Abstract
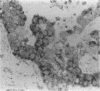
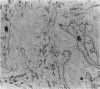
AIMS: To determine the correlation between cathepsin B expression and laminin distribution in pulmonary adenocarcinoma tissue. METHODS: The distribution of cathepsin B and laminin was examined in 28 formalin fixed, paraffin wax embedded specimens of pulmonary adenocarcinoma tissue, using a double immunostaining technique with commercially available antibodies to cathepsin B and laminin, respectively. RESULTS: Tumour cells in 23 (82%) cases reacted to cathepsin B: 13 cases were weakly positive and 10 were strongly positive. Laminin in tumour associated basement membrane produced various staining patterns: two cases had an almost continuous distribution of laminin in tumour associated basement membrane in the tumour tissues, while a moderately discontinuous laminin distribution pattern was found in 12 cases, and a highly fragmented pattern was found in 14 cases. The degree of cathepsin B expression in tumour cells was significantly correlated with the break up of laminin staining. In some cases a discontinuous pattern of tumour associated laminin was frequently observed adjacent to cathepsin B positive tumour cell nests. CONCLUSIONS: Considering that cathepsin B has the capacity to degrade basement membrane components, including laminin, the inverse correlation shown in this study between the increase in cathepsin B expression by tumour cells and the diminution of laminin in tumour associated basement membrane could reflect local progression and spread by pulmonary adenocarcinoma.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Docherty K., Carroll R. J., Steiner D. F. Conversion of proinsulin to insulin: involvement of a 31,500 molecular weight thiol protease. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4613–4617. doi: 10.1073/pnas.79.15.4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdel M., Trefz G., Spiess E., Habermaas S., Spring H., Lah T., Ebert W. Localization of cathepsin B in two human lung cancer cell lines. J Histochem Cytochem. 1990 Sep;38(9):1313–1321. doi: 10.1177/38.9.2201737. [DOI] [PubMed] [Google Scholar]
- Fligiel S. E., Rodriguez A. F., Knibbs R. N., McCoy J. P., Jr, Varani J. Characterization of laminin-stimulated adherence and motility in tumor cells. Oncology. 1985;42(4):265–271. doi: 10.1159/000226043. [DOI] [PubMed] [Google Scholar]
- GREENBAUM L. M., FRUTON J. S. Purification and properties of beef spleen cathepsin B. J Biol Chem. 1957 May;226(1):173–180. [PubMed] [Google Scholar]
- Graf M., Baici A., Sträuli P. Histochemical localization of cathepsin B at the invasion front of the rabbit V2 carcinoma. Lab Invest. 1981 Dec;45(6):587–596. [PubMed] [Google Scholar]
- Howie A. J., Burnett D., Crocker J. The distribution of cathepsin B in human tissues. J Pathol. 1985 Apr;145(4):307–314. doi: 10.1002/path.1711450404. [DOI] [PubMed] [Google Scholar]
- Katunuma N., Kominami E. Structures and functions of lysosomal thiol proteinases and their endogenous inhibitor. Curr Top Cell Regul. 1983;22:71–101. doi: 10.1016/b978-0-12-152822-5.50007-5. [DOI] [PubMed] [Google Scholar]
- Krepela E., Bártek J., Skalková D., Vicar J., Rasnick D., Taylor-Papadimitriou J., Hallowes R. C. Cytochemical and biochemical evidence of cathepsin B in malignant, transformed and normal breast epithelial cells. J Cell Sci. 1987 Feb;87(Pt 1):145–154. doi: 10.1242/jcs.87.1.145. [DOI] [PubMed] [Google Scholar]
- Köppel P., Baici A., Keist R., Matzku S., Keller R. Cathepsin B-like proteinase as a marker for metastatic tumor cell variants. Exp Cell Biol. 1984;52(5):293–299. doi: 10.1159/000163273. [DOI] [PubMed] [Google Scholar]
- Lah T. T., Buck M. R., Honn K. V., Crissman J. D., Rao N. C., Liotta L. A., Sloane B. F. Degradation of laminin by human tumor cathepsin B. Clin Exp Metastasis. 1989 Jul-Aug;7(4):461–468. doi: 10.1007/BF01753666. [DOI] [PubMed] [Google Scholar]
- Maciewicz R. A., Wardale R. J., Etherington D. J., Paraskeva C. Immunodetection of cathepsins B and L present in and secreted from human pre-malignant and malignant colorectal tumour cell lines. Int J Cancer. 1989 Mar 15;43(3):478–486. doi: 10.1002/ijc.2910430323. [DOI] [PubMed] [Google Scholar]
- Nishino T., Ishida T., Oka T., Yasumoto K., Sugimachi K. Prognostic significance of laminin in adenocarcinoma of the lung. J Surg Oncol. 1990 Apr;43(4):214–218. doi: 10.1002/jso.2930430405. [DOI] [PubMed] [Google Scholar]
- Poole A. R., Tiltman K. J., Recklies A. D., Stoker T. A. Differences in secretion of the proteinase cathepsin B at the edges of human breast carcinomas and fibroadenomas. Nature. 1978 Jun 15;273(5663):545–547. doi: 10.1038/273545a0. [DOI] [PubMed] [Google Scholar]
- Rozhin J., Robinson D., Stevens M. A., Lah T. T., Honn K. V., Ryan R. E., Sloane B. F. Properties of a plasma membrane-associated cathepsin B-like cysteine proteinase in metastatic B16 melanoma variants. Cancer Res. 1987 Dec 15;47(24 Pt 1):6620–6628. [PubMed] [Google Scholar]
- Sheahan K., Shuja S., Murnane M. J. Cysteine protease activities and tumor development in human colorectal carcinoma. Cancer Res. 1989 Jul 15;49(14):3809–3814. [PubMed] [Google Scholar]
- Shuja S., Sheahan K., Murnane M. J. Cysteine endopeptidase activity levels in normal human tissues, colorectal adenomas and carcinomas. Int J Cancer. 1991 Sep 30;49(3):341–346. doi: 10.1002/ijc.2910490305. [DOI] [PubMed] [Google Scholar]
- Sloane B. F., Honn K. V., Sadler J. G., Turner W. A., Kimpson J. J., Taylor J. D. Cathepsin B activity in B16 melanoma cells: a possible marker for metastatic potential. Cancer Res. 1982 Mar;42(3):980–986. [PubMed] [Google Scholar]
- Sloane B. F., Rozhin J., Johnson K., Taylor H., Crissman J. D., Honn K. V. Cathepsin B: association with plasma membrane in metastatic tumors. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2483–2487. doi: 10.1073/pnas.83.8.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkey J. R. Cell-matrix interactions during tumor invasion. Cancer Metastasis Rev. 1990 Sep;9(2):113–123. doi: 10.1007/BF00046338. [DOI] [PubMed] [Google Scholar]
- Watanabe M., Higashi T., Hashimoto M., Tomoda I., Tominaga S., Hashimoto N., Morimoto S., Yamauchi Y., Nakatsukasa H., Kobayashi M. Elevation of tissue cathepsin B and L activities in gastric cancer. Hepatogastroenterology. 1987 Jun;34(3):120–122. [PubMed] [Google Scholar]
- Weiss R. E., Liu B. C., Ahlering T., Dubeau L., Droller M. J. Mechanisms of human bladder tumor invasion: role of protease cathepsin B. J Urol. 1990 Sep;144(3):798–804. doi: 10.1016/s0022-5347(17)39595-2. [DOI] [PubMed] [Google Scholar]
- Zucker S., Lysik R. M., Wieman J., Wilkie D. P., Lane B. Diversity of human pancreatic cancer cell proteinases: role of cell membrane metalloproteinases in collagenolysis and cytolysis. Cancer Res. 1985 Dec;45(12 Pt 1):6168–6178. [PubMed] [Google Scholar]