Abstract
SON is a key component of the spliceosomal complex and a critical mediator of constitutive and alternative splicing. Additionally, SON has been shown to influence cell-cycle progression, genomic integrity, and maintenance of pluripotency in stem cell populations. The clear functional relevance of SON in coordinating essential cellular processes and its presence in diverse human tissues suggests that intact SON might be crucial for normal growth and development. However, the phenotypic effects of deleterious germline variants in SON have not been clearly defined. Herein, we describe seven unrelated individuals with de novo variants in SON and propose that deleterious variants in SON are associated with a severe multisystem disorder characterized by developmental delay, persistent feeding difficulties, and congenital malformations, including brain anomalies.
Main Text
Whole-exome sequencing (WES) is an essential tool in the diagnostic evaluation of individuals with suspected genetic disorders for which a genetic etiology has not been established by conventional approaches. Studies of large cohorts of individuals have demonstrated a diagnostic yield of 25%–30% when WES is applied to otherwise perplexing cases.1, 2, 3 The additional benefit of the unbiased sequencing approach of WES is the ability to ascertain genes in which variants have not been previously reported to cause disease. In our clinical WES cohort of over 6,000 unrelated individuals—the majority of whom have neurologic manifestations and are of pediatric age—we identified six individuals (subjects 1–6) with truncating variants in SON (SON DNA binding protein [MIM: 182465]) and overlapping clinical features. We analyzed parental samples by Sanger sequencing or WES and confirmed de novo status of all six variants. Subsequently, we ascertained one additional individual (subject 7) with two de novo missense variants in SON and similar features. Herein, we comprehensively phenotype all seven individuals and propose that deleterious variants in SON are associated with severe developmental outcomes.
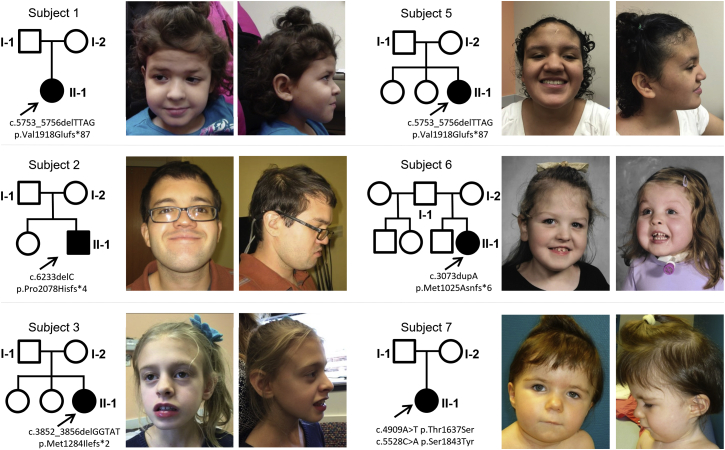
This study was performed in accordance with a protocol that was prospectively reviewed and approved by the Baylor College of Medicine Institutional Review Board. Written informed consent was obtained from all study participants. The key clinical features of our cohort are summarized in Table 1. Detailed clinical summaries for all subjects are provided in the Supplemental Data, and photographs are included in Figure 1. All subjects had dysmorphic features including, for example, mild midface retrusion with apparently deep-set eyes (n = 6), frontal bossing and bitemporal narrowing (n = 2), downslanting palpebral fissures (n = 5), and epicanthal folds (n = 3). All subjects had either a smooth or short philtrum (n = 7), and a subset had thin lips (n = 5) and/or a short mouth (n = 3). All subjects exhibited developmental delay, which appeared to progress with age into moderate to severe intellectual disability. All but one individual had additional neurological features including regression (n = 3), epilepsy or other electroencephalography (EEG) abnormalities (n = 4), autism spectrum disorder (n = 3), and hyper- or hypotonia (n = 5). Additionally, five of six subjects evaluated had abnormalities detected on brain imaging; features suggestive of volume loss specifically were seen in all five. Five subjects had congenital malformations. An atrial septal defect, ventricular septal defect, patent ductus arteriosus, left lung agenesis, single kidney, dysplastic kidney, and agenesis of the gallbladder were each seen in a single individual; several subjects had more than one malformation. All subjects had a history of feeding difficulties, which were evident as early as the neonatal period and associated with growth failure in most cases. Several subjects required a gastrostomy feeding tube. Most subjects also had ophthalmologic concerns including strabismus (n = 4) and vision loss (n = 2). Six subjects had skeletal abnormalities including joint laxity (n = 3), cervical ribs (n = 2), scoliosis (n = 1), and thumb agenesis (n = 1). Pregnancy and delivery complications were common in the cohort. Five of the seven subjects had intrauterine growth restriction, at least four had significant fetal distress requiring delivery via cesarean section, and five were born prematurely. Three subjects had a history of borderline low or frankly deficient immunoglobulin levels, and two subjects had episodes of suspected abnormal clotting, including unprovoked deep-vein thrombosis in subject 1 and a history of a right middle cerebral artery infarct and multiple transient ischemic attacks in subject 6.
Table 1.
Clinical Features of Subjects with De Novo SON Variants
| Subject 1 | Subject 2 | Subject 3 | Subject 4 | Subject 5 | Subject 6 | Subject 7 | |
|---|---|---|---|---|---|---|---|
| Current age | 6 years | 23 years | 9 years | 3 years | 15 years | 9 years | 3 years |
| Sex | female | male | female | female | female | female | female |
| Pregnancy | IUGR, placenta previa | maternal hypertension | IUGR | IUGR, maternal borderline diabetes, factor V deficiency | maternal hypertension | IUGR, oligohydramnios, pre-eclampsia, fetal anomalies | IUGR, fetal anomalies |
| Age at birth | 32 weeks | full term | full term | 33 weeks | 35 weeks | 36 weeks | 36 weeks |
| Delivery | C-section for fetal distress | C-section for fetal distress | wrapped cord, variable heart rate, failure to progress | C-section for fetal distress | C-section for maternal hypertension | vaginal delivery | C-section for fetal distress |
| Postnatal course | respiratory failure, feeding difficulties | feeding difficulties | feeding difficulties, hypoglycemia | respiratory failure, feeding difficulties | feeding difficulties, respiratory issues | feeding difficulties, respiratory issues | respiratory distress, feeding difficulties |
| Height | 2nd percentile | 40th percentile | 25th percentile | 75th percentile | 3rd percentile | −3 (Z score) | 1st percentile |
| Weight | 3rd percentile | 1st percentile | −2.29 (Z score) | 85th percentile | 12th percentile | 2nd percentile | −3 (Z score) |
| OFC | 2nd percentile | 50th percentile | −4 (Z score) | 60th percentile | 72nd percentile | 12th percentile | −2.5 (Z score) |
| Distinctive features | frontal bossing, bitemporal narrowing, epicanthal folds, thin lip, smooth philtrum | downslanting palpebral fissures, bifid uvula, submucous cleft palate, short philtrum | downslanting palpebral fissures, downturned mouth, short philtrum, thin lip, thin limbs | submucous and laryngeal cleft, frontal bossing, bitemporal narrowing, epicanthal folds, thin lip, smooth philtrum | downslanting palpebral fissures, laterally flared eyebrows, short philtrum | downslanting palpebral fissures, long face, full cheeks, short philtrum, thin lips | downslanting palpebral fissures, epicanthal folds, smooth philtrum, thin lips |
| Developmental delay | yes | yes | yes | yes | yes | yes | yes |
| Regression | yes | yes | no | yes | no | no | no |
| ASD | yes | yes | yes | NA | NA | no | no |
| Seizures | yes | yes | no (abnormal EEG) | staring spells | NA | no (abnormal EEG) | no |
| Tone | hypotonia | NA | hypotonia and spasticity | hypotonia | normal | hypotonia | hypotonia |
| Brain imaging | global volume loss, thin corpus callosum, mild periventricular gliosis | progressive ventricular and subarachnoid space dilatation, arachnoid cyst | unremarkable | periventricular leukomalacia with mild dilation of the lateral ventricle | prominent extra-axial spaces, dysgenesis of corpus callosum | evidence of prior MCA stroke, prominent ventricles | not done |
| Congenital malformation | atrial septal defect (resolved) | NA | NA | abnormal placement of carotid arteries in the neck | single kidney | dysplastic kidney, congenital lobar emphysema | VSD, PDA, agenesis of the left lung, gallbladder agenesis |
| Vision | exotropia, nystagmus | progressive vision loss, myopia, exotropia | NA | esotropia, CVI, blue sclera, segmental optic nerve hypoplasia | history of bilateral eye surgery | strabismus | no concerns |
| Hearing | PE tubes | auditory hallucination | PE tubes | PE tubes | NA | inconclusive hearing assessment | no concerns |
| Gastro-intestinal features | delayed gastric emptying, feeding difficulties | pancreatic lipase insufficiency, dysphagia | failure to thrive, chronic diarrhea, feeding difficulties | failure to thrive, G-tube feeding, diarrhea, reflux, gastric dysmotility | feeding difficulties | dysphagia, G-tube feeding | failure to thrive, G-tube recommended |
| Musculo-skeletal features | joint laxity | scoliosis, arachnodactyly, dolichostenomelia | joint laxity, cervical rib | joint laxity, cervical ribs, mild syndactyly | exaggerated lumbar lordosis | none | hemivertebrae, rib fusion, thumb agenesis, syndactyly |
| Hematologic features | DVT | NA | IgG and IgA deficiency, recurrent infection | IgA deficiency, recurrent infection | borderline IgG levels | prior MCA infarct, multiple TIAs | NA |
Abbreviations are as follows: ASD, autism spectrum disorder; C-section, cesarean section; CVI, cortical visual impairment; DVT, deep-vein thrombosis; EEG, electroencephalography; G-tube, gastrostomy tube; IgA, immunoglobulin A; IgG, immunoglobulin G; IUGR, intrauterine growth restriction; MCA, middle cerebral artery; NA, not ascertained; OFC, occipitofrontal circumference; PDA, patent ductus arteriosus; PE tubes, pressure-equalizing tubes; TIA, transient ischemic attack; and VSD, ventricular septal defect.
Figure 1.
Photographs and Pedigrees of Subjects with SON Variants
Photographs show subjects reported in this article, and pedigrees illustrate the de novo status of all detected SON variants. Shaded symbols represent affected individuals.
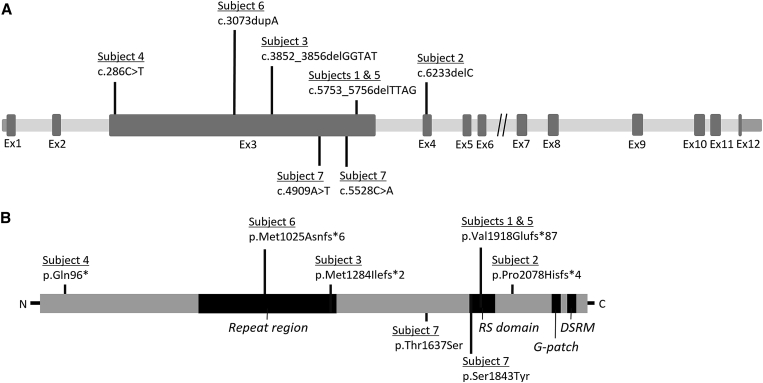
Sequencing results are summarized in Table 2. Variant nomenclature is consistent with SON transcript GenBank: NM_138927.2 (UCSC Genome Browser hg19). All truncating and missense variants were confirmed by Sanger sequencing and found to be de novo by parental testing. Subjects 1–6 had truncating variants including one premature stop variant in exon 3 (c.286C>T [p.Gln96∗]), three frameshift variants in exon 3 (c.3073dupA [p.Met1025Asnfs∗6], c.3852_3856delGGTAT [p.Met1284Ilefs∗2], and c.5753_5756delTTAG [p.Val1918Glufs∗87]), and one frameshift variant in exon 4 (c.6233delC [p.Pro2078Hisfs∗4]) (Figure 2). Of note, the c.5753_5756delTTAG (p.Val1918Glufs∗87) variant was observed in two unrelated subjects. Subject 7 had two de novo missense changes in cis configuration in exon 3 (Figure S1). SIFT and PolyPhen-2 predicted the c.4909A>T (p.Thr1637Ser) missense variant to be deleterious and benign, respectively, and the c.5528C>A (p.Ser1843Tyr) variant to be deleterious and damaging, respectively. It is unclear whether this is a complex allele or whether an individual variant contributes to the disease phenotype.
Table 2.
Putative Pathogenic Variants in SON
| Subject 1 | Subject 2 | Subject 3 | Subject 4 | Subject 5 | Subject 6 | Subject 7a | Subject 7a | |
|---|---|---|---|---|---|---|---|---|
| DNA variant | c.5753_5756delTTAG | c.6233delC | c.3852_3856delGGTAT | c.286C>T | c.5753_5756delTTAG | c.3073dupA | c.4909A>T | c.5528C>A |
| Protein change | p.Val1918Glufs∗87 | p.Pro2078Hisfs∗4 | p.Met1284Ilefs∗2 | p.Gln96∗ | p.Val1918Glufs∗87 | p.Met1025Asnfs∗6 | p.Thr1637Ser | p.Ser1843Tyr |
| Inheritance | de novo | de novo | de novo | de novo | de novo | de novo | de novo | de novo |
| ExAC Browser | novel | novel | novel | novel | novel | novel | novel | novel |
| SIFT | – | – | – | – | – | – | deleterious | deleterious |
| PolyPhen-2 | – | – | – | – | – | – | benign | damaging |
| CADD | – | – | – | – | – | – | 15.19 | 15.67 |
Variant nomenclature is based on GenBank: NM_138927.2.
These variants are in cis configuration, and both are confirmed de novo changes.
Figure 2.
Intragenic Location of SON Variants and Key Protein Functional Domains
(A) All but one of the SON variants in the described individuals localize to exon 3 of SON (GenBank: NM_138927.2).
(B) Approximate location of amino acid changes in relation to SON’s key functional domains, which include a unique central highly repetitive region, an RS-rich domain, a G-patch domain, and a double-stranded RNA-binding motif (DSRM). Data were extracted from GenBank: NP_620305.2. This panel was adapted from Hickey et al.4
SON is located in human chromosomal region 21q22.11 and consists of 12 exons.5 A striking feature of the gene’s structure is the size of exon 3, which accounts for 82% of the entire coding region (GenBank: NM_138927.2). According to the Exome Aggregation Consortium (ExAC) Browser, SON is predicted to be intolerant to loss-of-function mutations given that 49.1 loss-of-function variants are expected but only one loss-of-function variant is observed (pLI = 1.00).6 SON does not appear to be intolerant to missense variation,6 however, suggesting that cautious interpretation of the missense changes detected in subject 7 is warranted.
The canonical SON isoform (GenBank: NP_620305.2, isoform F) encoded by GenBank: NM_138927.2 is a 2,426 amino acid protein that is ubiquitously present in human tissues and highly conserved7, 8 and has an estimated 84% sequence homology between human SON and mouse Son.9 SON contains several recognizable domains implicating it as a modulator of RNA processing; these include an arginine/serine (RS)-rich domain, a G-patch domain, and a double-stranded RNA-binding motif (Figure 2).4, 9, 10 The RS domain is involved in protein-protein interactions and RNA processing.11, 12, 13, 14 Interestingly, the c.5528C>A substitution affects the serine at amino acid 1,843 within the RS region (Figure 2), thus altering the composition of a crucial functional domain of SON.
Previous analyses of murine and human cells have shown nuclear staining of SON in a stippled pattern consistent with localization to the nuclear speckle.9, 10, 15, 16 The nuclear speckle is a subcellular intranuclear compartment that is enriched with pre-mRNA splicing factors, including small nuclear ribonucleoprotein particles17 and SR protein family members known to be involved in RNA splicing.4 SON’s functional domains and its localization in the nuclear speckle suggest that it plays a role in pre-mRNA processing. Functional studies have confirmed that SON is an important mediator of both constitutive and alternative splicing4, 18 and that it is specifically involved in splicing short introns with suboptimal or weak splice sites.8, 19 Known targets of SON-mediated splicing include cell-cycle and microtubule genes, as well as genes involved in DNA repair.4, 19 Indeed, depletion of SON by RNAi leads to an array of adverse cellular consequences, including mitotic arrest, disordered spindle architecture with abnormal chromosomal alignment, aneuploidy in cells that continue to divide,16, 19 and loss of genomic integrity, as evidenced by increased double-stranded DNA breaks and micronuclei formation in cells lacking functional SON.19 In addition, the regulatory effect of SON on splicing has been shown to be essential for the maintenance of pluripotency and self-renewal in human embryonic stem cells.8
In spite of extensive work showing a critical functional role for SON in coordinating splicing and evidence that aberrant splicing contributes to human disease,20, 21 mutations in SON have not yet been definitively linked to a phenotype in humans. The first de novo truncating variant in SON was identified in a large cohort of individuals with severe intellectual disability.22 Zhu et al. later described another individual with a de novo truncating variant in SON.23 This individual had developmental delay, epilepsy, minor dysmorphic features, macrocephaly, brain white-matter abnormalities, intestinal atresia, and a ventricular septal defect. However, this individual also had a de novo missense change in a second candidate gene, C5AR1 (MIM: 113995), confounding the clinical relevance of the SON change. This published individual and the seven subjects in our cohort exhibit many of the same features, suggesting that deleterious variants in SON cause a consistent phenotype. Moreover, two of our subjects (1 and 5) share the same frameshift variant as the individual described by Zhu et al., indicating that this is a recurrent pathogenic change.
Variants in genes encoding other components of the spliceosomal machinery have been implicated in several developmental disorders, including Guion-Almeida type mandibulofacial dysostosis (MFDGA [MIM: 610536]) and Nager syndrome (MIM: 154400), among others (recently reviewed by Lehalle et al.24). MFDGA is caused by mutations in EFTUD2 (MIM: 603892), which encodes a highly conserved spliceosomal GTPase.24 The phenotype associated with MFDGA mirrors both in breadth and severity the features common to our cohort, including psychomotor delay, growth retardation, musculoskeletal anomalies, and cardiac, brain, and visceral malformations.24 Nager syndrome, which is caused by mutations in SF3B4 (MIM: 605593), is characterized by midface retrusion, downslanting palpebral fissures, and thumb anomalies.25 All of these features were present in one or more of our subjects with SON variants. This phenotypic overlap with established spliceosomal disorders confers plausibility to the hypothesis that defects in SON cause the features seen in our cohort.
Orthogonal evidence of the potential clinical relevance of SON haploinsufficiency derives from reports of individuals with copy-number variants (CNVs) involving this gene. Non-recurrent microdeletions encompassing 21q22.11, the locus harboring SON, have been extensively described in the literature.26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36 Roberson et al. performed genotype-phenotype correlations for 46 individuals with partial 21q monosomy and, consistent with other reports,27, 36 found that individuals with deletions encompassing the 21q22.11 locus manifest severe phenotypes.35 Lindstrand et al. compared 26 individuals who had partial 21q monosomy and for whom reliable molecular data were available.34 Alignment of the deleted regions and comparison of phenotypes showed a narrow 159 kb region of overlap in 21q22.11 among individuals with intellectual disability.34 This region contains only five genes and encompasses the entirety of all isoforms of SON, suggesting that loss of SON might contribute specifically to the intellectual disability in these individuals.34
To further explore the potential implications of SON copy-number loss, we queried our internal clinical database of chromosomal microarrays (n = ∼70,000 affected individuals) and identified an individual (subject 8; Table 3) with a ∼825 kb deletion encompassing SON and ten additional RefSeq genes. This individual was reported to have global developmental delay, seizures, and a congenital heart defect—features also seen in the described subjects with SON sequence variants. We then selectively reviewed published reports of phenotypically characterized individuals with <5 Mb 21q22.11 deletions that partially or completely involve SON (Figure S2)26, 27, 28, 29, 30, 31, 32, 33 and found substantial phenotypic overlap between individuals with deletions encompassing SON and the seven subjects with SON variants reported herein (Table 3). The individuals with microdeletions included both male and female probands, which is notable given the clear predominance of female subjects in our cohort. Seven of eight individuals with deletions of SON had developmental delay; all eight individuals had growth failure with short stature, seven of eight had brain anomalies, and six of eight had a history of intrauterine growth restriction and/or low birth weight. Four individuals were reported to have feeding difficulties, which required G-tube placement in three. Table 3 also includes a single individual with a small 341 kb de novo deletion reported in ClinVar (ClinVar: SCV000080160.5; dbVar: nssv577822). This individual is reported to have global developmental delay, seizures, and short stature—all features seen in our subjects with SON variants. Thus, although we cannot exclude the possibility that other genes in this region (e.g., GART [MIM: 138440], DONSON [MIM: 611428], CRYZL1 [MIM: 603920], and ITSN1 [MIM: 602442]) contribute to the phenotype in individuals with large deletions, the existing CNV data on this well-studied region strengthens the supposition that SON haploinsufficiency is in fact pathogenic.
Table 3.
SON-Associated Clinical Features in Reported Subjects with Deletions Encompassing SON
| Subjects 1–7 | Izumi et al.26 | Fukai et al.27 | Beri-Dexheimer et al.28(Patient 2) | Hoyer et al.29 | Shinawi et al.30(Patient 2) | Thevenon et al.31 | Carrascosa-Romero et al.32 | Katzaki et al.33(Patient 2) | dbVar: nssv577822 | Subject 8 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Deletion (Mb) | NA | 1.9 | 1.4 | 3.3 | 3 | 1.81 | 2.97 | 2.84 | 2.9 | 0.341 | 0.825 |
| Developmental delay | + (7/7) | NR | + | + | + | + | + | + | + | + | + |
| IUGR and/or low birth weight | + (5/6) | + | − | + | NR | + | + | + | + | NR | NR |
| Short stature | + (5/7) | + | + | + | + | + | + | + | + | + | + |
| Respiratory problems | + (5/7) | + | NR | NR | NR | NR | NR | + | NR | NR | NR |
| Feeding problems | + (7/7) | + (G-tube) | NR | NR | NR | + (G-tube) | + | + (G-tube) | NR | NR | − |
| Seizures | + (2/6) | NR | + | − | − | − | + | − | + | + | + |
| Abnormal tone | + (5/6) | + | + | + | NR | NR | + | + | NR | NR | − |
| Brain anomalies | + (5/6) | + | + | + | NR | + | + | + | + | NR | − |
| Congenital malformations | + (5/5) | heart | heart | heart | − | NR | heart | renal | − | NR | heart |
Abbreviations are as follows: G-tube, gastrostomy tube; NA, not applicable; and NR, not reported.
In summary, we have characterized a clinical phenotype associated with pathogenic variants involving SON. The similarity in phenotype between subjects with truncating variants and those with CNVs suggests that haploinsufficiency of SON could be the underlying disease mechanism. Although additional studies will be necessary to confirm the functional relevance of heterozygous loss of SON and to capture the full phenotypic spectrum, the available human data compellingly support the assertion that deleterious variants in SON are associated with a severe human phenotype.
Conflicts of Interest
The Department of Molecular and Human Genetics at the Baylor College of Medicine derives revenue from molecular genetic testing offered at the Baylor Miraca Genetics Laboratories.
Acknowledgments
We greatly appreciate the study participants and their families, without whom this work would not have been possible. We also express our gratitude to Mahshid Azamian for excellent assistance with sample collection.
Published: August 18, 2016
Footnotes
Supplemental Data include a detailed clinical history of each reported subject and two figures and can be found with this article online at http://dx.doi.org/10.1016/j.ajhg.2016.06.035.
Contributor Information
Christian P. Schaaf, Email: schaaf@bcm.edu.
Magdalena A. Walkiewicz, Email: mwalkiew@bcm.edu.
Accession Numbers
The accession numbers for the truncating variants reported in this paper are ClinVar: SCV000297718, SCV000297719, SCV000297720, SCV000297721, and SCV000297722.
Web Resources
ExAC Browser, http://exac.broadinstitute.org/
OMIM, http://www.omim.org/
UCSC Genome Browser, https://genome.ucsc.edu/
Supplemental Data
References
- 1.Yang Y., Muzny D.M., Xia F., Niu Z., Person R., Ding Y., Ward P., Braxton A., Wang M., Buhay C. Molecular findings among patients referred for clinical whole-exome sequencing. JAMA. 2014;312:1870–1879. doi: 10.1001/jama.2014.14601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee H., Deignan J.L., Dorrani N., Strom S.P., Kantarci S., Quintero-Rivera F., Das K., Toy T., Harry B., Yourshaw M. Clinical exome sequencing for genetic identification of rare Mendelian disorders. JAMA. 2014;312:1880–1887. doi: 10.1001/jama.2014.14604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sawyer S.L., Hartley T., Dyment D.A., Beaulieu C.L., Schwartzentruber J., Smith A., Bedford H.M., Bernard G., Bernier F.P., Brais B., FORGE Canada Consortium. Care4Rare Canada Consortium Utility of whole-exome sequencing for those near the end of the diagnostic odyssey: time to address gaps in care. Clin. Genet. 2016;89:275–284. doi: 10.1111/cge.12654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hickey C.J., Kim J.H., Ahn E.Y. New discoveries of old SON: a link between RNA splicing and cancer. J. Cell. Biochem. 2014;115:224–231. doi: 10.1002/jcb.24672. [DOI] [PubMed] [Google Scholar]
- 5.Khan I.M., Fisher R.A., Johnson K.J., Bailey M.E., Siciliano M.J., Kessling A.M., Farrer M., Carritt B., Kamalati T., Buluwela L. The SON gene encodes a conserved DNA binding protein mapping to human chromosome 21. Ann. Hum. Genet. 1994;58:25–34. doi: 10.1111/j.1469-1809.1994.tb00723.x. [DOI] [PubMed] [Google Scholar]
- 6.Lek M., Karczewski K., Minikel E., Samocha K., Banks E., Fennell T., O’Donnell-Luria A., Ware J., Hill A., Cummings B. Analysis of protein-coding genetic variation in 60,706 humans. bioRxiv. 2015 doi: 10.1038/nature19057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mattioni T., Hume C.R., Konigorski S., Hayes P., Osterweil Z., Lee J.S. A cDNA clone for a novel nuclear protein with DNA binding activity. Chromosoma. 1992;101:618–624. doi: 10.1007/BF00360539. [DOI] [PubMed] [Google Scholar]
- 8.Lu X., Göke J., Sachs F., Jacques P.E., Liang H., Feng B., Bourque G., Bubulya P.A., Ng H.H. SON connects the splicing-regulatory network with pluripotency in human embryonic stem cells. Nat. Cell Biol. 2013;15:1141–1152. doi: 10.1038/ncb2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wynn S.L., Fisher R.A., Pagel C., Price M., Liu Q.Y., Khan I.M., Zammit P., Dadrah K., Mazrani W., Kessling A. Organization and conservation of the GART/SON/DONSON locus in mouse and human genomes. Genomics. 2000;68:57–62. doi: 10.1006/geno.2000.6254. [DOI] [PubMed] [Google Scholar]
- 10.Saitoh N., Spahr C.S., Patterson S.D., Bubulya P., Neuwald A.F., Spector D.L. Proteomic analysis of interchromatin granule clusters. Mol. Biol. Cell. 2004;15:3876–3890. doi: 10.1091/mbc.E04-03-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Birney E., Kumar S., Krainer A.R. Analysis of the RNA-recognition motif and RS and RGG domains: conservation in metazoan pre-mRNA splicing factors. Nucleic Acids Res. 1993;21:5803–5816. doi: 10.1093/nar/21.25.5803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graveley B.R. Sorting out the complexity of SR protein functions. RNA. 2000;6:1197–1211. doi: 10.1017/s1355838200000960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sacco-Bubulya P., Spector D.L. Disassembly of interchromatin granule clusters alters the coordination of transcription and pre-mRNA splicing. J. Cell Biol. 2002;156:425–436. doi: 10.1083/jcb.200107017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhong X.Y., Wang P., Han J., Rosenfeld M.G., Fu X.D. SR proteins in vertical integration of gene expression from transcription to RNA processing to translation. Mol. Cell. 2009;35:1–10. doi: 10.1016/j.molcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma A., Markey M., Torres-Muñoz K., Varia S., Kadakia M., Bubulya A., Bubulya P.A. Son maintains accurate splicing for a subset of human pre-mRNAs. J. Cell Sci. 2011;124:4286–4298. doi: 10.1242/jcs.092239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huen M.S., Sy S.M., Leung K.M., Ching Y.P., Tipoe G.L., Man C., Dong S., Chen J. SON is a spliceosome-associated factor required for mitotic progression. Cell Cycle. 2010;9:2679–2685. doi: 10.4161/cc.9.13.12151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spector D.L., Lamond A.I. Nuclear speckles. Cold Spring Harb. Perspect. Biol. 2011;3 doi: 10.1101/cshperspect.a000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu X., Ng H.H., Bubulya P.A. The role of SON in splicing, development, and disease. Wiley Interdiscip. Rev. RNA. 2014;5:637–646. doi: 10.1002/wrna.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahn E.Y., DeKelver R.C., Lo M.C., Nguyen T.A., Matsuura S., Boyapati A., Pandit S., Fu X.D., Zhang D.E. SON controls cell-cycle progression by coordinated regulation of RNA splicing. Mol. Cell. 2011;42:185–198. doi: 10.1016/j.molcel.2011.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang G.S., Cooper T.A. Splicing in disease: disruption of the splicing code and the decoding machinery. Nat. Rev. Genet. 2007;8:749–761. doi: 10.1038/nrg2164. [DOI] [PubMed] [Google Scholar]
- 21.Padgett R.A. New connections between splicing and human disease. Trends Genet. 2012;28:147–154. doi: 10.1016/j.tig.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gilissen C., Hehir-Kwa J.Y., Thung D.T., van de Vorst M., van Bon B.W., Willemsen M.H., Kwint M., Janssen I.M., Hoischen A., Schenck A. Genome sequencing identifies major causes of severe intellectual disability. Nature. 2014;511:344–347. doi: 10.1038/nature13394. [DOI] [PubMed] [Google Scholar]
- 23.Zhu X., Petrovski S., Xie P., Ruzzo E.K., Lu Y.F., McSweeney K.M., Ben-Zeev B., Nissenkorn A., Anikster Y., Oz-Levi D. Whole-exome sequencing in undiagnosed genetic diseases: interpreting 119 trios. Genet. Med. 2015;17:774–781. doi: 10.1038/gim.2014.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lehalle D., Wieczorek D., Zechi-Ceide R.M., Passos-Bueno M.R., Lyonnet S., Amiel J., Gordon C.T. A review of craniofacial disorders caused by spliceosomal defects. Clin. Genet. 2015;88:405–415. doi: 10.1111/cge.12596. [DOI] [PubMed] [Google Scholar]
- 25.Bernier F.P., Caluseriu O., Ng S., Schwartzentruber J., Buckingham K.J., Innes A.M., Jabs E.W., Innis J.W., Schuette J.L., Gorski J.L., FORGE Canada Consortium Haploinsufficiency of SF3B4, a component of the pre-mRNA spliceosomal complex, causes Nager syndrome. Am. J. Hum. Genet. 2012;90:925–933. doi: 10.1016/j.ajhg.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Izumi K., Brooks S.S., Feret H.A., Zackai E.H. 1.9 Mb microdeletion of 21q22.11 within Braddock-Carey contiguous gene deletion syndrome region: dissecting the phenotype. Am. J. Med. Genet. A. 2012;158A:1535–1541. doi: 10.1002/ajmg.a.35368. [DOI] [PubMed] [Google Scholar]
- 27.Fukai R., Hiraki Y., Nishimura G., Nakashima M., Tsurusaki Y., Saitsu H., Matsumoto N., Miyake N. A de novo 1.4-Mb deletion at 21q22.11 in a boy with developmental delay. Am. J. Med. Genet. A. 2014;164A:1021–1028. doi: 10.1002/ajmg.a.36377. [DOI] [PubMed] [Google Scholar]
- 28.Béri-Dexheimer M., Latger-Cannard V., Philippe C., Bonnet C., Chambon P., Roth V., Grégoire M.J., Bordigoni P., Lecompte T., Leheup B., Jonveaux P. Clinical phenotype of germline RUNX1 haploinsufficiency: from point mutations to large genomic deletions. Eur. J. Hum. Genet. 2008;16:1014–1018. doi: 10.1038/ejhg.2008.89. [DOI] [PubMed] [Google Scholar]
- 29.Hoyer J., Dreweke A., Becker C., Göhring I., Thiel C.T., Peippo M.M., Rauch R., Hofbeck M., Trautmann U., Zweier C. Molecular karyotyping in patients with mental retardation using 100K single-nucleotide polymorphism arrays. J. Med. Genet. 2007;44:629–636. doi: 10.1136/jmg.2007.050914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shinawi M., Erez A., Shardy D.L., Lee B., Naeem R., Weissenberger G., Chinault A.C., Cheung S.W., Plon S.E. Syndromic thrombocytopenia and predisposition to acute myelogenous leukemia caused by constitutional microdeletions on chromosome 21q. Blood. 2008;112:1042–1047. doi: 10.1182/blood-2008-01-135970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thevenon J., Callier P., Thauvin-Robinet C., Mejean N., Falcon-Eicher S., Maynadie M., de Maistre E., Bidot S., Huet F., Beri-Dexheimer M. De Novo 21q22.1q22.2 deletion including RUNX1 mimicking a congenital infection. Am. J. Med. Genet. A. 2011;155A:126–129. doi: 10.1002/ajmg.a.33809. [DOI] [PubMed] [Google Scholar]
- 32.Carrascosa-Romero M.C., Suela J., Pardal-Fernández J.M., Bermejo-Sánchez E., Vidal-Company A., MacDonald A., Tébar-Gil R., Martínez-Fernández M.L., Martínez-Frías M.L. A 2.84 Mb deletion at 21q22.11 in a patient clinically diagnosed with Marden-Walker syndrome. Am. J. Med. Genet. A. 2013;161A:2281–2290. doi: 10.1002/ajmg.a.35862. [DOI] [PubMed] [Google Scholar]
- 33.Katzaki E., Morin G., Pollazzon M., Papa F.T., Buoni S., Hayek J., Andrieux J., Lecerf L., Popovici C., Receveur A. Syndromic mental retardation with thrombocytopenia due to 21q22.11q22.12 deletion: Report of three patients. Am. J. Med. Genet. A. 2010;152A:1711–1717. doi: 10.1002/ajmg.a.33478. [DOI] [PubMed] [Google Scholar]
- 34.Lindstrand A., Malmgren H., Sahlén S., Schoumans J., Nordgren A., Ergander U., Holm E., Anderlid B.M., Blennow E. Detailed molecular and clinical characterization of three patients with 21q deletions. Clin. Genet. 2010;77:145–154. doi: 10.1111/j.1399-0004.2009.01289.x. [DOI] [PubMed] [Google Scholar]
- 35.Roberson E.D., Wohler E.S., Hoover-Fong J.E., Lisi E., Stevens E.L., Thomas G.H., Leonard J., Hamosh A., Pevsner J. Genomic analysis of partial 21q monosomies with variable phenotypes. Eur. J. Hum. Genet. 2011;19:235–238. doi: 10.1038/ejhg.2010.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lyle R., Béna F., Gagos S., Gehrig C., Lopez G., Schinzel A., Lespinasse J., Bottani A., Dahoun S., Taine L. Genotype-phenotype correlations in Down syndrome identified by array CGH in 30 cases of partial trisomy and partial monosomy chromosome 21. Eur. J. Hum. Genet. 2009;17:454–466. doi: 10.1038/ejhg.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.