Abstract
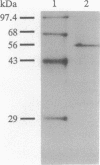
We isolated cDNA clones containing the entire coding region of the putative oncogene AKT2. Sequence analysis and in vitro translation demonstrated that AKT2 encodes a 56-kDa protein with homology to serine/threonine kinases; moreover, this protein contains a Src homology 2-like domain. AKT2 was shown to be amplified and overexpressed in 2 of 8 ovarian carcinoma cell lines and 2 of 15 primary ovarian tumors. AKT2 was mapped to chromosome region 19q13.1-q13.2 by fluorescence in situ hybridization. In the two ovarian carcinoma cell lines exhibiting amplification of AKT2, the amplified sequences were localized within homogeneously staining regions. We conclude that AKT2 belongs to a distinct subfamily of protein-serine/threonine kinases containing Src homology 2-like domains and that alterations of AKT2 may contribute to the pathogenesis of ovarian carcinomas.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beebe S. J., Oyen O., Sandberg M., Frøysa A., Hansson V., Jahnsen T. Molecular cloning of a tissue-specific protein kinase (C gamma) from human testis--representing a third isoform for the catalytic subunit of cAMP-dependent protein kinase. Mol Endocrinol. 1990 Mar;4(3):465–475. doi: 10.1210/mend-4-3-465. [DOI] [PubMed] [Google Scholar]
- Bellacosa A., Testa J. R., Staal S. P., Tsichlis P. N. A retroviral oncogene, akt, encoding a serine-threonine kinase containing an SH2-like region. Science. 1991 Oct 11;254(5029):274–277. doi: 10.1126/science.254.5029.274. [DOI] [PubMed] [Google Scholar]
- Bishop J. M. The molecular genetics of cancer. Science. 1987 Jan 16;235(4786):305–311. doi: 10.1126/science.3541204. [DOI] [PubMed] [Google Scholar]
- Brodeur G. M., Seeger R. C., Schwab M., Varmus H. E., Bishop J. M. Amplification of N-myc in untreated human neuroblastomas correlates with advanced disease stage. Science. 1984 Jun 8;224(4653):1121–1124. doi: 10.1126/science.6719137. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eva A., Robbins K. C., Andersen P. R., Srinivasan A., Tronick S. R., Reddy E. P., Ellmore N. W., Galen A. T., Lautenberger J. A., Papas T. S. Cellular genes analogous to retroviral onc genes are transcribed in human tumour cells. Nature. 1982 Jan 14;295(5845):116–119. doi: 10.1038/295116a0. [DOI] [PubMed] [Google Scholar]
- Fan Y. S., Davis L. M., Shows T. B. Mapping small DNA sequences by fluorescence in situ hybridization directly on banded metaphase chromosomes. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6223–6227. doi: 10.1073/pnas.87.16.6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filmus J. E., Buick R. N. Stability of c-K-ras amplification during progression in a patient with adenocarcinoma of the ovary. Cancer Res. 1985 Sep;45(9):4468–4472. [PubMed] [Google Scholar]
- Finkenzeller G., Marmé D., Hug H. Sequence of human protein kinase C alpha. Nucleic Acids Res. 1990 Apr 25;18(8):2183–2183. doi: 10.1093/nar/18.8.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto M., Estensen R. D., Sha L., Oakley G. J., Twiggs L. B., Adcock L. L., Carson L. F., Roninson I. B. Association of Ki-ras with amplified DNA sequences, detected in human ovarian carcinomas by a modified in-gel renaturation assay. Cancer Res. 1989 Apr 1;49(7):1693–1697. [PubMed] [Google Scholar]
- Hamilton T. C., Lai G. M., Rothenberg M. L., Fojo A. T., Young R. C., Ozols R. F. Mechanisms of resistance to cisplatin and alkylating agents. Cancer Treat Res. 1989;48:151–169. doi: 10.1007/978-1-4613-1601-5_10. [DOI] [PubMed] [Google Scholar]
- Hamilton T. C., Young R. C., McKoy W. M., Grotzinger K. R., Green J. A., Chu E. W., Whang-Peng J., Rogan A. M., Green W. R., Ozols R. F. Characterization of a human ovarian carcinoma cell line (NIH:OVCAR-3) with androgen and estrogen receptors. Cancer Res. 1983 Nov;43(11):5379–5389. [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M., Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988 Jul 1;241(4861):42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Jones P. F., Jakubowicz T., Hemmings B. A. Molecular cloning of a second form of rac protein kinase. Cell Regul. 1991 Dec;2(12):1001–1009. doi: 10.1091/mbc.2.12.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P. F., Jakubowicz T., Pitossi F. J., Maurer F., Hemmings B. A. Molecular cloning and identification of a serine/threonine protein kinase of the second-messenger subfamily. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4171–4175. doi: 10.1073/pnas.88.10.4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch C. A., Anderson D., Moran M. F., Ellis C., Pawson T. SH2 and SH3 domains: elements that control interactions of cytoplasmic signaling proteins. Science. 1991 May 3;252(5006):668–674. doi: 10.1126/science.1708916. [DOI] [PubMed] [Google Scholar]
- Masuda H., Battifora H., Yokota J., Meltzer S., Cline M. J. Specificity of proto-oncogene amplification in human malignant diseases. Mol Biol Med. 1987 Aug;4(4):213–227. [PubMed] [Google Scholar]
- Mitelman F., Kaneko Y., Trent J. M. Report of the committee on chromosome changes in neoplasia. Cytogenet Cell Genet. 1990;55(1-4):358–386. doi: 10.1159/000133022. [DOI] [PubMed] [Google Scholar]
- Miura I., Siegfried J. M., Resau J., Keller S. M., Zhou J. Y., Testa J. R. Chromosome alterations in 21 non-small cell lung carcinomas. Genes Chromosomes Cancer. 1990 Nov;2(4):328–338. doi: 10.1002/gcc.2870020411. [DOI] [PubMed] [Google Scholar]
- Mohrenweiser H. W., Carrano A. V., Fertitta A., Perry B., Thompson L. H., Tucker J. D., Weber C. A. Refined mapping of the three DNA repair genes, ERCC1, ERCC2, and XRCC1, on human chromosome 19. Cytogenet Cell Genet. 1989;52(1-2):11–14. doi: 10.1159/000132829. [DOI] [PubMed] [Google Scholar]
- Ohno H., Takimoto G., McKeithan T. W. The candidate proto-oncogene bcl-3 is related to genes implicated in cell lineage determination and cell cycle control. Cell. 1990 Mar 23;60(6):991–997. doi: 10.1016/0092-8674(90)90347-h. [DOI] [PubMed] [Google Scholar]
- Ohno S., Kawasaki H., Konno Y., Inagaki M., Hidaka H., Suzuki K. A fourth type of rabbit protein kinase C. Biochemistry. 1988 Mar 22;27(6):2083–2087. doi: 10.1021/bi00406a040. [DOI] [PubMed] [Google Scholar]
- Ono Y., Fujii T., Ogita K., Kikkawa U., Igarashi K., Nishizuka Y. The structure, expression, and properties of additional members of the protein kinase C family. J Biol Chem. 1988 May 15;263(14):6927–6932. [PubMed] [Google Scholar]
- Pawson T. Non-catalytic domains of cytoplasmic protein-tyrosine kinases: regulatory elements in signal transduction. Oncogene. 1988 Nov;3(5):491–495. [PubMed] [Google Scholar]
- Pejovic T., Heim S., Mandahl N., Baldetorp B., Elmfors B., Flodérus U. M., Furgyik S., Helm G., Himmelmann A., Willén H. Chromosome aberrations in 35 primary ovarian carcinomas. Genes Chromosomes Cancer. 1992 Jan;4(1):58–68. doi: 10.1002/gcc.2870040108. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slamon D. J., Clark G. M., Wong S. G., Levin W. J., Ullrich A., McGuire W. L. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987 Jan 9;235(4785):177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- Staal S. P., Hartley J. W., Rowe W. P. Isolation of transforming murine leukemia viruses from mice with a high incidence of spontaneous lymphoma. Proc Natl Acad Sci U S A. 1977 Jul;74(7):3065–3067. doi: 10.1073/pnas.74.7.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staal S. P., Hartley J. W. Thymic lymphoma induction by the AKT8 murine retrovirus. J Exp Med. 1988 Mar 1;167(3):1259–1264. doi: 10.1084/jem.167.3.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staal S. P. Molecular cloning of the akt oncogene and its human homologues AKT1 and AKT2: amplification of AKT1 in a primary human gastric adenocarcinoma. Proc Natl Acad Sci U S A. 1987 Jul;84(14):5034–5037. doi: 10.1073/pnas.84.14.5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota J., Tsunetsugu-Yokota Y., Battifora H., Le Fevre C., Cline M. J. Alterations of myc, myb, and rasHa proto-oncogenes in cancers are frequent and show clinical correlation. Science. 1986 Jan 17;231(4735):261–265. doi: 10.1126/science.3941898. [DOI] [PubMed] [Google Scholar]
- van 't Veer L. J., Hermens R., van den Berg-Bakker L. A., Cheng N. C., Fleuren G. J., Bos J. L., Cleton F. J., Schrier P. I. ras oncogene activation in human ovarian carcinoma. Oncogene. 1988 Feb;2(2):157–165. [PubMed] [Google Scholar]