Abstract
Familial adenomatous polyposis (FAP) is a dominantly inherited syndrome caused by germline mutations in the APC gene and characterized by the development of multiple colorectal adenomas and a high risk of developing colorectal cancer (CRC). The severity of polyposis is correlated with the site of the APC mutation. However, there is also phenotypic variability within families with the same underlying APC mutation, suggesting that additional factors influence the severity of polyposis. Genome-wide association studies identified several single nucleotide polymorphisms (SNPs) that are associated with CRC. We assessed whether these SNPs are associated with polyp multiplicity in proven APC mutation carriers. Sixteen CRC-associated SNPs were analysed in a cohort of 419 APC germline mutation carriers from 182 families. Clinical data were retrieved from the Dutch Polyposis Registry. Allele frequencies of the SNPs were compared for patients with <100 colorectal adenomas versus patients with ≥100 adenomas, using generalized estimating equations with the APC genotype as a covariate. We found a trend of association of two of the tested SNPs with the ≥100 adenoma phenotype: the C alleles of rs16892766 at 8q23.3 (OR 1.71, 95 % CI 1.05–2.76, p = 0.03, dominant model) and rs3802842 at 11q23.1 (OR 1.51, 95 % CI 1.03–2.22, p = 0.04, dominant model). We identified two risk variants that are associated with a more severe phenotype in APC mutation carriers. These risk variants may partly explain the phenotypic variability in families with the same APC gene defect. Further studies with a larger sample size are recommended to evaluate and confirm the phenotypic effect of these SNPs in FAP.
Keywords: Familial adenomatous polyposis, Cancer genetics, Colonic adenomas, Genetic polymorphisms
Introduction
Familial adenomatous polyposis (FAP) is a hereditary colorectal cancer (CRC) susceptibility syndrome, caused by germline mutations in the adenomatous polyposis coli (APC) gene, which is located on chromosome 5. Carriers of mutations in the APC gene develop multiple colorectal adenomas and consequently have a high risk of developing CRC. The risk of CRC in these individuals is related to the number of colorectal adenomas [1]. The severity of polyposis, reflected by the number of colorectal adenomas and the age of onset, is correlated with the site of the APC mutation [2]. Most patients with mutations in the codon 1250–1464 region develop thousands of colorectal adenomas in the first or second decades of life. Patients with a mutation at either end or in a specific splice site region of the APC gene (codons <157, 312–412, >1595) usually have an attenuated polyposis phenotype, with less than a hundred polyps and an age of onset in the third or fourth decades. The majority of FAP patients have mutations in the remainder of the gene and develop hundreds to thousands of polyps from the second decade of life onwards. However, there is also phenotypic variability within FAP families with the same underlying gene defect, suggesting that beside the APC genotype, other factors also play a role in determining the severity of polyposis and the risk of CRC.
Both environmental and genetic factors are known to influence CRC risk [3]. To date, several single nucleotide polymorphisms (SNPs) that show an association with sporadic CRC have been identified by genome-wide association studies (GWAS) [4–10]. Furthermore, gene-environmental interactions may play a role in the effect of SNPs on CRC predisposition [11].
Two of these CRC-associated SNPs (rs16892766 and rs3802842) have been shown to be significantly associated with the risk of CRC and/or age of CRC development in patients with Lynch syndrome [12–14].
We hypothesized that SNPs associated with sporadic CRC may play a role in polyp formation in patients with a germline APC mutation. In the present study, we assessed whether known CRC-associated SNPs influence the disease phenotype in patients with a germline APC mutation.
Methods
Patients
A total of 419 patients from 182 families with a proven germline APC mutation were selected from the polyposis database of the Netherlands Foundation for the Detection of Hereditary Tumors. All patients gave informed consent for registration in the database and for use of their medical data for research purposes. All patients had also given written consent for use of their DNA in further institutional ethics-approved research into their condition before the study. The following data were collected: gender, mode of diagnosis (symptomatic or by screening), age at diagnosis of polyposis and CRC, cumulative number of colorectal adenomas, age at colorectal surgery, date and status of last follow-up. Based on the APC mutation site, patients were categorized into attenuated, intermediate or severe genotype groups, as described in the introduction [2].
Genotyping of SNPs
DNA was extracted from peripheral lymphocytes using an automated procedure (Gentra Systems, Minneapolis, USA) and quantified using Picogreen (Invitrogen, California, USA). Genotyping of the SNPs was performed with the KASPar genotyping system, and outsourced to KBioscience (http://www.kbioscience.co.uk).
Statistical analysis
The Hardy–Weinberg equilibrium of the SNPs was first tested using PLINK, version 1.07 [15]. Further analyses were performed using PASW Statistics 20. The patients were categorized according to the number of colorectal adenomas. We defined two groups: the first group with less than 100 adenomas, and the second group with 100 or more adenomas. The allele frequency of the SNPs was compared between the two groups. To assess association between phenotype and SNP, genotypic odds ratios (OR) and 95 % confidence intervals (CI) were computed using the Generalized Estimating Equation, with exchangeable as working covariance structure for observations within families. A general model for the risk alleles was used for assessing statistical significance, where a dominant model was used in case of rare alleles. As a second step, we also fitted dominant and recessive models to provide further information. For testing, Wald tests were applied. APC mutation site, categorized as genotype group, was included in the model as a covariate. For all statistical analysis, a p value of <0.05 was considered to show a trend of association. When Bonferroni multiple testing correction was applied for 15 SNPs at thirteen susceptibility loci, p < 0.004 should be considered as cut off point for significance.
Results
A total of 419 APC mutation-positive patients were included, of which 188 (44.9 %) had more than 100 colorectal adenomas. The clinical and demographic characteristics of the study subjects are shown in Table 1.
Table 1.
Clinical and demographic characteristics of 419 APC mutation carriers
| <100 adenomas (N = 231) | ≥100 adenomas (N = 188) | |
|---|---|---|
| Gender | ||
| Male (%) | 111 (48 %) | 99 (53 %) |
| Polyposis | ||
| Mean age at diagnosis, years | 26.5 | 27.6 |
| Mode of diagnosis | ||
| Symptomatic (%) | 34 (15 %) | 72 (38 %) |
| Screening (%) | 197 (85 %) | 116 (62 %) |
| CRC (%) | 19 (8 %) | 30 (16 %) |
| Mean age at CRC, years (range) | 43.4 | 40.4 |
| Mutation group | ||
| Attenuated (%) | 50 (22 %) | 20 (11 %) |
| Intermediate (%) | 172 (74 %) | 141 (75 %) |
| Severe (%) | 9 (4 %) | 27 (14 %) |
| Last follow-up | ||
| Age, years | 34.7 | 40.4 |
| Status at last follow-up | ||
| Alive (%) | 221 (96 %) | 165 (88 %) |
| Dead due to CRC (%) | 9 (4 %) | 14 (7 %) |
| Dead due to other cause (%) | 1 (0.4 %) | 9 (5 %) |
Regarding differences between groups, more patients with >100 colorectal adenomas (38 %) were symptomatic on diagnosis compared to the other group (15 %). In addition, the frequency of CRC in the >100 adenoma group was significantly higher than the other group. About 75 % of patients from both phenotype groups had an intermediate phenotype but the proportion of patients with mutations belonging to the attenuated genotype group was twice as high in <100 adenoma as the >100 adenoma group (Table 1).
Of the 16 SNPs tested, fifteen SNPs were in Hardy–Weinberg equilibrium (Table 2). One SNP, rs4939827, showed borderline significant deviance and was excluded from further analyses.
Table 2.
Test for Hardy–Weinberg equilibrium
| SNP | Chromosome region | Alleles major/minor | Risk allele | HWE P value | MAFa (allele) | Gene | Reference |
|---|---|---|---|---|---|---|---|
| rs6691170 | 1q41 | G/T | T | 0.2182 | 0.321 (T) | DUSP10 | [9] |
| rs6687758 | 1q41 | A/G | G | 0.1461 | 0.160 (G) | DUSP10 | [9] |
| rs10936599 | 3q26.2 | C/T | C | 0.8902 | 0.229 (T) | MYNN | [9] |
| rs16892766 | 8q23.3 | A/C | C | 0.5592 | 0.091 (C) | EIF3H | [4] |
| rs6983267 | 8q24.21 | G/T | G | 0.2798 | 0.461 (T) | MYC | [5] |
| rs10795668 | 10p14 | G/A | G | 0.1723 | 0.311 (A) | Unknown | |
| rs3802842 | 11q23.1 | A/C | C | 0.6216 | 0.265 (C) | POU2AF1 | [6] |
| rs7136702 | 12q13.13 | C/T | T | 0.8298 | 0.346 (T) | LARP4 | [9] |
| rs11169552 | 12q13.13 | C/T | C | 0.6966 | 0.247 (T) | DIP2B | [9] |
| rs4444235 | 14q22.2 | T/C | C | 0.2362 | 0.432 (C) | BMP4 | [7] |
| rs4779584 | 15q13.3 | C/T | T | 1 | 0.159 (T) | GREM1 | [8] |
| rs9929218 | 16q22.1 | G/A | G | 0.4207 | 0.304 (A) | CDH1 | [7] |
| rs4939827 | 18q21.1 | C/T | T | 0.04911 | 0.435 (T) | SMAD7 | [10] |
| rs10411210 | 19q13.11 | C/T | C | 0.07355 | 0.127 (T) | RHPN2 | [7] |
| rs961253 | 20p12.3 | C/A | A | 0.1397 | 0.311 (A) | BMP2 | [7] |
| rs4925386 | 20q13.33 | C/T | C | 0.4955 | 0.311 (T) | LAMA5 | [9] |
aMinor allele frequency (MAF) in patients included in this study
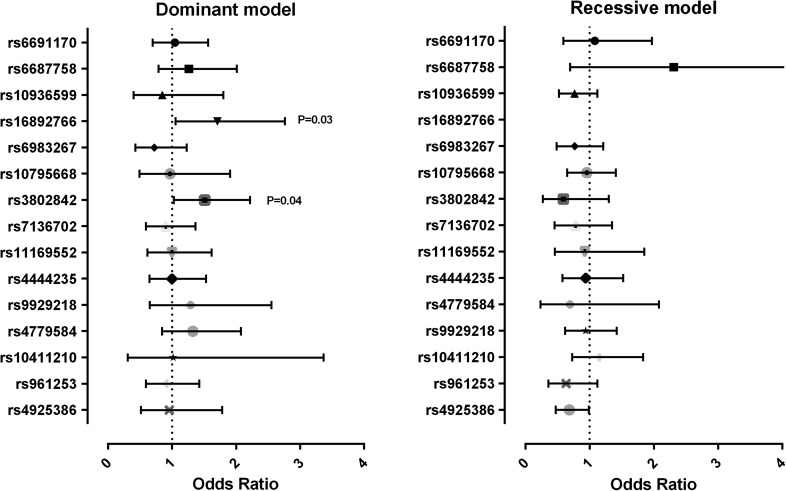
The association of all 15 SNPs with disease phenotype in APC mutation carriers was modelled by Generalized Estimating Equilibrium with exchangeable variance structure. Allelic distribution, genotypic ORs and the corresponding 95 % CIs for each SNP are shown in Table 3 (general inheritance model) and Fig. 1 (dominant and recessive inheritance models). Due to the low number of patients with the CC genotype for rs16892766, the genotypic OR for the CC could not be estimated and therefore the dominant model was applied.
Table 3.
Results for 15 CRC susceptibility SNPs in patients with ≥100 polyps and <100 polyps, under a codominant inheritance model
| SNP | Chromosome position | Genotype | Total (%) | ≥100 polyps (%) | Odds ratio | 95 % CI | p value Wald 1 df | p value Wald 2 df |
|---|---|---|---|---|---|---|---|---|
| rs6691170 | 1q41 | 410 (100) | 182 | 0.96 | ||||
| GG | 195 (47.6) | 86 (47.3) | 1 | |||||
| TG | 167 (40.7) | 74 (40.7) | 1.03 | 0.68–1.56 | 0.89 | |||
| TT | 48 (11.7) | 22 (12.0) | 1.01 | 0.57–2.13 | 0.78 | |||
| rs6687758 | 1q41 | 419 (100) | 188 | 0.35 | ||||
| AA | 300 (71.6) | 133 (70.7) | 1 | |||||
| GA | 104 (24.8) | 46 (24.5) | 2.42 | 0.71–1.87 | 0.56 | |||
| GG | 15 (3.6) | 9 (4.8) | 2.10 | 0.72–8.17 | 0.16 | |||
| rs10936599 | 3q26.2 | 410 (100) | 180 | 0.39 | ||||
| TT | 21 (5.1) | 10 (5.5) | 1 | |||||
| TC | 146 (35.6) | 68 (37.8) | 0.99 | 0.46–2.13 | 0.98 | |||
| CC | 243 (59.3) | 102 (56.7) | 0.76 | 0.35–1.65 | 0.49 | |||
| rs16892766 | 8q23.3 | 417 (100) | 187 | |||||
| AA | 343 (82.3) | 146 (78.1) | 1 | |||||
| CA and CCa | 74 (17.7) | 41 (21.9) | 1.71 | 1.05–2.76 | 0.03 | |||
| [CC] | [2] | [2] | ||||||
| rs6983267 | 8q24.21 | 408 (100) | 179 | 0.32 | ||||
| TT | 92 (22.5) | 45 (25.1) | 1 | |||||
| TG | 192 (47.1) | 84 (46.9) | 0.77 | 0.43–1.40 | 0.40 | |||
| GG | 124 (30.4) | 50 (27.9) | 0.64 | 0.36–1.14 | 0.13 | |||
| rs10795668 | 10p14 | 417 (100) | 187 | 0.98 | ||||
| AA | 33 (7.9) | 14 (7.5) | 1 | |||||
| GA | 193 (46.3) | 85 (45.4) | 0.99 | 0.49–1.98 | 0.97 | |||
| GG | 191 (45.8) | 88 (47.1) | 0.95 | 0.46–1.94 | 0.88 | |||
| rs3802842 | 11q23.1 | 415 (100) | 185 | 0.02 | ||||
| AA | 226 (54.5) | 91 (49.2) | 1 | |||||
| CA | 158 (38.1) | 84 (45.4) | 1.70 | 1.13–2.55 | 0.01 | |||
| CC | 31 (7.5) | 10 (5.4) | 0.76 | 0.34–1.68 | 0.49 | |||
| rs7136702 | 12q13.13 | 413 (100) | 185 | 0.65 | ||||
| CC | 175 (42.4) | 82 (44.3) | 1 | |||||
| TC | 190 (46.0) | 84 (45.4) | 0.94 | 0.60–1.46 | 0.78 | |||
| TT | 48 (11.6) | 19 (10.3) | 0.75 | 0.42–1.37 | 0.35 | |||
| rs11169552 | 12q13.13 | 415 (100) | 185 | 0.97 | ||||
| CC | 237 (57.1) | 106 (57.3) | 1 | |||||
| TC | 151 (36.4) | 68 (36.8) | 1.01 | 0.61–1.68 | 0.97 | |||
| TT | 27 (6.5) | 11 (5.9) | 0.93 | 0.45–1.93 | 0.84 | |||
| rs4444235 | 14q22.2 | 415 (100) | 184 | 0.97 | ||||
| TT | 128 (30.8) | 57 (31.0) | 1 | |||||
| CT | 215 (51.8) | 94 (51.1) | 1.01 | 0.65–1.58 | 0.96 | |||
| CC | 72 (17.3) | 33 (17.9) | 0.95 | 0.53–1.69 | 0.85 | |||
| rs4779584 | 15q13.3 | 411 (100) | 183 | 0.27 | ||||
| CC | 290 (70.6) | 123 (67.2) | 1 | |||||
| CT | 111 (27.0) | 57 (31.1) | 1.77 | 0.60–5.22 | 0.30 | |||
| TT | 10 (2.4) | 3 (1.6) | 1.28 | 0.42–3.86 | 0.67 | |||
| rs9929218 | 16q22.1 | 415 (100) | 186 | 0.66 | ||||
| AA | 34 (8.2) | 12 (6.5) | 1 | |||||
| GA | 184 (44.3) | 86 (46.2) | 1.36 | 0.68–2.73 | 0.39 | |||
| GG | 197 (47.5) | 88 (47.3) | 1.22 | 0.59–2.52 | 0.60 | |||
| rs10411210 | 19q13.11 | 418 (100) | 188 | 0.83 | ||||
| TT | 11 (2.6) | 5 (2.7) | 1 | |||||
| CT | 84 (20.1) | 33 (17.6) | 0.90 | 0.25–3.22 | 0.88 | |||
| CC | 323 (77.3) | 150 (79.8) | 1.05 | 0.32–3.46 | 0.93 | |||
| rs961253 | 20p12.3 | 412 (100) | 184 | 0.29 | ||||
| CC | 202 (49.0) | 94 (51.0) | 1 | |||||
| CA | 164 (39.8) | 75 (40.8) | 1.00 | 0.62–1.63 | 0.99 | |||
| AA | 46 (11.2) | 15 (8.2) | 0.64 | 0.35–1.16 | 0.14 | |||
| rs4925386 | 20q13.33 | 413 (100) | 182 | 0.10 | ||||
| TT | 43 (10.4) | 18 (9.9) | 1 | |||||
| TC | 171 (41.4) | 83 (45.6) | 1.15 | 0.61–2.17 | 0.66 | |||
| CC | 199 (48.2) | 81 (44.5) | 0.77 | 0.39–1.51 | 0.44 |
aDue to the low frequency, the CC genotype of rs16892766 could not be assessed; the CC and CA genotypes were combined for this SNP
Fig. 1.
Forest plot: results for 15 susceptibility SNPs in patients with ≥100 polyps and <100 polyps, under recessive and dominant inheritance models
For rs16892766, carriage of the C allele showed a trend of association with a more severe phenotype (OR 1.71, 95 % CI 1.05–2.76, p = 0.03, dominant model). At 11q23.1 (rs3802842), a borderline association was observed in the codominant inheritance model (Wald 2df p value =0.02), and when tested for the recessive and dominant models of inheritance, carriers of the risk allele of this SNP were also more frequent in the ≥100 polyp group (OR 1.51, 95 % CI 1.03–2.22, p = 0.04, dominant model). The other SNPs showed no associations.
When the joint association of the two SNPs (rs16892766 and rs3802842) was tested, both remained borderline significant using dominant mode of inheritance (p = 0.04 and p = 0.03, respectively), however the interaction of the two SNPs was not significant (p = 0.80).
When the total number of sporadic CRC risk alleles in individuals of both groups was compared, the mean number of risk alleles was similar (mean of 13.11 risk alleles for the <100 and 12.90 for the ≥100 group).
Discussion
In this study, we examined the role of CRC-associated SNPs in disease phenotype in APC mutation carriers. Although a correlation between the mutation site in the APC gene and the phenotype of FAP is well-established [2], the phenotypic variability observed in patients with the same underlying gene defect suggests that other factors must play a role in modifying disease expression in APC mutation carriers. The role of modifier genes in disease severity in FAP patients has been investigated and several modifiers, such as N-acetyl transferases, have been suggested [16–19].
In recent years, several SNPs have been identified that influence CRC risk in the general population. In this study, we investigated whether these SNPs influence the phenotype of patients carrying a pathogenic APC mutation. Two variants were found to be associated with the disease phenotype: under a dominant inheritance model, the C alleles of both rs16892766 and rs3802842 showed a trend of association with a phenotype of more than 100 adenomas.
A previous study demonstrated that individuals carrying the risk (C) allele of rs16892766 (8q23.3) present with a more advanced stage of CRC at diagnosis [20]. Tomlinson et al. found that the risk allele of rs16892766 was associated with CRC in younger individuals [4]. In other studies, the risk allele of rs16892766 correlated with an increased CRC risk and/or age of CRC diagnosis in Lynch syndrome [12–14]. In our study, the C allele of this SNP was associated with a more severe FAP phenotype (≥100 polyps) in APC mutation carriers. The higher polyp number associated with the C allele of rs16892766 could be explained by the location of this SNP in the EIF3H gene, which increases cell proliferation, growth, and survival when overexpressed. However, Carvajal-Carmona et al. [21] suggested that UTP23, rather than EIF3H, is the most likely target of the genetic variation associated with CRC in the 8q23.3 region, but also proposed that both of these genes may play a role in CRC development, given that they have related roles in mRNA translation. UTP23 is thought to be involved in ribosome biogenesis [22].
The risk allele of rs3802842 (11q23.1) has been associated with early-onset CRC (<50 years old) and a family history of CRC [20, 23]. Moreover, this SNP is also known to be associated with increased CRC risk in patients with Lynch syndrome [12–14]. A recent study described the association of rs3802842 with disease in patients with unexplained polyposis [20, 24]. In the present study, rs3802842 showed a borderline association with the more severe phenotype of ≥100 polyps in the codominant model of inheritance with two degrees of freedom. When this SNP was tested under recessive and dominant inheritance models, a trend of association was observed between risk allele carriage and the ≥100 polyp phenotype (dominant inheritance model). Functionally, rs3802842 is located within a gene-rich region of chromosome 11q23 that includes four open reading frames (ORFs) within 100 kb: COLCA1, COLCA2, POU2AF1 and C11orf53 (6). The exact function of this SNP is still unknown; one study assessed whether rs3802842 might have cis-regulatory effects on these neighbouring genes, but found no evidence for a relationship. These authors suggested that the underlying sequence change defined by this SNP might exert regulatory effects on genes mapping outside 11q23.1 [25]. Another study suggested that rs3802842 is not itself a functional SNP but is in linkage disequilibrium with a functional SNP [26].
SNPs associated with CRC susceptibility could increase CRC risk by promoting initiation of adenoma formation or promoting growth and/or progression from the adenoma to carcinoma stage, or be involved in both. Theoretically, initiation-promoting SNPs are expected to be more frequent in patients with multiple adenomas and in CRC-free patients with adenoma. A recent study found eight known CRC-associated SNPs, including rs3802842, to be overrepresented in CRC-free patients with adenoma [27]. In relation to the effect of SNPs on the above-mentioned stages, only the association of a CRC-associated SNP at 8q24.21 (rs6983267) with adenoma multiplicity and the association of rs3802842 and rs4779584 with unexplained polyposis have been described to date [6, 24]. Based on these literature reports and the outcome of our study, we hypothesize that rs3802842 is involved in the initiation stage of adenoma development.
An association between the total number of CRC-associated risk alleles and familial CRC has been suggested in two previous studies [28, 29]. Therefore, we investigated whether there was a difference in total number of risk alleles between the two groups. We found the mean number of risk alleles to be similar in the two groups.
Recently, one study examined the severity of polyposis in 64 patients and found no evidence of association in any of their tested SNPs [30], however as stated by Talseth-Palmer et al. [31] large cohorts are required to examine the role of modifiers in severity of disease phenotype in FAP patients.
In conclusion, we identified two CRC-associated SNPs, rs16892766 (8q23.3) and rs3802842 (11q23.1), which show an association with adenoma number in APC mutation carriers. In order to evaluate and confirm the effect of these SNPs on the phenotype of FAP, further studies with larger sample sizes are now recommended.
Acknowledgments
Association of International Cancer Research, Grant 2010-0619 and Dutch Cancer Society, Grant KWF-UL-2010-4656.
References
- 1.Debinski HS, Love S, Spigelman AD, Phillips RK. Colorectal polyp counts and cancer risk in familial adenomatous polyposis. Gastroenterology. 1996;110(4):1028–1030. doi: 10.1053/gast.1996.v110.pm8612989. [DOI] [PubMed] [Google Scholar]
- 2.Nieuwenhuis MH, Vasen HF. Correlations between mutation site in APC and phenotype of familial adenomatous polyposis (FAP): a review of the literature. Crit Rev Oncol Hematol. 2007;61(2):153–161. doi: 10.1016/j.critrevonc.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Watson AJ, Collins PD. Colon cancer: a civilization disorder. Dig Dis. 2011;29(2):222–228. doi: 10.1159/000323926. [DOI] [PubMed] [Google Scholar]
- 4.Tomlinson IP, Webb E, Carvajal-Carmona L, et al. A genome-wide association study identifies colorectal cancer susceptibility loci on chromosomes 10p14 and 8q23.3. Nat Genet. 2008;40(5):623–630. doi: 10.1038/ng.111. [DOI] [PubMed] [Google Scholar]
- 5.Tomlinson I, Webb E, Carvajal-Carmona L, et al. A genome-wide association scan of tag SNPs identifies a susceptibility variant for colorectal cancer at 8q24.21. Nat Genet. 2007;39(8):984–988. doi: 10.1038/ng2085. [DOI] [PubMed] [Google Scholar]
- 6.Tenesa A, Farrington SM, Prendergast JG, et al. Genome-wide association scan identifies a colorectal cancer susceptibility locus on 11q23 and replicates risk loci at 8q24 and 18q21. Nat Genet. 2008;40(5):631–637. doi: 10.1038/ng.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Study C, Houlston RS, Webb E, et al. Meta-analysis of genome-wide association data identifies four new susceptibility loci for colorectal cancer. Nat Genet. 2008;40(12):1426–1435. doi: 10.1038/ng.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jaeger E, Webb E, Howarth K, et al. Common genetic variants at the CRAC1 (HMPS) locus on chromosome 15q13.3 influence colorectal cancer risk. Nat Genet. 2008;40(1):26–28. doi: 10.1038/ng.2007.41. [DOI] [PubMed] [Google Scholar]
- 9.Houlston RS, Cheadle J, Dobbins SE, et al. Meta-analysis of three genome-wide association studies identifies susceptibility loci for colorectal cancer at 1q41, 3q26.2, 12q13.13 and 20q13.33. Nat Genet. 2010;42(11):973–977. doi: 10.1038/ng.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Broderick P, Carvajal-Carmona L, Pittman AM, et al. A genome-wide association study shows that common alleles of SMAD7 influence colorectal cancer risk. Nat Genet. 2007;39(11):1315–1317. doi: 10.1038/ng.2007.18. [DOI] [PubMed] [Google Scholar]
- 11.Hutter CM, Chang-Claude J, Slattery ML, et al. Characterization of gene-environment interactions for colorectal cancer susceptibility loci. Cancer Res. 2012;72(8):2036–2044. doi: 10.1158/0008-5472.CAN-11-4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wijnen JT, Brohet RM, van Eijk R, et al. Chromosome 8q23.3 and 11q23.1 variants modify colorectal cancer risk in Lynch syndrome. Gastroenterology. 2009;136(1):131–137. doi: 10.1053/j.gastro.2008.09.033. [DOI] [PubMed] [Google Scholar]
- 13.Talseth-Palmer BA, Scott RJ, Vasen HF, Wijnen JT. 8q23.3 and 11q23.1 as modifying loci influencing the risk for CRC in Lynch syndrome. Eur J Hum Genet. 2012;20(5):487–488. doi: 10.1038/ejhg.2011.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Talseth-Palmer BA, Brenne IS, Ashton KA, et al. Colorectal cancer susceptibility loci on chromosome 8q23.3 and 11q23.1 as modifiers for disease expression in Lynch syndrome. J Med Genet. 2011;48(4):279–284. doi: 10.1136/jmg.2010.079962. [DOI] [PubMed] [Google Scholar]
- 15.Purcell S, Neale B, Todd-Brown K, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yanaru-Fujisawa R, Matsumoto T, Kukita Y, et al. Impact of Phospholipase A2 group IIa gene polymorphism on phenotypic features of patients with familial adenomatous polyposis. Dis Colon Rectum. 2007;50(2):223–231. doi: 10.1007/s10350-006-0780-2. [DOI] [PubMed] [Google Scholar]
- 17.Houlston R, Crabtree M, Phillips R, Crabtree M, Tomlinson I. Explaining differences in the severity of familial adenomatous polyposis and the search for modifier genes. Gut. 2001;48(1):1–5. doi: 10.1136/gut.48.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crabtree MD, Fletcher C, Churchman M, et al. Analysis of candidate modifier loci for the severity of colonic familial adenomatous polyposis, with evidence for the importance of the N-acetyl transferases. Gut. 2004;53(2):271–276. doi: 10.1136/gut.2003.015586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crabtree MD, Tomlinson IP, Hodgson SV, Neale K, Phillips RK, Houlston RS. Explaining variation in familial adenomatous polyposis: relationship between genotype and phenotype and evidence for modifier genes. Gut. 2002;51(3):420–423. doi: 10.1136/gut.51.3.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abuli A, Bessa X, Gonzalez JR, et al. Susceptibility genetic variants associated with colorectal cancer risk correlate with cancer phenotype. Gastroenterology. 2010;139(3):788–796. doi: 10.1053/j.gastro.2010.05.072. [DOI] [PubMed] [Google Scholar]
- 21.Carvajal-Carmona LG, Cazier JB, Jones AM, et al. Fine-mapping of colorectal cancer susceptibility loci at 8q23.3, 16q22.1 and 19q13.11: refinement of association signals and use of in silico analysis to suggest functional variation and unexpected candidate target genes. Hum Mol Genet. 2011;20(14):2879–2888. doi: 10.1093/hmg/ddr190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bleichert F, Granneman S, Osheim YN, Beyer AL, Baserga SJ. The PINc domain protein Utp24, a putative nuclease, is required for the early cleavage steps in 18S rRNA maturation. Proc Natl Acad Sci USA. 2006;103(25):9464–9469. doi: 10.1073/pnas.0603673103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giraldez MD, Lopez-Doriga A, Bujanda L, et al. Susceptibility genetic variants associated with early-onset colorectal cancer. Carcinogenesis. 2012;33(3):613–619. doi: 10.1093/carcin/bgs009. [DOI] [PubMed] [Google Scholar]
- 24.Hes FJ, Ruano D, Nieuwenhuis M, et al. Colorectal cancer risk variants on 11q23 and 15q13 are associated with unexplained adenomatous polyposis. J Med Genet. 2014;51(1):55–60. doi: 10.1136/jmedgenet-2013-102000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pittman AM, Webb E, Carvajal-Carmona L, et al. Refinement of the basis and impact of common 11q23.1 variation to the risk of developing colorectal cancer. Hum Mol Genet. 2008;17(23):3720–3727. doi: 10.1093/hmg/ddn267. [DOI] [PubMed] [Google Scholar]
- 26.Biancolella M, Fortini BK, Tring S, et al. Identification and characterization of functional risk variants for colorectal cancer mapping to chromosome 11q23.1. Hum Mol Genet. 2014;23(8):2198–2209. doi: 10.1093/hmg/ddt584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carvajal-Carmona LG, Zauber AG, Jones AM, et al. Much of the genetic risk of colorectal cancer is likely to be mediated through susceptibility to adenomas. Gastroenterology. 2013;144(1):53–55. doi: 10.1053/j.gastro.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Middeldorp A, Jagmohan-Changur S, van Eijk R, et al. Enrichment of low penetrance susceptibility loci in a Dutch familial colorectal cancer cohort. Cancer Epidemiol Biomarkers Prev. 2009;18(11):3062–3067. doi: 10.1158/1055-9965.EPI-09-0601. [DOI] [PubMed] [Google Scholar]
- 29.Niittymaki I, Kaasinen E, Tuupanen S, et al. Low-penetrance susceptibility variants in familial colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2010;19(6):1478–1483. doi: 10.1158/1055-9965.EPI-09-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng TH, Gorman M, Martin L, et al. Common colorectal cancer risk alleles contribute to the multiple colorectal adenoma phenotype, but do not influence colonic polyposis in FAP. Eur J Hum Genet. 2015;23(2):260–263. doi: 10.1038/ejhg.2014.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Talseth-Palmer BA, Wijnen JT, Andreassen EK, et al. The importance of a large sample cohort for studies on modifier genes influencing disease severity in FAP patients. Hered Cancer Clin Pract. 2013;11(1):20. doi: 10.1186/1897-4287-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]