277.2009.01009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Gutierrez MS, Navarrete F, Aracil A, Bartoll A, Martinez-Gras I, Lanciego JL, Rubio G, Manzanares J. Increased vulnerability to ethanol consumption in adolescent maternal separated mice. Addict Biol. 2015 doi: 10.1111/adb.12266. [DOI] [PubMed] [Google Scholar]
- Gatto GJ, McBride WJ, Murphy JM, Lumeng L, Li TK. Ethanol self-infusion into the ventral tegmental area by alcohol-preferring rats. Alcohol. 1994;11:557–564. doi: 10.1016/0741-8329(94)90083-3. [DOI] [PubMed] [Google Scholar]
- Gessa GL, Muntoni F, Collu M, Vargiu L, Mereu G. Low doses of ethanol activate dopaminergic neurons in the ventral tegmental area. Brain Res. 1985;348:201–203. doi: 10.1016/0006-8993(85)90381-6. [DOI] [PubMed] [Google Scholar]
- Gudsnuk K, Champagne FA. Epigenetic influence of stress and the social environment. ILAR J. 2012;53:279–288. doi: 10.1093/ilar.53.3-4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey SC, Foster KL, McKay PF, Carroll MR, Seyoum R, Woods JE, 2nd, Grey C, Jones CM, McCane S, Cummings R, Mason D, Ma C, Cook JM, June HL. The GABA(A) receptor alpha1 subtype in the ventral pallidum regulates alcohol-seeking behaviors. J Neurosci. 2002;22:3765–3775. doi: 10.1523/JNEUROSCI.22-09-03765.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Nixon K, Shetty AK, Crews FT. Chronic alcohol exposure reduces hippocampal neurogenesis and dendritic growth of newborn neurons. Eur J Neurosci. 2005;21:2711–2720. doi: 10.1111/j.1460-9568.2005.04120.x. [DOI] [PubMed] [Google Scholar]
- Hulshof HJ, Novati A, Sgoifo A, Luiten PG, den Boer JA, Meerlo P. Maternal separation decreases adult hippocampal cell proliferation and impairs cognitive performance but has little effect on stress sensitivity and anxiety in adult Wistar rats. Behav Brain Res. 2011;216:552–560. doi: 10.1016/j.bbr.2010.08.038. [DOI] [PubMed] [Google Scholar]
- Huot RL, Plotsky PM, Lenox RH, McNamara RK. Neonatal maternal separation reduces hippocampal mossy fiber density in adult Long Evans rats. Brain Res. 2002;950:52–63. doi: 10.1016/s0006-8993(02)02985-2. [DOI] [PubMed] [Google Scholar]
- Huot RL, Thrivikraman KV, Meaney MJ, Plotsky PM. Development of adult ethanol preference and anxiety as a consequence of neonatal maternal separation in Long Evans rats and reversal with antidepressant treatment. Psychopharmacology (Berl) 2001;158:366–373. doi: 10.1007/s002130100701. [DOI] [PubMed] [Google Scholar]
- Jawahar MC, Murgatroyd C, Harrison EL, Baune BT. Epigenetic alterations following early postnatal stress: a review on novel aetiological mechanisms of common psychiatric disorders. Clin Epigenetics. 2015;7:122. doi: 10.1186/s13148-015-0156-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia N, Yang K, Sun Q, Cai Q, Li H, Cheng D, Fan X, Zhu Z. Prenatal stress causes dendritic atrophy of pyramidal neurons in hippocampal CA3 region by glutamate in offspring rats. Dev Neurobiol. 2010;70:114–125. doi: 10.1002/dneu.20766. [DOI] [PubMed] [Google Scholar]
- June HL, Eiler WJA. Handbook of Contemporary Neuropharmacology. John Wiley & Sons, Inc; 2007. Dopaminergic and GABAergic Regulation of Alcohol-Motivated Behaviors: Novel Neuroanatomical Substrates. [Google Scholar]
- Kimelberg HK, Nedergaard M. Functions of astrocytes and their potential as therapeutic targets. Neurotherapeutics. 2010;7:338–353. doi: 10.1016/j.nurt.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Brain stress systems in the amygdala and addiction. Brain Res. 2009;1293:61–75. doi: 10.1016/j.brainres.2009.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J. The amygdala. Curr Biol. 2007;17:R868–874. doi: 10.1016/j.cub.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Kim JW, Yim SV, Kim MJ, Kim SA, Kim YJ, Kim CJ, Chung JH. Fluoxetine enhances cell proliferation and prevents apoptosis in dentate gyrus of maternally separated rats. Mol Psychiatry. 2001;6:610, 725–618. doi: 10.1038/sj.mp.4000954. [DOI] [PubMed] [Google Scholar]
- Leventopoulos M, Ruedi-Bettschen D, Knuesel I, Feldon J, Pryce CR, Opacka-Juffry J. Long-term effects of early life deprivation on brain glia in Fischer rats. Brain Res. 2007;1142:119–126. doi: 10.1016/j.brainres.2007.01.039. [DOI] [PubMed] [Google Scholar]
- Liu D, Caldji C, Sharma S, Plotsky PM, Meaney MJ. Influence of neonatal rearing conditions on stress-induced adrenocorticotropin responses and norepinepherine release in the hypothalamic paraventricular nucleus. J Neuroendocrinol. 2000;12:5–12. doi: 10.1046/j.1365-2826.2000.00422.x. [DOI] [PubMed] [Google Scholar]
- Liu J, Yang AR, Kelly T, Puche A, Esoga C, June HL, Jr, Elnabawi A, Merchenthaler I, Sieghart W, June HL, Sr, Aurelian L. Binge alcohol drinking is associated with GABAA alpha2-regulated Toll-like receptor 4 (TLR4) expression in the central amygdala. Proc Natl Acad Sci U S A. 2011;108:4465–4470. doi: 10.1073/pnas.1019020108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorente R, Gallardo ML, Berzal AL, Prada C, Garcia-Segura LM, Viveros MP. Early maternal deprivation in rats induces gender-dependent effects on developing hippocampal and cerebellar cells. Int J Dev Neurosci. 2009;27:233–241. doi: 10.1016/j.ijdevneu.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Manaye KF, Mouton PR, Xu G, Drew A, Lei DL, Sharma Y, Rebeck GW, Turner S. Age-related loss of noradrenergic neurons in the brains of triple transgenic mice. Age (Dordr) 2013;35:139–147. doi: 10.1007/s11357-011-9343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride WJ, Murphy JM, Gatto GJ, Levy AD, Yoshimoto K, Lumeng L, Li TK. CNS mechanisms of alcohol self-administration. Alcohol Alcohol Suppl. 1993;2:463–467. [PubMed] [Google Scholar]
- Michaeli A, Matzner H, Poltyrev T, Yaka R. Modifications of the input currents on VTA dopamine neurons following acute versus chronic cocaine exposure. Neuropharmacology. 2012;62:1834–1840. doi: 10.1016/j.neuropharm.2011.12.005. [DOI] [PubMed] [Google Scholar]
- Monroy E, Hernandez-Torres E, Flores G. Maternal separation disrupts dendritic morphology of neurons in prefrontal cortex, hippocampus, and nucleus accumbens in male rat offspring. J Chem Neuroanat. 2010;40:93–101. doi: 10.1016/j.jchemneu.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Morzorati SL. VTA dopamine neuron activity distinguishes alcohol-preferring (P) rats from Wistar rats. Alcohol Clin Exp Res. 1998;22:854–857. [PubMed] [Google Scholar]
- Mouton PR, Gokhale AM, Ward NL, West MJ. Stereological length estimation using spherical probes. J Microsc. 2002;206:54–64. doi: 10.1046/j.1365-2818.2002.01006.x. [DOI] [PubMed] [Google Scholar]
- Murmu MS, Salomon S, Biala Y, Weinstock M, Braun K, Bock J. Changes of spine density and dendritic complexity in the prefrontal cortex in offspring of mothers exposed to stress during pregnancy. Eur J Neurosci. 2006;24:1477–1487. doi: 10.1111/j.1460-9568.2006.05024.x. [DOI] [PubMed] [Google Scholar]
- Oomen CA, Soeters H, Audureau N, Vermunt L, van Hasselt FN, Manders EM, Joels M, Krugers H, Lucassen PJ. Early maternal deprivation affects dentate gyrus structure and emotional learning in adult female rats. Psychopharmacology (Berl) 2011;214:249–260. doi: 10.1007/s00213-010-1922-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakkenberg B, Gundersen HJ. New stereological method for obtaining unbiased and efficient estimates of total nerve cell number in human brain areas. Exemplified by the mediodorsal thalamic nucleus in schizophrenics. APMIS. 1989;97:677–681. doi: 10.1111/j.1699-0463.1989.tb00462.x. [DOI] [PubMed] [Google Scholar]
- Penasco S, Mela V, Lopez-Moreno JA, Viveros MP, Marco EM. Early maternal deprivation enhances voluntary alcohol intake induced by exposure to stressful events later in life. Neural Plast. 2015;2015:342761. doi: 10.1155/2015/342761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotsky PM, Thrivikraman KV, Nemeroff CB, Caldji C, Sharma S, Meaney MJ. Long-term consequences of neonatal rearing on central corticotropin-releasing factor systems in adult male rat offspring. Neuropsychopharmacology. 2005;30:2192–2204. doi: 10.1038/sj.npp.1300769. [DOI] [PubMed] [Google Scholar]
- Pryce CR, Ruedi-Bettschen D, Dettling AC, Weston A, Russig H, Ferger B, Feldon J. Long-term effects of early-life environmental manipulations in rodents and primates: Potential animal models in depression research. Neurosci Biobehav Rev. 2005;29:649–674. doi: 10.1016/j.neubiorev.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Qin S, Young CB, Duan X, Chen T, Supekar K, Menon V. Amygdala subregional structure and intrinsic functional connectivity predicts individual differences in anxiety during early childhood. Biol Psychiatry. 2014;75:892–900. doi: 10.1016/j.biopsych.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raftogianni A, Stamatakis A, Diamantopoulou A, Kollia AM, Stylianopoulou F. Effects of an early experience of reward through maternal contact or its denial on the dopaminergic system of the rat brain. Neuroscience. 2014;269:11–20. doi: 10.1016/j.neuroscience.2014.03.035. [DOI] [PubMed] [Google Scholar]
- Raineki C, Cortes MR, Belnoue L, Sullivan RM. Effects of early-life abuse differ across development: infant social behavior deficits are followed by adolescent depressive-like behaviors mediated by the amygdala. J Neurosci. 2012;32:7758–7765. doi: 10.1523/JNEUROSCI.5843-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, McQueen VK, Davids MR, Hsu CC, Murphy JM, Li TK, Lumeng L, McBride WJ. Chronic ethanol drinking by alcohol-preferring rats increases the sensitivity of the posterior ventral tegmental area to the reinforcing effects of ethanol. Alcohol Clin Exp Res. 2005a;29:358–366. doi: 10.1097/01.alc.0000156127.30983.9d. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, McQueen VK, Davids MR, Hsu CC, Murphy JM, Li TK, Lumeng L, McBride WJ. Prolonged increase in the sensitivity of the posterior ventral tegmental area to the reinforcing effects of ethanol following repeated exposure to cycles of ethanol access and deprivation. J Pharmacol Exp Ther. 2005b;315:648–657. doi: 10.1124/jpet.105.084350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman E, Gustafsson L, Hyytia P, Nylander I. Short and prolonged periods of maternal separation and voluntary ethanol intake in male and female ethanol-preferring AA and ethanol-avoiding ANA rats. Alcohol Clin Exp Res. 2005;29:591–601. doi: 10.1097/01.alc.0000158933.70242.fc. [DOI] [PubMed] [Google Scholar]
- Roman E, Ploj K, Nylander I. Maternal separation has no effect on voluntary ethanol intake in female Wistar rats. Alcohol. 2004;33:31–39. doi: 10.1016/j.alcohol.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Romano-Lopez QF, Mendez-Diaz MD, Garcia FGD, Regalado-Santiago CD, Ruiz Contreras AED, Prospero-Garcia OD. Maternal separation and early stress cause long-lasting effects on dopaminergic and endocannabinergic systems and alters dendritic morphology in the nucleus accumbens and frontal cortex in rats. Dev Neurobiol. 2015 doi: 10.1002/dneu.22361. [DOI] [PubMed] [Google Scholar]
- Ruedi-Bettschen D, Zhang W, Russig H, Ferger B, Weston A, Pedersen EM, Feldon J, Pryce CR. Early deprivation leads to altered behavioural, autonomic and endocrine responses to environmental challenge in adult Fischer rats. Eur J Neurosci. 2006;24:2879–2893. doi: 10.1111/j.1460-9568.2006.05158.x. [DOI] [PubMed] [Google Scholar]
- Smith AD, Weiss F. Ethanol exposure differentially alters central monoamine neurotransmission in alcohol-preferring versus -nonpreferring rats. J Pharmacol Exp Ther. 1999;288:1223–1228. [PubMed] [Google Scholar]
- Strekalova T, Steinbusch HW. Measuring behavior in mice with chronic stress depression paradigm. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:348–361. doi: 10.1016/j.pnpbp.2009.12.014. [DOI] [PubMed] [Google Scholar]
- Stuhrmann A, Dohm K, Kugel H, Zwanzger P, Redlich R, Grotegerd D, Rauch AV, Arolt V, Heindel W, Suslow T, Zwitserlood P, Dannlowski U. Mood-congruent amygdala responses to subliminally presented facial expressions in major depression: associations with anhedonia. J Psychiatry Neurosci. 2013;38:249–258. doi: 10.1503/jpn.120060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda H, Boku S, Nakagawa S, Inoue T, Kato A, Takamura N, Song N, Nibuya M, Koyama T, Kusumi I. Maternal separation enhances conditioned fear and decreases the mRNA levels of the neurotensin receptor 1 gene with hypermethylation of this gene in the rat amygdala. PLoS One. 2014;9:e97421. doi: 10.1371/journal.pone.0097421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaiserman AM. Epigenetic programming by early-life stress: Evidence from human populations. Dev Dyn. 2015;244:254–265. doi: 10.1002/dvdy.24211. [DOI] [PubMed] [Google Scholar]
- Vazquez V, Giros B, Dauge V. Maternal deprivation specifically enhances vulnerability to opiate dependence. Behav Pharmacol. 2006;17:715–724. doi: 10.1097/FBP.0b013e3280116e6f. [DOI] [PubMed] [Google Scholar]
- Wang A, Nie W, Li H, Hou Y, Yu Z, Fan Q, Sun R. Epigenetic upregulation of corticotrophin-releasing hormone mediates postnatal maternal separation-induced memory deficiency. PLoS One. 2014;9:e94394. doi: 10.1371/journal.pone.0094394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Gondre-Lewis MC. Prenatal nicotine and maternal deprivation stress de-regulate the development of CA1, CA3, and dentate gyrus neurons in hippocampus of infant rats. PLoS One. 2013;8:e65517. doi: 10.1371/journal.pone.0065517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Dual roles of dopamine in food and drug seeking: the drive-reward paradox. Biol Psychiatry. 2013;73:819–826. doi: 10.1016/j.biopsych.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Morales M. A ventral tegmental CRF-glutamate-dopamine interaction in addiction. Brain Res. 2010;1314:38–43. doi: 10.1016/j.brainres.2009.09.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JK, Heinbockel T, Gondre-Lewis MC. Astrocyte fatty acid binding protein-7 is a marker for neurogenic niches in the rat hippocampus. Hippocampus. 2013;23:1476–1483. doi: 10.1002/hipo.22200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FC, Anthony B, Dunn KW, Lindquist WB, Xu ZC, Deng P. Chronic alcohol drinking alters neuronal dendritic spines in the brain reward center nucleus accumbens. Brain Res. 2007;1134:148–161. doi: 10.1016/j.brainres.2006.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]