Summary
Periodontal disease exemplifies a chronic and recurrent infection with a necessary biofilm component. Mucosal inflammation is a hallmark response of the host seen in chronic diseases, such as colitis, gingivitis and periodontitis (and the related disorder peri-implantitis). We have taken advantage of our recently developed rat model of human peri-implantitis that recapitulates osteolysis, the requirement of biofilm formation and the perpetuation of the bona fide disease state, to test a new therapeutic modality with two novel components. First we utilized hyperimmune antiserum directed against the DNABII family of proteins, now known to be a critical component of the extracellular matrix of bacterial biofilms. Second we delivered the antiserum as cargo in biodegradable microspheres to the site of the biofilm infection. We demonstrated that delivery of a single dose of anti-DNABII in Poly(Lactic-co-glycolic acid) (PLGA) microspheres induced significant resolution of experimental peri-implantitis, including marked reduction of inflammation. These data support the continued development of a DNABII protein-targeted therapeutic for peri-implantitis and other chronic inflammatory pathologies of the oral cavity in animals and humans.
Keywords: Inflammation, Biofilm, IHF, DNABII protein, A. actinomycetemcomitans
Introduction
Biofilm mediated diseases such as gingivitis and periodontitis are considered the most common chronic inflammatory conditions of humans (Offenbacher et al. 2007). Half of the adult population presents with periodontitis that is a major cause of tooth loss in adults. Peri-implantitis is a similar inflammatory osteolytic infection that affects dental implants (Heitz-Mayfield and Lang 2010). Periodontitis and peri-implantitis have associated microbial biofilm etiology, including Aggregatibacter actinomycetemcomitans (Aa), Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia (Socransky et al. 1998). The initial host response against these pathogenic bacteria causes transient local inflammation, commonly called gingivitis. It is increasingly evident that unresolved acute inflammation leads to chronic inflammation and neutrophil mediated destruction of the periodontal ligaments and bone surrounding the affected tooth (Baloul et al. 2011; Van Dyke and Kornman 2008). While periodontal disease is locally manifested on tooth supporting structures, there is increasing evidence that chronic inflammation modulates a patient’s risk for developing systemic diseases including cardiovascular diseases, atherosclerosis, diabetes mellitus, osteoporosis and possibly rheumatoid arthritis that result from the entry of oral microbes, microbial antigens, cytokines and other proinflammatory mediators into circulation (Cairo et al. 2004; Chun et al. 2005; Couper et al. 2008; Janket et al. 2008; Kaur et al. 2013; Kornman 2008; Williams et al. 2008).
More than 700 prokaryote species have been identified and sequenced as part of the human oral microbiome (Dewhirst et al. 2010). The host is able to maintain a homeostatic state between commensals and pathogenic flora in healthy individuals, however when this interactive relationship that maintains homeostasis is lost, disease initiates. A known factor in the initiation and progression of periodontal inflammatory diseases is an increase in the proportion of Gram-negative bacteria present in the oral microbiome (Teles et al. 2010). One of the most important etiological agents in chronic and aggressive periodontitis in humans is Aa (Muller et al. 1998; Nalbant and Zadeh 2002; Papapanou 2002; Slots 1984; Umeda et al. 1998). Multiple virulence factors including leukotoxins, chemotaxis inhibitors, immunosuppressive proteins, collagenase, lymphocyte suppressive factor, and bone resorption agents facilitate survival of Aa in the oral cavity and enable it to circumvent the host’s immune response (Fives-Taylor et al. 1999; Henderson et al. 2003).
Periodontal debridement (PD) in combination with several adjunctive therapies including local use of antimicrobial agents, providone iodine during debridement (Greenstein 1999; Sahrmann et al. 2010), chlorhexidine rinses (Southard et al. 1989), subgingival gels, and systemic antibiotics have been used in the treatment of periodontitis with only moderate improvement in clinical outcomes. (Drisko 2014; Karlsson et al. 2008; Schwarz et al. 2008; Sgolastra et al. 2012a; 2012b). The limited efficacy of the current treatment options and the need for repeated treatments demand the need for development of novel methods to effectively treat periodontitis due to biofilms and prevent the associated tooth loss.
One of the targets for the treatment of biofilm-mediated infectious diseases is the extracellular matrix that contains an abundant amount of extracellular DNA (eDNA) (Flemming and Wingender 2010). Extracellular DNA plays an integral role as a structural component of the biofilm and has been believed to be an impenetrable barrier to both the immune system and antimicrobials (Flemming and Wingender 2010; Nickel et al. 1985; Slinger et al. 2006). Therapeutic approaches for bacterial infections have failed in “biofilm-related diseases”, partially due to the protection rendered by the biofilm matrix. Interestingly, a family of proteins referred to as DNABII proteins have been ubiquitously found in the biofilm matrix of multiple human bacterial pathogens (Boleij et al. 2009; Kim et al. 2002; Lunsford et al. 1996; Winters et al. 1993) and identified as critical to maintaining the structural integrity of eDNA in the biofilm matrix (Brockson et al. 2014; Goodman et al. 2011; Gustave et al. 2013; Justice et al. 2012; Novotny et al. 2013). This family includes the ubiquitous eubacterial protein HU and integration host factor (IHF) found in α- and γ-proteobacteria (Rouviere-Yaniv and Gros 1975; Swinger and Rice 2004), with Aa possessing 3 DNABII alleles (D7S_00047, ihfA; D7S_00171, ihfB; D7S_00989, HU). We have shown hyperimmune polyclonal antiserum against one of the family members IHF (isolated from E. coli, anti-IHFEc), to effectively disrupt pre-formed biofilms of several pathogenic bacteria in vitro (Brockson et al. 2014; Goodman et al. 2011; Novotny et al. 2013) and even disperse polymicrobial sputum aggregates recovered from cystic fibrosis patients ex-vivo (Gustave et al. 2013). Furthermore, we have found that immunization of chinchillas with E. coli IHF in a model of experimental otitis media results in significantly more rapid resolution of disease and clearance of non-typeable Haemophilus influenzae (NTHI) biofilms from the middle ear (Goodman et al. 2011). In a mouse model of urinary tract infection, we have shown, using mutants of uropathogenic E. coli (UPEC) strain UTI89 that lacks either subunit of IHF, is defective in colonization of the mouse bladder and kidney (Justice et al. 2012). More recently we have also demonstrated that DNABII proteins play a crucial role in UPEC biofilm development (Devaraj et al. 2015) and also identified eDNA and DNABII proteins in polymicrobial otorrhea solids (Idicula et al. 2016). These studies underscore the importance of DNABII proteins in maintaining the structural stability of eDNA in the extracellular matrix and the therapeutic potential of strategies that target these proteins to mediate biofilm disruption in multiple biofilm-mediated diseases.
In this study we employed a previously developed animal model (Freire et al. 2011) of a disease state that is closely related to periodontitis, namely peri-implantitis. This model takes advantage of an Aa biofilm preformed on the surface of titanium implants, to investigate the therapeutic potential of hyperimmune antiserum directed against E. coli IHF (anti-IHFEc). We encapsulated anti-IHFEc in Poly(lactic-co-glycolic acid) (PLGA) microspheres as a means to limit diffusion of the antibody away from the site of infection as well as to mediate sustained release at that site. PLGA is biodegradable and has gained attention in drug delivery systems due to several attractive properties such as minimal systemic toxicity, protection of cargo from degradation, capacity for sustained release of drug, compatibility with a wide array of small molecules and macromolecules, and the ability to target contents to a specific tissue/organ (Danhier et al. 2012). Most importantly PLGA is FDA approved for drug delivery in humans and has been used in a myriad of in vivo pre-clinical studies including cerebral and cardiovascular diseases, osteoporosis, diabetes, cancer, inflammation, and regenerative medicine (Danhier et al. 2012). In fact minocycline loaded PLGA microspheres registered under the name Arestin™ (Meinberg et al. 2002; Renvert et al. 2008) is currently used as an adjunct to scaling and root planing in limiting but not reversing the bacterial nidus that causes periodontitis.
In this study, we demonstrated that treatment of a pre-formed Aa biofilm with anti-IHFEc encapsulated in PLGA microspheres resulted in significant disruption of the biofilms in vitro. Furthermore, using our recently developed rat model of Aa biofilm-induced oral osteolytic lesion (peri-implantitis), we have demonstrated that a single dose of anti-IHFEc in PLGA microspheres applied topically to implants which carried pre-formed Aa biofilms facilitated rapid resolution of the pathogenic state including a significant reduction in inflammation and increased stability of the implants within bone in the oral cavity. Our studies emphasize the therapeutic potential of anti-DNABII approach in Aa-induced osteolytic infections, including peri-implantitis and periodontitis.
Materials and Methods
Aggregatibacter actinomycetemcomitans (Aa) culture conditions for in vivo experiments
Aa strain D7S-1 was originally recovered from a patient with aggressive periodontitis (Chen et al. 2010; Wang et al. 2002). Strain D7S-1 serotype A was grown for 48 hours in modified tryptic soy agar (3% tryptic soy broth with 0.6% yeast extract; 1.5% agar), 5 to 10 colonies were transferred to 5 ml of liquid mTSB then vortexed to disperse the bacteria. The suspension was transferred to 2 ml of fresh mTSB at 1:20 dilution and incubated at 37°C in an atmosphere of 5% CO2.
Antibodies
Hyperimmune rabbit antiserum directed against purified Escherichia coli IHF (anti-IHFEc) was described previously (Goodman et al. 2011; Gustave et al. 2013).
Encapsulation of the antibody in Poly(lactic-co-glycolic acid), PLGA
PLGA microspheres were created using a modified double emulsion technique (Beer et al. 1998). First 1% Polyvinyl alcohol (PVA, 31,500–50,000 molecular weight, Sigma-Aldrich, St. Louis, MO) was dissolved in 10 ml phosphate-buffered saline (PBS) for one hour with stirring at 65°C. Next, a 20% PLGA solution (40,000–70,000 molecular weight, Sigma-Aldrich, St. Louis, MO) was made in 1 ml dichloromethane by vortexing for 10 minutes until the PLGA was dissolved. To form the microspheres, the PLGA solution was vortexed while 100 µl of either undiluted naïve rabbit serum or anti-IHFEc was added and then vortexed for an additional 30 seconds. The PLGA- anti-IHFEc or PLGA-naïve serum suspension was then slowly pipetted into 2 ml of the PVA solution while vortexing for an additional 30 seconds. The PLGA/PVA was then quickly added to 100 ml of 5% isopropyl alcohol with continuous stirring, then stirred for an additional three hours. The mixture was transferred to 50 ml centrifuge tubes and spun at 515 x g for 10 minutes at 4°C. The supernatant was removed and the microspheres were washed four times with 25 ml PBS. After the final wash, 1 ml of PBS was added to the microspheres and 100 µl aliquot stocks were prepared. The microspheres were stored at 4°C for immediate use or −20°C for long-term storage.
Quantification of IgG in PLGA microspheres
The Easy-Titer IgG assay kit (Thermo Fisher Scientific, Waltham, MA) was used to determine the amount of total IgG encapsulated within the microspheres. PLGA microspheres containing anti-IHFEc were melted at 60°C for 30 minutes to release and quantify encapsulated IgG as per manufacturer’s instructions. This assay was repeated twice, with an average value of 734 ng anti-IHFEc IgG /ml.
In vitro biofilm assay
In vitro biofilm assay was as previously described (Goodman et al. 2011). Aa was cultured in mTSB for 48 hours at 37°C, in a humidified atmosphere containing 5% CO2. The culture was vortexed for one minute to resuspend the aggregates, then diluted 1:10 in mTSB and 200 µl of this bacterial suspension was inoculated into each well of an eight-well chamber slide (Thermo Fisher Scientific, Waltham, MA). After 16 hours of incubation at 37°C, 5% CO2, the medium in each well was replaced with fresh medium. After an additional 8 hour incubation period, the medium was removed and replaced with one of the following: pre-warmed medium, medium that contained a 1:50 dilution of serum (naïve or anti-IHFEc) or a 1:10 dilution of microspheres (average value of 734 ng anti-IHFEc IgG /ml) that contained either naïve serum or anti-IHFEc. Chamber slides were then incubated for an additional 16 hours as described above. After incubation with serum or microspheres that contained serum, the medium was carefully removed; the biofilm was washed twice with 0.9% saline and stained with LIVE/DEAD® stain (Molecular Probes, Eugene, OR) for 15 minutes at room temperature as per manufacturer’s instructions. To determine the distribution of extracellular IHF, fixed Aa biofilms were incubated with anti-IHFEc and then treated with goat anti-rabbit IgG conjugated to AlexaFluor® 647. Aa biofilms were visualized with LIVE/DEAD® stain. The biofilms were then imaged using a 63X objective on a Zeiss 510 Meta-laser scanning confocal microscope (Carl Zeiss, Thornwood, NY).
Biofilm Imaging & Quantification
After biofilms were washed twice with 0.9% saline, samples were fixed with a solution of 1.6% paraformaldehyde, 0.025% glutaraldehyde, and 4.0% acetic acid in phosphate buffer at pH 7.4. The biofilms were imaged using a 63X objective on a Zeiss 510 Meta-laser scanning confocal microscope, CSLM, (Carl Zeiss, Oberkochen, Germany). All in vitro biofilm assays were repeated a minimum of three times on separate days and all individual biofilm assays were done in duplicate on each assay day. The efficacy of treatment was assessed based on differences in biofilm height, thickness and biomass as determined by COMSTAT analysis (Heydorn et al. 2000). Values of percent reduction in biofilm architectural parameters upon exposure to immune serum were compared to those obtained upon exposure to naïve serum.
Animals
Sprague-Dawley, virgin, 6 week old female rats (n=83) (Charles River Laboratories, Hollister, CA) were housed in a laboratory at 20 – 24°C under a 12-hours light and 12-hours dark cycle and fed ad libitum (Purina Laboratory Rodent Chow, Purina Milk, Richmond, IN). Animal care was in accordance to the NIH Guide for the Care and Use of Laboratory Animals prepared by the Institute of Laboratory Animal Resources, National Research Council, all applicable government regulations, and university policies governing the care and use of laboratory animals. All protocols and procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Southern California and are in accordance with the Panel on Euthanasia of the American Veterinary Medical Association.
In vivo inflammation model
Titanium implant screw (1.2 × 4.5 mm) surfaces were modified by grit blasting with Al2O3 (100 µm) and HCl etching (pH 3, 20 minutes, 80°C) to produce a rough surface before being inoculated with Aa as previously described (Freire et al. 2011). Briefly, the heads of implants (supragingival portion) were partially submerged in either sterile medium (mTSB) or mTSB inoculated with Aa D7S-1 followed by incubation for two days at 37°C with 5% CO2 (Figure 1). Rats were anesthetized with isoflurane and either two Aa biofilm-inoculated implants or two control implants were transmucosally placed into the hard palate adjacent to the maxillary alveolar ridge on either side of the oral cavity (n=32). Seven days post-surgery, PLGA microspheres (20 µl) containing naïve or anti-IHFEc antisera (734 ng anti-IHFEc IgG /ml) were applied topically at the site of implant insertion. After three days of treatment, peri-implant mucosal inflammation and bleeding, were evaluated blindly. Tissue inflammation was classified clinically on a scale of 0 to 3 as follows: 0 - no bleeding; 1 - slight bleeding; 2 -moderate bleeding; 3 - severe bleeding (Renvert et al. 2008). After evaluation, rats were euthanized by CO2 asphyxiation and the skulls were harvested and stored in 10% buffered formalin for histological analysis.
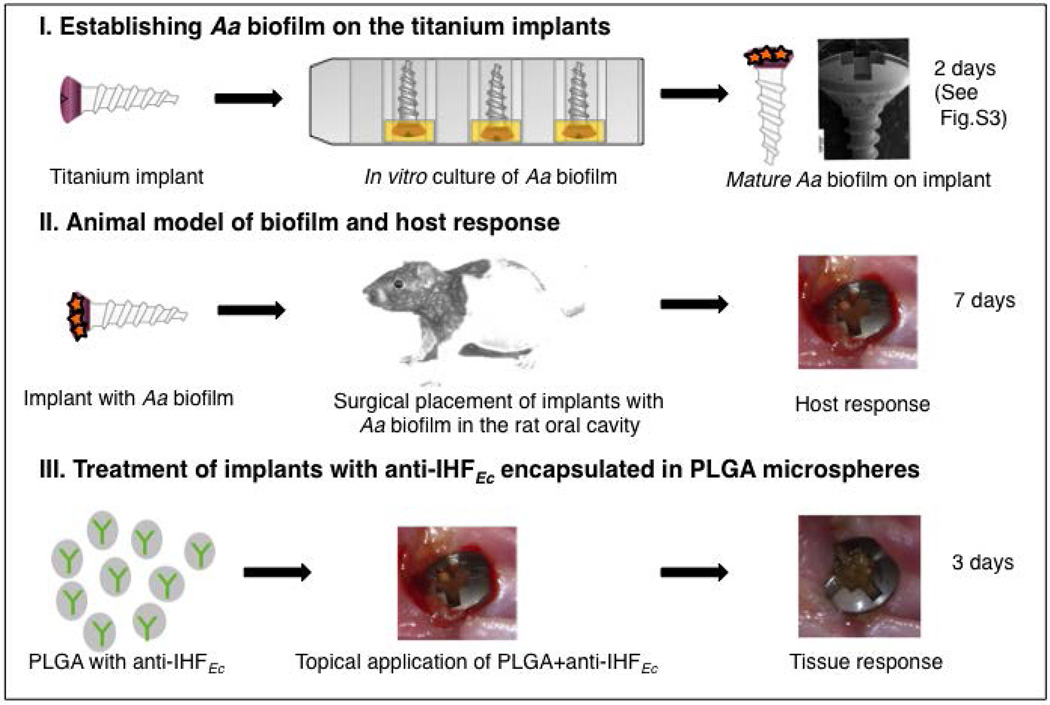
Figure 1. Schematic representation of the animal model used to investigate the host response to implants that carried preformed Aa biofilms.
(I) The heads of the titanium implants were partially submerged in sterile medium (mTSB) or mTSB inoculated with Aa for two days at 37°C with 5% CO2 (to produce a uniform biofilm on the surface of the implant head; see Figure S3). (II) Rats were anesthetized and either Aa biofilm-inoculated implants or control implants were transmucosally placed into the palate adjacent to the maxillary alveolar ridge. (III) After seven days post-surgery animals were treated topically with PLGA microspheres containing naïve serum or anti-IHFEc. After three days of treatment, clinical evaluation of peri-implant mucosal inflammation, bleeding, and implant mobility was recorded.
Implant stability
To investigate the stability of the implants surgically placed into the hard palate of the rats, clinical assessment of relative mobility of the implant was determined with a periodontal probe as well as via reverse torque analysis of the implants. Seven days after surgical placement of either sterile implants or those with pre-formed Aa biofilms into the bone of the hard palate of a separate cohort of animals (n=19), the implants were treated with either naïve serum (n=5) or anti-IHFEc (n=8) for three days. At the end of three days, the animals were anaesthetized and the implants were analyzed with a periodontal probe by naked eye by a single observer (MOF) for relative ability to move them in both a coronal or palatal plane. The implants were considered unstable if they were positive for any movement (Newman et al. 2002). For reverse torque analysis, animals that were surgically implanted with either control implants or those with pre-formed Aa biofilms-and treated with anti-IHFEc (n=3) or naïve serum (n=3) were euthanized by CO2 asphyxiation after three days and samples were fixed in 10% formalin. After maxillary tissue dissection and stabilization, a counterclockwise (reverse) force was applied to the implant with the aid of a computerized torque driver to remove the implant. The torque required to remove the implant was recorded (Jividen and Misch 2000).
Histology
Bone-implant specimens (n=32) were fixed with 10% neutral buffered formalin (Richard-Allan Scientific) for 24 hours at 4°C. Each tissue was trimmed in the coronal plane and the resulting samples were processed to an individual plastic block (Technovit 7200VLC). One ground and polished section was created per block from the longitudinal through the midline of the implant. Samples were dehydrated in graded ethanol (70%, 95%, and 100%) and embedded in paraffin. Five-micrometer sections were cut and stained with hematoxylin and eosin (H&E) (Zijnge et al. 2010). Stained histological slides were imaged using a Zeiss Axio Cam with a 10X objective.
Statistics
All data were analyzed and graphed using GraphPad Prism. Means and SEM were evaluated by t test (Figures 2 and 5) and Mann-Whitney test (Figures 3 and 4). A p-value ≤ 0.05 was considered significant. A p-value of 0.01–0.05 is represented by *, p-value less than 0.01 is represented by **, p-value less than 0.001 is *** and p-value less than 0.0001 is represented by ****.
Figure 2. anti-IHFEc antibodies diminished pre-formed Aa biofilms in vitro.
Aa biofilms were pre-formed in vitro for 24 hours in mTSB and treated with one of the following: (A) medium only, (B) naïve serum, or (C) anti-IHFEc. In addition to direct treatment, similar treatments were performed with (D) PLGA microspheres that contained either (E) naïve serum (F) or anti-IHFEc. Representative images of Aa biofilms are shown. COMSTAT analysis was employed to calculate maximum height (G), average thickness (H) and biomass (I). (J) CFU/ml in biofilm and planktonic state was enumerated. All in vitro biofilm assays were repeated a minimum of three times on separate days, and all individual biofilm assays were done in duplicates on each assay day. Data are presented as mean values ± standard deviation. A p-value ≤ 0.05 was considered significant. p-value of 0.01–0.05 is represented by *, p-value less than 0.01 is represented by **, p-value less than 0.001 is *** and p-value less than 0.0001 is represented by ****.
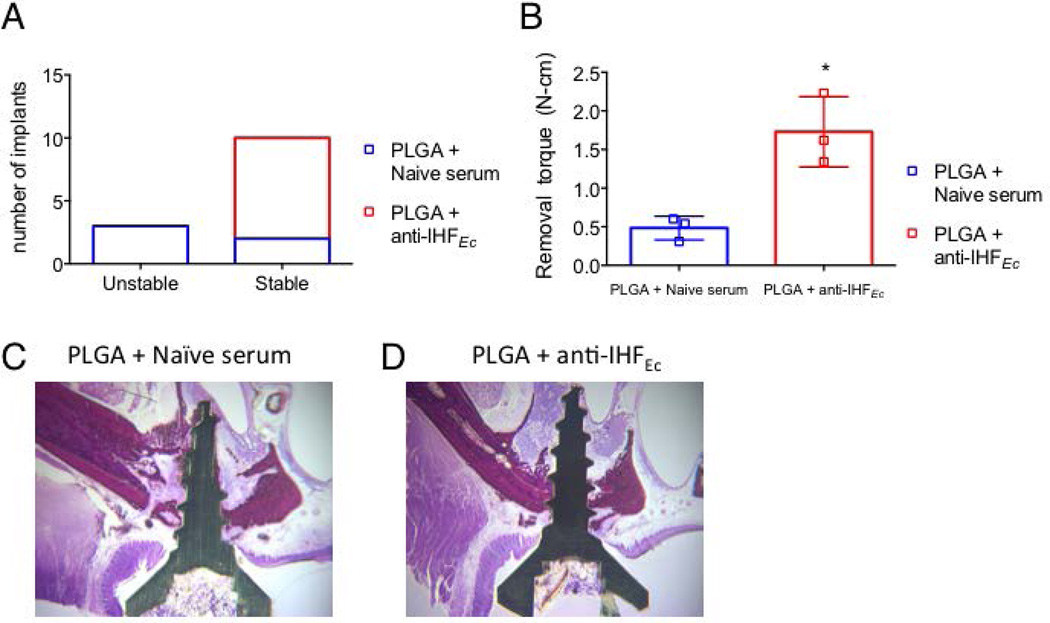
Figure 5. Biomechanical evaluation of stability of implants in bone of the rat oral cavity.
(A) Two Aa biofilm-inoculated implants were placed into the anterior region of the first molars on the right and left sides of the oral cavity. Seven days after placement of the biofilm-inoculated implant, the implants were treated with either naïve serum or anti-IHFEc encapsulated in PLGA microspheres. The clinical stability of the implant was evaluated by moving the implants coronally or palatally with a periodontal probe in anesthetized animals. The implants were considered stable if no movement was observed. (B) Animals that were surgically implanted with Aa biofilm-inoculated implants and treated with anti-IHFEc (n=3) or naïve serum (n=3) were euthanized after three days and the stability of the implant was evaluated by reverse torque analysis. Representative images of H&E staining of the bone (dark red) upon treatment with naïve serum (C) or anti-IHFEc (D) are shown. Data is represented as mean ± standard deviation. A p-value of 0.01–0.05 is represented by *.
Figure 3. Aa biofilm effects on surrounding tissue.
Control implants or Aa biofilm-inoculated implants were placed into the anterior region of the first molars in maxillae on the right and left sides of the oral cavity. Representative images of peri-implant tissues with control (A) or Aa biofilm inoculated implants (B) seven days after surgical placement of titanium implants are illustrated. Clinical evaluation of peri-implant mucosal inflammation and bleeding were recorded from 1–7 days and tissue inflammation (mean ± standard deviation) was classified clinically with score values: 0–3 (0 - no bleeding; 1 - slight bleeding; 2 - moderate bleeding; 3 - severe bleeding). (C) Inflammation and bleeding score after seven days post-surgery. Statistical significance was evaluated by Mann-Whitney test and a p-value of 0.01–0.05 is represented by *.
Figure 4. Effect of anti-IHFEc on Aa biofilm Induced peri-implant mucosal inflammation.
Two Aa biofilm-inoculated implants were placed into the anterior region of the first molars in maxillae on the right and left sides of the oral cavity. (A, C) Representative images of peri-implant tissues seven days after surgical placement of titanium implants and before treatment are illustrated. Representative images of the tissue response of rat mucosa three days after treatment with either naïve serum (B) or anti-IHFEc (D) encapsulated in PLGA microspheres are illustrated. (E) After three days of treatment, clinical evaluation of peri-implant mucosal inflammation and bleeding, were recorded and tissue inflammation was classified clinically with score values: 0–3 (0 - no bleeding; 1 - slight bleeding; 2 -moderate bleeding; 3 - severe bleeding). Inflammation and bleeding score is represented as mean ± standard deviation. Statistical significance was evaluated by Mann-Whitney test and a p-value less than 0.01 is represented by **.
Results
Hyperimmune antiserum to E. coli IHF disrupted pre-formed Aa biofilms in vitro
We have previously demonstrated that hyperimmune antiserum directed against E. coli IHF (anti-IHFEc) disrupts biofilms formed by multiple known human pathogens under laboratory conditions and that sequestration of extra-bacterial IHF from the bulk media surrounding the extracellular matrix increases sensitivity of bacteria to antimicrobials (Goodman et al. 2011). To now determine whether IHF is within the extracellular matrix, we performed immunofluorescence by incubating fixed Aa biofilms with anti-IHFEc followed by goat anti-rabbit IgG conjugated to AlexaFluor® 647 (Invitrogen). Aa biofilms were further visualized with LIVE/DEAD® stain. We observed labeling (white) throughout the biofilm (Figure S1) that confirmed the presence of IHF within the extracellular matrix of Aa biofilms. To test the ability of anti-IHFEc to disrupt preformed Aa biofilms, we treated in vitro pre-formed Aa biofilms (Figure 2A) with either naïve rabbit serum (Figure 2B) or anti-IHFEc at an optimal dilution of 1:50 (Figure S2) for 16 hours (Figure 2C). As shown in Figure 2B, when compared to a biofilm incubated with naïve serum, treatment with anti-IHFEc significantly disrupted the Aa biofilms (Figure 2C). Quantification of biofilm parameters with COMSTAT revealed that treatment with anti-IHFEc significantly decreased the maximum height by 18%, average thickness by 47% and biomass by 45% respectively (Figure 2G–I). The enumeration of bacteria in the planktonic and biofilm state revealed that while the total bacteria (planktonic +biofilm) upon treatment with anti-IHFEc was comparable to that in the presence of naïve serum, there was a significant increase in planktonic bacteria in the presence of anti-IHFEc compared to treatment with naïve serum (Figure 2J). This outcome suggested that the treatment with anti-IHFEc simply released bacteria from the biofilm to the planktonic state (Figure 2J). In a parallel experiment where in we treated dental implants with an Aa biofilm with either medium or anti-IHFEc also demonstrated disruption of Aa biofilm upon treatment with anti-IHFEc (Figure S3) suggesting this treatment was not dependent on the attached surface.
In addition to the direct treatment of Aa biofilms with anti-IHFEc, we tested the effect of exposure to anti-IHFEc that had been encapsulated in biodegradable PLGA microspheres as a possible means to limit diffusion of the antibody and target the antibody for sustained release at the site of infection for later use in our animal model (Freire et al. 2011). Consistent with the previous observation, in vitro pre-formed Aa biofilms were disrupted by anti-IHFEc encapsulated in PLGA microspheres, in comparison to the treatment with naïve serum encapsulated in PLGA microspheres (Figure 2B, C, and E-I). These data further demonstrated that PLGA microspheres could be used as a mode of delivery of the antibody in an in vivo model of experimental peri-implantitis.
Aa induced robust inflammation in a rat model of biofilm-mediated peri-implantitis
To characterize the host response to Aa, we established a 48-hour biofilm on the supragingival portion of the implant (Figure 1 and Figure S3) and transmucosally placed either a control implant with no exogenous biofilm (implant incubated in sterile medium) or implants with pre-formed Aa biofilms into the hard palate adjacent to the maxillary alveolar ridge (Figure 1). Implants were blindly evaluated for inflammation and bleeding every day for seven days. As shown in Figure 3A, the control implants without pre-formed Aa biofilm presented with healthy peri-implant mucosal tissue, as noted by the absence of inflammation. Conversely, clinical manifestations of inflammation including bleeding, redness and swelling of the mucosal tissue surrounding implants were observed in the case of implants with pre-formed Aa biofilms (Figure 3B, C). While inflammation and bleeding due to the surgical procedure ceased two days after surgery and healthy mucosa was maintained for up to seven days in those animals implanted with control implants (n=16; Figure 3C), those that received implants with pre-formed Aa biofilms (n=16) exhibited severe peri-mucosal inflammation throughout the seven day observation period (Figure 3C). Taken together with our previous studies, these data demonstrated that the implants on which Aa biofilms were present induced inflammation with tissue destruction and could thereby be employed to investigate the host immune responses to biofilms formed by Aa.
Aa biofilm-induced peri-implant mucosal inflammation was resolved by treatment with anti-IHFEc
Next, we employed the rat model described above to evaluate the ability of anti-IHFEc to resolve mucosal inflammation caused by the implants with pre-formed Aa biofilms. First, inflammation was clinically evaluated seven days after the surgical placement of implants into the oral cavity of rats (Figure 4A, C, E). At the end of seven days, treatment with a single topical dose of either naïve or anti-IHFEc encapsulated in PLGA microspheres was applied to the implant and the surrounding tissue. Mucosal inflammation was evaluated three days after treatment. While the implants with Aa biofilms that were treated with naïve serum demonstrated persistence of inflammation as shown in Figure 4B, the implants with Aa biofilms that were treated with anti-IHFEc showed a significant decrease in inflammation (Figure 4D). The severity of the bleeding, erythema and edema after treatment with either naïve or anti-IHFEc was also scored and the results demonstrated that while implants that carried pre-formed Aa biofilms that had been treated with naïve serum encapsulated in PLGA microspheres received moderate to high inflammation scores (1.8 ± 1.0, n=5), those implants treated with anti-IHFEc encapsulated in PLGA microspheres showed no signs of inflammation as shown in Figure 4E (n=8). These data demonstrated the efficacy of topical treatment of anti-IHFEc encapsulated in PLGA microspheres against Aa biofilm-mediated mucosal inflammation in a rat model of experimental peri-implantitis.
Treatment of Aa biofilm-coated implants with anti-IHFEc increased the stability of the implant in a rat model of experimental peri-implantitis
Titanium implants are biocompatible and bioinert (Beder and Eade 1956; Branemark et al. 1977). We have demonstrated that the inflammation induced during implantation was transient and resolves within two days (Figure 3C). In contrast, naturally occurring or experimentally induced pathogenic biofilm formation on implants is associated with progressive loss of stability leading to implant failure (Freire et al. 2011; Hultin et al. 2002; Renvert et al. 2008). Also, any micro-movement resulting from continuous inflammation interferes with collagen deposition of the connective tissue that is responsible for mucosal healing and bone deposition that is required for bone healing (Vandamme et al. 2007). Here, we evaluated the effect of treatment of anti-IHFEc encapsulated in PLGA microspheres on the stability of implants with pre-formed Aa biofilms in the oral cavity of rats. As described above, seven days after surgical placement of the Aa biofilm-coated implants into the oral cavity, the implants were treated with a single dose of either naïve or anti-IHFEc encapsulated in PLGA microspheres for three days. The clinical stability of the implant was evaluated by application of light lateral force with a periodontal probe in anesthetized animals. The implants were considered stable if no movement was observed. As shown in Figure 5A, while all of the implants treated with anti-IHFEc encapsulated in PLGA microspheres were stable, only about 40% of those treated with naïve serum encapsulated in PLGA microspheres were stable.
To further determine the stability of the implants the torque required to remove the implant was evaluated after the rats were euthanized. As shown in Figure 5B, while the implants treated with naïve serum exhibited a lower implant removal torque (0.5N/cm2 ± 0.2, n=3) consistent with reduced implant stability, implants treated with anti-IHFEc exhibited about a 4-fold greater torque required to remove the implant (1.8N/cm2 ± 0.4, n=3). To investigate the effect of treatment of implants that contained pre-formed Aa biofilms with anti-IHFEc on bone healing, histological analysis of the peri-implant tissues was performed. As shown in representative images (Figure 5C), while bone surrounding the implant with Aa biofilm that had been treated with a single dose of naïve serum exhibited extensive disintegration, bone surrounding the implant that had been treated with a single dose of anti-IHFEc (Figure 5D) was intact. These data demonstrated that treatment with anti-IHFEc allowed resolution of inflammation with healing that resulted in increased stability of implants that contained pre-formed Aa biofilms and further underscored the therapeutic potential of anti-IHFEc in an animal model of experimental peri-implantitis.
Discussion
Homeostasis is the key to maintaining a healthy stable environment between the commensal microbiota and the host. Pathogenic bacterial biofilms such as those formed by Aa disrupt this homeostasis causing dysbiosis and resulting in disease (Bezerra Bde et al. 2012; Cairo et al. 2004; Fives-Taylor et al. 1999; Freire et al. 2011; Henderson et al. 2003; Nalbant and Zadeh 2002; Slots 1984). Uncontrolled mucosal inflammation is a hallmark sign of chronic diseases of the oral cavity, including oral cancer, tonsillitis, gingivitis, peri-implantitis, and periodontitis. As unresolved acute inflammation results in chronic inflammation and subsequent destruction of the periodontal ligament and bone, it is imperative to control and resolve the inflammation to prevent tooth loss. Hence there is an urgent need for the development of targeted therapies to minimize pathogenic oral flora and thereby facilitate the reversal of dysbiosis caused by the presence of a dominant pathogenic biofilm.
Animal models of periodontitis often involve introduction of bacteria into the oral cavity through oral gavage or direct injection of the pathogenic bacteria into the oral tissues (Trombone et al. 2009). The pathogenic bacteria used for inoculation are typically in the planktonic state (Kinane and Hajishengallis 2009) and hence retention of these bacteria in the oral cavity is greatly diminished, as these pathogenic bacteria must compete with the existing oral microbiota and resist the host immune response. Although retention of bacteria can be achieved by the placement of cotton or silk ligatures around the teeth or implants (Albouy et al. 2009; Berglundh et al. 2007; Duarte et al. 2010; Lindhe et al. 1992), the tissue destruction caused by the placement of the ligature fails to mimic in vivo pathogenesis (Karimbux et al. 1998; Wilensky et al. 2009). To circumvent these barriers, we have previously developed an animal model wherein we employed titanium implants as a colonizing surface for Aa biofilm formation in vitro, subsequently implanting these biofilm-coated implants transmucosally in the rat jaw (Freire et al. 2011). This technique allows for successful colonization and establishment of Aa biofilm in the absence of antagonistic commensals and the immune response prior to placement in submucosal space in addition to eliciting a subsequent robust immune response characterized by inflammation and bleeding (Figure 3) that persisted for weeks and resulting in exfoliation of the implant (Freire et al. 2011). In the present study we employed this model to investigate the effect of a hyperimmune polyclonal antiserum against one of the DNA binding proteins, IHF on an Aa biofilm and demonstrated that a single dose of topical application of the antiserum encapsulated in PLGA microspheres promoted the resolution of the disease state including demonstrating greater stability of the implants in the oral cavity suggesting that this approach could serve as an effective treatment of periodontitis.
In this study, we encapsulated anti-IHFEc in PLGA microspheres as a means to limit diffusion of the antibody and target the antibody for sustained release at the site of infection in our animal model. There are potentially two mechanisms by which antiserum encapsulated in PLGA microspheres could mediate debulking of Aa biofilm. One possibility is that the degradation of PLGA microspheres resulted in release of the antiserum at the site of infection. PLGA microspheres degrade by hydrolysis or by enzyme-mediated cleavage of the backbone ester bonds (Park 1995) that contribute to the release of the cargo. The process of degradation of these microspheres is influenced by several factors including the ratio of glycolic to lactic acid, crystallinity, average molecular weight of the polymer, type of drug encapsulated and other environmental factors and therefore the rate of release of the encapsulated molecule can range from a few days to several weeks (Makadia and Siegel 2011). The second possibility is that the microspheres simply served as a ‘sponge’ to sequester the IHF from the Aa biofilm. Indeed, we have recently demonstrated, using a transwell system, that treatment of in vitro pre-formed NTHI (a related bacterium from the same family Pasteurellaceae) biofilms with anti-IHFEc causes release of the resident bacteria by sequestering free/unbound IHF in the vicinity of the biofilm without the need of direct contact (Brockson et al. 2014). It is this sequestration that causes a shift in equilibrium between the free and bound IHF that eventually results in destabilization of the biofilm. It is possible that the PLGA microspheres containing anti-IHFEc served as a ‘sponge’ to sequester free IHF in a biofilm analogous to the transwell system to mediate disruption of the Aa biofilm. The fact that a single dose of the antibody encapsulated in these microspheres was capable of disrupting pre-formed Aa biofilm in vitro (Figure 2) suggest the potential use of these microspheres for delivery of the antibody for a robust and rapid therapeutic effect. Importantly, this platform mimics Arestin™, a PLGA based therapeutic containing an antimicrobial that is already an accepted approach in stabilizing the progression of periodontal disease (Meinberg et al. 2002; Renvert et al. 2008). Since we have already shown that antimicrobials are synergistic for biofilm resolution with anti-IHFEc (Brandstetter et al. 2013; Brockson et al. 2014; Goodman et al. 2011; Novotny et al. 2013) future experiments will test a combined version of Arestin with anti-IHFEc for improved efficacy and eradication of periodontal biofilms.
Inflammation is fundamentally a host defense mechanism, however if uncontrolled, it leads to tissue destruction and damage. Traditionally non-steroidal anti-inflammatory drugs (NSAIDs) are employed to inhibit inflammatory responses (Van Dyke and Serhan 2003). In recent years pro-resolving molecules such as lipoxins, resolvins, and protectins that act as agonists to stimulate the resolution of inflammation have been actively investigated for the treatment of periodontitis (Hasturk et al. 2007; Serhan and Chiang 2008). Although these molecules could effectively control inflammation, they fail to target the underlying cause of the inflammatory response that is mediated by pathogenic biofilms. In our animal model we have directly targeted the pathogenic biofilm with a hyperimmune antiserum directed against one of the members of DNABII family and demonstrated that a single dose of the anti-IHFEc encapsulated in PLGA microspheres effectively resolved the inflammation in just three days (Figure 4). Since this approach directly targets the pathogenic biofilm and resolves inflammation associated with the early stages of a disease state that mimics periodontitis, this approach holds great potential as a therapeutic as well as a prophylactic to the disease state prior to significant bone loss.
Periodontitis progresses from inflammation of gingiva without any bone loss termed as gingivitis, to inflammation of gingiva and the surrounding tissues with moderate to severe bone loss that ultimately results in tooth loss. Aa biofilms have been shown to promote osteoclastogenesis and promote bone resorption in humans and in experimental models (Bezerra Bde et al. 2012; Fives-Taylor et al. 1999; Freire et al. 2011; Schreiner et al. 2003). The treatment of biofilm-inoculated implants with anti-IHFEc encapsulated in PLGA microspheres increased the stability of the implant in the oral cavity as measured by the reverse torque analysis (Figure 5B). While histological analysis of implants treated with naïve serum revealed bone disintegration, intact bone was evident in implants treated with anti-IHFEc (Figure 5C, 5D). These data suggest that treatment with anti-IHFEc could potentially ameliorate inflammation and thus accelerate healing. Future work will include a more thorough analysis of this restoration process including the relative changes in cytokines and other proinflammatory markers.
The DNABII family of proteins has been shown to be critical for the stability of biofilm matrix formed by several pathogenic bacteria both in vitro and in vivo (Brockson et al. 2014; Goodman et al. 2011; Novotny et al. 2013). We have previously demonstrated that antiserum directed against one of the DNABII proteins results in release of the resident bacteria and these released bacteria are more sensitive to the action of both antibiotics and the immune system (Goodman et al. 2011; Novotny et al. 2013). Hence anti-IHFEc encapsulated in PLGA microspheres has a great potential in the treatment of periodontitis as it could potentially enhance the ability of the immune system to clear the bacterial biofilms that cause the disease state as well as quiet the host’s inflammatory response that results in collateral tissue damage.
Supplementary Material
Fixed 16 hr biofilm formed by Aa was stained with LIVE/DEAD® stain and immunolabeled with (A) naïve serum or (B) rabbit anti-IHFEc serum to show the presence of IHF (white color) within the biofilm. All images captured with a 63X objective.
Aa biofilms were pre-formed in vitro for 24 hours in mTSB and treated with either naïve serum or anti-IHFEc at various concentrations. COMSTAT analysis was employed to calculate average thickness (A) and biomass (B). All in vitro biofilm assays were repeated a minimum of three times on separate days. Data are presented as mean values ± SEM. A p-value ≤ 0.05 was considered significant. p-value of 0.01-0.05 is represented by *, p-value less than 0.01 is represented by **, p-value less than 0.001 is ***.
Aa biofilms were pre-formed on titanium implants in vitro for 24 hours in mTSB and treated with one of the following: (A) medium only, (B) anti-IHFEc for 16 hours. Implants were washed with saline and stained with BD cell viability kit to visualize bacteria. Representative images of Aa biofilms are shown.
Acknowledgments
This work was supported by NIH grant R01DC011818 to SDG and LOB, NIH grant DE012212 to CC and HZ and Ideas Empowered Program, University of Southern California to SDG. We thank Joseph Jursicek for imaging the histology slides and Lauren Mashburn-Warren for critical reading of the manuscript. LOB and SDG own equity in a company, ProclaRx, outside the submitted work. In addition, LOB and SDG have a patent “Compositions and methods for the removal of biofilms” issued to ProclaRx.
Footnotes
Author contributions
Conceived and designed the experiments: MOF, JSD, CC, LOB, HHZ, SDG Performed the experiments: MOF, AD, JSD, AY, JBN. Analyzed the data: MOF, AD, AY, JBN. Contributed reagents/materials/analysis tools: HHZ, CC, SDG, LOB. Wrote the paper: MOF, AD.
References
- Albouy JP, Abrahamsson I, Persson LG, Berglundh T. Spontaneous progression of ligatured induced peri-implantitis at implants with different surface characteristics. An experimental study in dogs II: histological observations. Clin Oral Implants Res. 2009;20:366–371. doi: 10.1111/j.1600-0501.2008.01645.x. [DOI] [PubMed] [Google Scholar]
- Baloul SS, Gerstenfeld LC, Morgan EF, Carvalho RS, Van Dyke TE, Kantarci A. Mechanism of action and morphologic changes in the alveolar bone in response to selective alveolar decortication-facilitated tooth movement. Am J Orthod Dentofacial Orthop. 2011;139:S83–S101. doi: 10.1016/j.ajodo.2010.09.026. [DOI] [PubMed] [Google Scholar]
- Beder OE, Eade G. An investigation of tissue tolerance to titanium metal implants in dogs. Surgery. 1956;39:470–473. [PubMed] [Google Scholar]
- Beer SJ, Matthews CB, Stein CS, Ross BD, Hilfinger JM, Davidson BL. Poly (lactic-glycolic) acid copolymer encapsulation of recombinant adenovirus reduces immunogenicity in vivo. Gene Ther. 1998;5:740–746. doi: 10.1038/sj.gt.3300647. [DOI] [PubMed] [Google Scholar]
- Berglundh T, Gotfredsen K, Zitzmann NU, Lang NP, Lindhe J. Spontaneous progression of ligature induced peri-implantitis at implants with different surface roughness: an experimental study in dogs. Clin Oral Implants Res. 2007;18:655–661. doi: 10.1111/j.1600-0501.2007.01397.x. [DOI] [PubMed] [Google Scholar]
- Bezerra Bde B, Andriankaja O, Kang J, et al. A. actinomycetemcomitans-induced periodontal disease promotes systemic and local responses in rat periodontium. J Clin Periodontol. 2012;39:333–341. doi: 10.1111/j.1600-051X.2011.01847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boleij ASchaeps RM, de Kleijn S, et al. Surface-exposed histone-like protein a modulates adherence of Streptococcus gallolyticus to colon adenocarcinoma cells. Infect Immun. 2009;77:5519–5527. doi: 10.1128/IAI.00384-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandstetter KA, Jurcisek JA, Goodman SD, Bakaletz LO, Das S. Antibodies directed against integration host factor mediate biofilm clearance from Nasopore. Laryngoscope. 2013;123:2626–2632. doi: 10.1002/lary.24183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branemark PI, Hansson BO, Adell R, et al. Osseointegrated implants in the treatment of the edentulous jaw. Experience from a 10-year period. Scand J Plast Reconstr Surg Suppl. 1977;16:1–132. [PubMed] [Google Scholar]
- Brockson ME, Novotny LA, Mokrzan EM, et al. Evaluation of the kinetics and mechanism of action of anti-integration host factor-mediated disruption of bacterial biofilms. Mol Microbiol. 2014;93:1246–1258. doi: 10.1111/mmi.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairo F, Gaeta C, Dorigo W, et al. Periodontal pathogens in atheromatous plaques. A controlled clinical and laboratory trial. J Periodontal Res. 2004;39:442–446. doi: 10.1111/j.1600-0765.2004.00761.x. [DOI] [PubMed] [Google Scholar]
- Chen C, Kittichotirat W, Chen W, Downey JS, Si Y, Bumgarner R. Genome sequence of naturally competent Aggregatibacter actinomycetemcomitans serotype a strain D7S-1. J Bacteriol. 2010;192:2643–2644. doi: 10.1128/JB.00157-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun YH, Chun KR, Olguin D, Wang HL. Biological foundation for periodontitis as a potential risk factor for atherosclerosis. J Periodontal Res. 2005;40:87–95. doi: 10.1111/j.1600-0765.2004.00771.x. [DOI] [PubMed] [Google Scholar]
- Couper DJ, Beck JD, Falkner KL, et al. The Periodontitis and Vascular Events (PAVE) pilot study: recruitment, retention, and community care controls. J Periodontol. 2008;79:80–89. doi: 10.1902/jop.2008.070216. [DOI] [PubMed] [Google Scholar]
- Danhier F, Ansorena E, Silva JM, Coco R, Le Breton A, Preat V. PLGA-based nanoparticles: an overview of biomedical applications. J Control Release. 2012;161:505–522. doi: 10.1016/j.jconrel.2012.01.043. [DOI] [PubMed] [Google Scholar]
- Devaraj A, Justice SS, Bakaletz LO, Goodman SD. DNABII proteins play a central role in UPEC biofilm structure. Mol Microbiol. 2015;96:1119–1135. doi: 10.1111/mmi.12994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewhirst FE, Chen T, Izard J, et al. The human oral microbiome. J Bacteriol. 2010;192:5002–5017. doi: 10.1128/JB.00542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drisko CL. Periodontal debridement: still the treatment of choice. J Evid Based Dent Pract. 2014;(14 Suppl):33–41 e1. doi: 10.1016/j.jebdp.2014.02.007. [DOI] [PubMed] [Google Scholar]
- Duarte PM, Tezolin KR, Figueiredo LC, Feres M, Bastos MF. Microbial profile of ligature-induced periodontitis in rats. Arch Oral Biol. 2010;55:142–147. doi: 10.1016/j.archoralbio.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Fives-Taylor PM, Meyer DH, Mintz KP, Brissette C. Virulence factors of Actinobacillus actinomycetemcomitans. Periodontol 2000. 1999;20:136–167. doi: 10.1111/j.1600-0757.1999.tb00161.x. [DOI] [PubMed] [Google Scholar]
- Flemming HC, Wingender J. The biofilm matrix. Nat Rev Microbiol. 2010;8:623–633. doi: 10.1038/nrmicro2415. [DOI] [PubMed] [Google Scholar]
- Freire MO, Sedghizadeh PP, Schaudinn C, et al. Development of an animal model for Aggregatibacter actinomycetemcomitans biofilm-mediated oral osteolytic infection: a preliminary study. J Periodontol. 2011;82:778–789. doi: 10.1902/jop.2010.100263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman SD, Obergfell KP, Jurcisek JA, et al. Biofilms can be dispersed by focusing the immune system on a common family of bacterial nucleoid-associated proteins. Mucosal Immunol. 2011;4:625–637. doi: 10.1038/mi.2011.27. [DOI] [PubMed] [Google Scholar]
- Greenstein G. Povidone-iodine’s effects and role in the management of periodontal diseases: a review. J Periodontol. 1999;70:1397–1405. doi: 10.1902/jop.1999.70.11.1397. [DOI] [PubMed] [Google Scholar]
- Gustave JE, Jurcisek JA, McCoy KS, Goodman SD, Bakaletz LO. Targeting bacterial integration host factor to disrupt biofilms associated with cystic fibrosis. J Cyst Fibros. 2013;12:384–389. doi: 10.1016/j.jcf.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasturk H, Kantarci A, Goguet-Surmenian E, et al. Resolvin E1 regulates inflammation at the cellular and tissue level and restores tissue homeostasis in vivo. J Immunol. 2007;179:7021–7029. doi: 10.4049/jimmunol.179.10.7021. [DOI] [PubMed] [Google Scholar]
- Heitz-Mayfield LJ, Lang NP. Comparative biology of chronic and aggressive periodontitis vs. peri-implantitis. Periodontol 2000. 2010;53:167–181. doi: 10.1111/j.1600-0757.2010.00348.x. [DOI] [PubMed] [Google Scholar]
- Henderson B, Nair SP, Ward JM, Wilson M. Molecular pathogenicity of the oral opportunistic pathogen Actinobacillus actinomycetemcomitans. Annu Rev Microbiol. 2003;57:29–55. doi: 10.1146/annurev.micro.57.030502.090908. [DOI] [PubMed] [Google Scholar]
- Heydorn A, Ersboll BK, Hentzer M, Parsek MR, Givskov M, Molin S. Experimental reproducibility in flow-chamber biofilms. Microbiology. 2000;146(Pt 10):2409–2415. doi: 10.1099/00221287-146-10-2409. [DOI] [PubMed] [Google Scholar]
- Hultin M, Gustafsson A, Hallstrom H, Johansson LA, Ekfeldt A, Klinge B. Microbiological findings and host response in patients with peri-implantitis. Clin Oral Implants Res. 2002;13:349–358. doi: 10.1034/j.1600-0501.2002.130402.x. [DOI] [PubMed] [Google Scholar]
- Idicula WA, et al. AtIdentification of biofilms in post-tympanostomy tube otorrhea. Laryngoscope In Press. 2016 doi: 10.1002/lary.25826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janket SJ, Jones JA, Meurman JH, Baird AE, Van Dyke TE. Oral infection, hyperglycemia, and endothelial dysfunction. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105:173–179. doi: 10.1016/j.tripleo.2007.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jividen G, Jr, Misch CE. Reverse torque testing and early loading failures: help or hindrance? J Oral Implantol. 2000;26:82–90. doi: 10.1563/1548-1336(2000)026<0082:RTTAEL>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- Justice SS, Downey JS, Dabdoub SM, et al. Aberrant community architecture and attenuated persistence of uropathogenic Escherichia coli in the absence of individual IHF subunits. PLoS One. 2012;7:e48349. doi: 10.1371/journal.pone.0048349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimbux NY, Ramamurthy NS, Golub LM, Nishimura I. The expression of collagen I and XII mRNAs in Porphyromonas gingivalis-induced periodontitis in rats: the effect of doxycycline and chemically modified tetracycline. J Periodontol. 1998;69:34–40. doi: 10.1902/jop.1998.69.1.34. [DOI] [PubMed] [Google Scholar]
- Karlsson MR, Diogo Lofgren CI, Jansson HM. The effect of laser therapy as an adjunct to non-surgical periodontal treatment in subjects with chronic periodontitis: a systematic review. J Periodontol. 2008;79:2021–2028. doi: 10.1902/jop.2008.080197. [DOI] [PubMed] [Google Scholar]
- Kaur S, White S, Bartold PM. Periodontal disease and rheumatoid arthritis: a systematic review. J Dent Res. 2013;92:399–408. doi: 10.1177/0022034513483142. [DOI] [PubMed] [Google Scholar]
- Kim N, Weeks DL, Shin JM, Scott DR, Young MK, Sachs G. Proteins released by Helicobacter pylori in vitro. J Bacteriol. 2002;184:6155–6162. doi: 10.1128/JB.184.22.6155-6162.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinane DF, Hajishengallis G. Polymicrobial infections, biofilms, and beyond. J Clin Periodontol. 2009;36:404–405. doi: 10.1111/j.1600-051X.2009.01396.x. [DOI] [PubMed] [Google Scholar]
- Kornman KS. Mapping the pathogenesis of periodontitis: a new look. J Periodontol. 2008;79:1560–1568. doi: 10.1902/jop.2008.080213. [DOI] [PubMed] [Google Scholar]
- Lindhe J, Berglundh T, Ericsson I, Liljenberg B, Marinello C. Experimental breakdown of peri-implant and periodontal tissues. A study in the beagle dog. Clin Oral Implants Res. 1992;3:9–16. doi: 10.1034/j.1600-0501.1992.030102.x. [DOI] [PubMed] [Google Scholar]
- Lunsford RD, Nguyen N, London J. DNA-binding activities in Streptococcus gordonii: identification of a receptor-nickase and a histonelike protein. Curr Microbiol. 1996;32:95–100. doi: 10.1007/s002849900017. [DOI] [PubMed] [Google Scholar]
- Makadia HK, Siegel SJ. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers (Basel) 2011;3:1377–1397. doi: 10.3390/polym3031377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinberg TA, Barnes CM, Dunning DG, Reinhardt RA. Comparison of conventional periodontal maintenance versus scaling and root planing with subgingival minocycline. J Periodontol. 2002;73:167–172. doi: 10.1902/jop.2002.73.2.167. [DOI] [PubMed] [Google Scholar]
- Muller HP, Heinecke A, Borneff M. A statistical approach to the ecology of Actinobacillus actinomycetemcomitans in subgingival plaque. Eur J Oral Sci. 1998;106:945–952. doi: 10.1046/j.0909-8836.1998.eos106507.x. [DOI] [PubMed] [Google Scholar]
- Nalbant A, Zadeh HH. Actinobacillus actinomycetemcomitans induces apoptosis of T lymphocytes by the Fas and Fas ligand pathway. Oral Microbiol Immunol. 2002;17:277–284. doi: 10.1034/j.1399-302x.2002.170503.x. [DOI] [PubMed] [Google Scholar]
- Newman MG, Takei HH, Carranza FA. Clinical Diagnosis. In: Newman Michael G, Carranza HHT, Fermin A, editors. Carranza’s Clinical Periodontology. 9. Philadelphia: W.B: Saunders Company; 2002. p. 1033. [Google Scholar]
- Nickel JC, Ruseska I, Wright JB, Costerton JW. Tobramycin resistance of Pseudomonas aeruginosa cells growing as a biofilm on urinary catheter material. Antimicrob Agents Chemother. 1985;27:619–624. doi: 10.1128/aac.27.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotny LA, Amer AO, Brockson ME, Goodman SD, Bakaletz LO. Structural stability of Burkholderia cenocepacia biofilms is reliant on eDNA structure and presence of a bacterial nucleic acid binding protein. PLoS One. 2013;8:e67629. doi: 10.1371/journal.pone.0067629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offenbacher S, Barros SP, Singer RE, Moss K, Williams RC, Beck JD. Periodontal disease at the biofilm-gingival interface. J Periodontol. 2007;78:1911–1925. doi: 10.1902/jop.2007.060465. [DOI] [PubMed] [Google Scholar]
- Papapanou PN. Population studies of microbial ecology in periodontal health and disease. Ann Periodontol. 2002;7:54–61. doi: 10.1902/annals.2002.7.1.54. [DOI] [PubMed] [Google Scholar]
- Park TG. Degradation of poly(lactic-co-glycolic acid) microspheres: effect of copolymer composition. Biomaterials. 1995;16:1123–1130. doi: 10.1016/0142-9612(95)93575-x. [DOI] [PubMed] [Google Scholar]
- Renvert S, Lessem J, Dahlen G, Renvert H, Lindahl C. Mechanical and repeated antimicrobial therapy using a local drug delivery system in the treatment of peri-implantitis: a randomized clinical trial. J Periodontol. 2008;79:836–844. doi: 10.1902/jop.2008.070347. [DOI] [PubMed] [Google Scholar]
- Rouviere-Yaniv J, Gros F. Characterization of a novel, low-molecular-weight DNA-binding protein from Escherichia coli. Proc Natl Acad Sci U S A. 1975;72:3428–3432. doi: 10.1073/pnas.72.9.3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahrmann P, Puhan MA, Attin T, Schmidlin PR. Systematic review on the effect of rinsing with povidone-iodine during nonsurgical periodontal therapy. J Periodontal Res. 2010;45:153–164. doi: 10.1111/j.1600-0765.2009.01232.x. [DOI] [PubMed] [Google Scholar]
- Schreiner HC, Sinatra K, Kaplan JB, et al. Tight-adherence genes of Actinobacillus actinomycetemcomitans are required for virulence in a rat model. Proc Natl Acad Sci U S A. 2003;100:7295–7300. doi: 10.1073/pnas.1237223100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz F, Aoki A, Becker J, Sculean A. Laser application in non-surgical periodontal therapy: a systematic review. J Clin Periodontol. 2008;35:29–44. doi: 10.1111/j.1600-051X.2008.01259.x. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Chiang N. Endogenous pro-resolving and anti-inflammatory lipid mediators: a new pharmacologic genus. Br J Pharmacol. 2008;153(Suppl 1):S200–S215. doi: 10.1038/sj.bjp.0707489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgolastra F, Petrucci A, Gatto R, Monaco A. Effectiveness of systemic amoxicillin/metronidazole as an adjunctive therapy to full-mouth scaling and root planing in the treatment of aggressive periodontitis: a systematic review and meta-analysis. J Periodontol. 2012a;83:731–743. doi: 10.1902/jop.2011.110432. [DOI] [PubMed] [Google Scholar]
- Sgolastra F, Petrucci A, Gatto R, Monaco A. Efficacy of Er:YAG laser in the treatment of chronic periodontitis: systematic review and meta-analysis. Lasers Med Sci. 2012b;27:661–673. doi: 10.1007/s10103-011-0928-8. [DOI] [PubMed] [Google Scholar]
- Slinger R, Chan F, Ferris W, et al. Multiple combination antibiotic susceptibility testing of nontypeable Haemophilus influenzae biofilms. Diagn Microbiol Infect Dis. 2006;56:247–253. doi: 10.1016/j.diagmicrobio.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Slots J. Actinobacillus actinomycetemcomitans and Bacteroides gingivalis in advanced periodontitis in man. Dtsch Zahnarztl Z. 1984;39:615–622. [PubMed] [Google Scholar]
- Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL., Jr Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25:134–144. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- Southard SR, Drisko CL, Killoy WJ, Cobb CM, Tira DE. The effect of 2% chlorhexidine digluconate irrigation on clinical parameters and the level of Bacteroides gingivalis in periodontal pockets. J Periodontol. 1989;60:302–309. doi: 10.1902/jop.1989.60.6.302. [DOI] [PubMed] [Google Scholar]
- Swinger KK, Rice PA. IHF and HU: flexible architects of bent DNA. Curr Opin Struct Biol. 2004;14:28–35. doi: 10.1016/j.sbi.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Teles R, Gursky LC, Faveri M, et al. Relationships among gingival crevicular fluid biomarkers, clinical parameters of periodontal disease, and the subgingival microbiota. J Periodontol. 2010;81:89–98. doi: 10.1902/jop.2009.090397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trombone AP, Ferreira SB, Jr, Raimundo FM, et al. Experimental periodontitis in mice selected for maximal or minimal inflammatory reactions: increased inflammatory immune responsiveness drives increased alveolar bone loss without enhancing the control of periodontal infection. J Periodontal Res. 2009;44:443–451. doi: 10.1111/j.1600-0765.2008.01133.x. [DOI] [PubMed] [Google Scholar]
- Umeda M, Chen C, Bakker I, Contreras A, Morrison JL, Slots J. Risk indicators for harboring periodontal pathogens. J Periodontol. 1998;69:1111–1118. doi: 10.1902/jop.1998.69.10.1111. [DOI] [PubMed] [Google Scholar]
- Van Dyke TE, Kornman KS. Inflammation and factors that may regulate inflammatory response. J Periodontol. 2008;79:1503–1507. doi: 10.1902/jop.2008.080239. [DOI] [PubMed] [Google Scholar]
- Van Dyke TE, Serhan CN. Resolution of inflammation: a new paradigm for the pathogenesis of periodontal diseases. J Dent Res. 2003;82:82–90. doi: 10.1177/154405910308200202. [DOI] [PubMed] [Google Scholar]
- Vandamme K, Naert I, Geris L, Vander Sloten J, Puers R, Duyck J. The effect of micro-motion on the tissue response around immediately loaded roughened titanium implants in the rabbit. Eur J Oral Sci. 2007;115:21–29. doi: 10.1111/j.1600-0722.2007.00416.x. [DOI] [PubMed] [Google Scholar]
- Wang Y, Goodman SD, Redfield RJ, Chen C. Natural transformation and DNA uptake signal sequences in Actinobacillus actinomycetemcomitans. J Bacteriol. 2002;184:3442–3449. doi: 10.1128/JB.184.13.3442-3449.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilensky A, Polak D, Awawdi S, Halabi A, Shapira L, Houri-Haddad Y. Strain-dependent activation of the mouse immune response is correlated with Porphyromonas gingivalis-induced experimental periodontitis. J Clin Periodontol. 2009;36:915–921. doi: 10.1111/j.1600-051X.2009.01464.x. [DOI] [PubMed] [Google Scholar]
- Williams RC, Barnett AH, Claffey N, et al. The potential impact of periodontal disease on general health: a consensus view. Curr Med Res Opin. 2008;24:1635–1643. doi: 10.1185/03007990802131215. [DOI] [PubMed] [Google Scholar]
- Winters BD, Ramasubbu N, Stinson MW. Isolation and characterization of a Streptococcus pyogenes protein that binds to basal laminae of human cardiac muscle. Infect Immun. 1993;61:3259–3264. doi: 10.1128/iai.61.8.3259-3264.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijnge V, et al. Oral biofilm architecture on natural teeth. PLoS One. 2010;5:e9321. doi: 10.1371/journal.pone.0009321. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fixed 16 hr biofilm formed by Aa was stained with LIVE/DEAD® stain and immunolabeled with (A) naïve serum or (B) rabbit anti-IHFEc serum to show the presence of IHF (white color) within the biofilm. All images captured with a 63X objective.
Aa biofilms were pre-formed in vitro for 24 hours in mTSB and treated with either naïve serum or anti-IHFEc at various concentrations. COMSTAT analysis was employed to calculate average thickness (A) and biomass (B). All in vitro biofilm assays were repeated a minimum of three times on separate days. Data are presented as mean values ± SEM. A p-value ≤ 0.05 was considered significant. p-value of 0.01-0.05 is represented by *, p-value less than 0.01 is represented by **, p-value less than 0.001 is ***.
Aa biofilms were pre-formed on titanium implants in vitro for 24 hours in mTSB and treated with one of the following: (A) medium only, (B) anti-IHFEc for 16 hours. Implants were washed with saline and stained with BD cell viability kit to visualize bacteria. Representative images of Aa biofilms are shown.