Abstract
Background
Insects are well known vectors of human and animal pathogens and millions of people are killed by mosquito-borne diseases every year. The use of insecticides to target insect vectors has been hampered by the issues of toxicity to the environment and by the selection of resistant insects. Therefore, biocontrol strategies based on naturally occurring microbial pathogens emerged as a promising control alternative. The entomopathogenic fungus Beauveria bassiana is well characterized and have been approved by the United States Environmental Protection Agency as a pest biological control method. However, thousands of other fungi are unexploited and it is important to identify and use different fungi for biocontrol with possibly some vector specific strains. The aim of this study was to identify new fungal entomopathogens that may be used as potential mosquito biocontrol agents.
Methods
Cadavers of arthropods were collected from pesticide free areas and the fungi associated isolated, cultured and identified. Then the ability of each isolate to kill laboratory insects was assayed and compared to that of B. bassiana.
Results
In total we have isolated and identified 42 fungal strains from 17 different arthropod cadavers. Twenty four fungal isolates were cultivated in the laboratory and were able to induce sporulation. When fungal spores were microinjected into Drosophila melanogaster, eight isolates proved to be highly pathogenic while the remaining strains showed moderate or no pathogenicity. Then a selection of isolates was tested against Aedes mosquitoes in a model mimicking natural infections. Only one fungus (Aspergillus nomius) was as pathogenic as B. bassiana and able to kill 100 % of the mosquitoes.
Conclusion
The obtained results are encouraging and demonstrate the feasibility of this simple approach for the identification of new potential mosquito killers. Indeed, it is essential to anticipate and prepare biocontrol methods to fight the expansion of mosquitoes’ habitat predicted in certain geographical areas in association with the occurring climatic changes.
Electronic supplementary material
The online version of this article (doi:10.1186/s13071-016-1763-3) contains supplementary material, which is available to authorized users.
Keywords: Fungi, Entomopathogen, Vector control, Beauveria bassiana, Drosophila, Aedes, Infection
Background
Insects are an essential component of all ecosystems. However, they can be detrimental to crop production and more dramatically many insects are disease-vectors for plants (herbivores) and for animals (blood-feeding). Mosquitoes for instance, are vectors of several deadly human diseases like malaria, dengue, chikungunya and more recently emerging Zika [1–4]. Billions of human lives are threatened by mosquito-borne diseases especially in tropical and sub-tropical zones. In Lebanon, several mosquito species are present, some of which are known to be vectors of disease including mosquitoes of the Culex group and the Asian tiger mosquito Aedes albopictus [5]. Climate warming may lead to the spread of mosquito-borne diseases in the near future. Indeed, A. albopictus occurrence was reported for the first time in Lebanon about 10 years ago and its population size and geographical distribution has considerably increased since then. Therefore several factors place Mediterranean countries at an increased risk of epidemics [6]. The recent reports of patients infected with chikungunya virus (spread by Aedes mosquitoes) in the south of France are an example [7]. The frequent travel and massive mobility of people associated with modern life and the presence of endogenous mosquito vectors places some areas such as the Mediterranean countries at high risk of epidemics.
The use of insecticides to target mosquitoes has been hampered by the issues of environmental contamination and risks for human health and by the emergence of resistance problems [8]. Therefore, biocontrol strategies are desirable, and the use of the endosymbiotic bacterium Wolbachia has been proposed as a possibility [9–12]. Also, control strategies based on naturally occurring microbial pathogens emerged as another promising alternative to control insects. Fungi are the most common and the most studied cause of insect disease in nature and approximately 1000 fungal species are reported to kill insects, aphids, mites etc. [13]. Spores of the fungus Beauveria bassiana have been approved by the United States Environmental Protection Agency as a pest biological control method.
Several laboratory studies have shown that insects are sensitive to infections with B. bassiana and this fungus is commonly used to study insect immunity particularly in the model organism Drosophila melanogaster [14]. The genetic dissection of the regulation of the immune response in Drosophila and in mosquitoes has made a breakthrough in the understanding of the innate immune system in Diptera but also in mammals [15]. Insects depend only on innate defences to fight pathogens: the process is initiated when microbial determinant called Pathogen-Associated Molecular Patterns (PAMPs) are recognized by the host Pattern Recognition Receptors (PRRs) leading to the activation of genetic pathways and triggering effector responses. These studies have shown that despite the fact that these immune reactions are innate, they are adapted to the type of invading pathogen [14]. Anti-fungal responses depend on the detection of fungal cell wall components by the PRR GNBP3 and on a parallel pathway involving the serine protease Persephone that needs to be processed by secreted fungal proteases to activate the Toll pathway [16]. Although entomopathogenic fungi are widespread within the Eumycetes, the focus has been put on Ascomycota and in particular the family of the Chordicipitaceae with its two representative genera Metharyzium and Beauveria [17–19]. Therefore, most of the data collected in Drosophila rely on challenge with B. bassiana as a fungal model, and infections with other entomopathogenic fungi may be needed to identify additional host response molecules.
Since both Metharizium and Beauveria are well characterized, the presence of their spores on the cuticle often serves as a visual indicator during the collection of insect cadavers. Hence, many studies report isolates of already known species of both fungi and most of them are stocked in the ARSEF collection of the US Department of Agriculture (USDA) [20]. An example is the isolation of a Beauveria species in an attempt aiming to identify a natural killer of the invasive sawfly Cephalcia tannourinensis which infests Cedar trees in Lebanon [21]. In the present study, the aim was to identify new fungal entomopathogens that may be used as potential mosquito biocontrol agent.
Methods
Drosophila and mosquito strains
Drosophila melanogaster W1118 strain was used in infection experiments as wild-type flies. Stocks were reared in 50 ml vials containing standard cornmeal agar food prepared according to the Drosophila Bloomington Stock Center recipe. Flies were kept at 24 °C, 45 % relative humidity on a 12 h light/dark photocycle. Aedes albopictus (Sarba strain) a local mosquito strain was reared in the insectary at 28 °C, 70 % relative humidity on a 12 h light/dark photocycle. Mosquito cages were supplemented with a cup of tap water and a cotton pad soaked in 10 % sucrose. Eggs were collected 4 days after a blood meal and allowed to air dry for 2 weeks before hatching. Dried eggs were hatched by immersion into deoxygenated water. Larvae were reared in pans containing tap water and fed on beer-brewing yeast for the first day after hatching then on fish pellets till pupation.
Fungus strain
Beauveria bassiana strain 80.2 (a gift from Dominique Ferrandon) was used as a control in all survival experiments.
Arthropod cadaver collection
Two series of dead animals were analyzed: the first series was collected in July 2014 from the American University of Beirut campus; the temperature range was 27–32 °C and relative humidity of 70–80 %. The second series was obtained in May 2015 from Nabatieh area (south of Lebanon); the temperature range was 20–26 °C and humidity around 70 %. Areas where insecticides may have been used were avoided and cadavers in the vicinity of spider nets or incandescent lights were also disregarded.
Fungus isolation
Carcasses were suspended in water containing 5 % Tween and shaken vigorously to resuspend spores or mycelium fragments present on the cuticle surface. Ten μl of different dilutions of this suspension was plated on standard PDA/chloramphenicol medium. After one or two days incubation at 27 °C, individual germinations (or mycelium regeneration) were transferred to a new plate. For each insect, only one isolate per group of morphologically identical thalli was selected. These isolates were submitted to several rounds of purification in order to follow morphological stability after the successive transfers. Conidial species such as Aspergillus spp. and Penicillium spp. were submitted to single spore purification.
DNA extraction and sequencing
Fungal isolates were grown on cellophane/PDA for two to four days at 27 °C. DNA was extracted as in [22]. ITS sequences were PCR amplified with the following universal primers: ITS1 (5'- TCC GTA GGT GAA CCT GCG G-3') and ITS4 (5'-TCC TCC GCT TAT TGA TAT GC-3'). Amplification and direct sequencing of fungal ribosomal RNA genes was as in [23]. Sequences (Additional file 1: Table S1) were blasted against NCBI GenBank for identification purposes [24].
Spore purification
Fungal spores were extracted from four week-old PDA plates by adding 25 ml sterile distilled water to each plate and scrapping the surface. A sterile funnel containing autoclaved glass wool was used to separate the spores from other mycelia structures. The collected spore suspension was centrifuged at 4000× g and washed three times with distilled water and finally resuspended in 0.5 ml water. Spores were then counted using a hemocytometer and diluted to the desired concentration. Freshly prepared fungal spore solutions were used for all Drosophila and mosquito challenges.
Infection and survival assays
Survival experiments were performed on batches of 15 wild-type flies or 20 mosquitoes. In all experiments, 3 to 7 day-old females were used. Mosquitoes were only sugar-fed (no blood meal). Two types of infection were performed: microinjections and infections by spraying. For microinjections, flies were anesthetized on a CO2 flow bed and 32 nl of water containing 100 fungal spores were injected into the thorax using a NanodropII microinjector (Drummond Scientific, California, USA). For infections by spraying, a suspension of 50 × 106 spores/ml was sprayed on anesthetized mosquitoes. Vials (for Drosophila melanogaster) or cups (for Aedes spp.) containing the challenged animals were then put in an incubator at 29 °C and the surviving flies counted every few hours. Flies that died within the first 2 h after injection were disregarded since their death is considered to be due to the needle injury. Each experiment was repeated at least three independent times, and a representative result is shown.
Statistical analysis
For statistical analysis of the survival data, Gehan-Breslow-Wilcoxon test was performed. Results with a P-value of less than 0.05 were considered as significant. Detailed analysis report is provided in Additional file 2: Table S2.
Results
Dead arthropod collection and identification
Dead arthropod identification was based on morphological criteria and determined to lowest taxonomic rank possible. Depending on the preservation of the specimen, the size of its group and the presence of distinctive features, variable rank levels could be determined with confidence for different animals. Dead arthropods 1 to 6 were collected in Beirut, and cadavers 7 to 17 were sampled from a more rural area in the south of Lebanon. Specimens collected were from the orders Coleoptera, Lepidoptera, Hemiptera, Hymenoptera, Thysanura, Isopoda, Aranea, Polydesmida and Diptera (see Table 1 and Additional file 3: Figure S1).
Table 1.
List of the collected dead arthropods and the corresponding fungi. Fungus # refers to the arthropod it was isolated from and letters correspond to different fungal isolates. Arthropod order is given in parentheses. The last column summarizes the results of spore microinjection: + denotes a pathogenic fungus (killing Drosophila with no statistically significant difference than B. bassiana, P > 0.05); − denotes a mildly pathogenic or non-pathogenic fungus (killing at a statistically significant different rate compared to B. bassiana, P < 0.05)
| Fungus # | Fungus species | Carrier arthropod | Pathogenicity |
|---|---|---|---|
| 1a | Aspergillus ustus | Buprestidae (Coleoptera) | + |
| 1b | Aspergillus candidus | Buprestidae (Coleoptera) | – |
| 1c | Aspergillus sclerotium | Buprestidae (Coleoptera) | + |
| 1d | Aspergillus candidus | Buprestidae (Coleoptera) | – |
| 1e | Aspergillus nomius | Buprestidae (Coleoptera) | + |
| 1f | Aspergillus sclerotium | Buprestidae (Coleoptera) | + |
| 2a | Wallemia sp. | Culex sp. Culicidae (Diptera) | + |
| 3a | Aspergillus sclerotium | Curculionidae (Coleoptera) | nt |
| 3b | Scopulariopsis brevicaulis | Curculionidae (Coleoptera) | – |
| 3c | Aspergillus sclerotium | Curculionidae (Coleoptera) | nt |
| 4a | Aspergillus fumigatus | Dermestidae (Coleoptera) | nt |
| 4b | Aspergillus ruber | Dermestidae (Coleoptera) | nt |
| 4c | Aspergillus ruber | Dermestidae (Coleoptera) | + |
| 4d | Aspergillus glaucus | Dermestidae (Coleoptera) | – |
| 5a | Chaetomium globosum | Lepismatidae (Thysanura) | – |
| 6a | Pyrenophora dictyoides | Miridae (Hemiptera) | nt |
| 6b | Fusarim tricinctum | Miridae (Hemiptera) | nt |
| 7a | Botrytis cinerea | Apis mellifera, Apidae (Hymenoptera) | nt |
| 7b | Alternaria alternata | Apis mellifera, Apidae (Hymenoptera) | + |
| 7c | Fomes fomentarius | Apis mellifera, Apidae (Hymenoptera) | – |
| 8a | Talaromyces amestolkiae | Pyrrhocoridae (Hemiptera) | nt |
| 8b | Cladosporium cladosporioides | Pyrrhocoridae (Hemiptera) | – |
| 8c | Stachybotrys chartarum | Pyrrhocoridae (Hemiptera) | nt |
| 8d | Ascomycota sp. | Pyrrhocoridae (Hemiptera) | nt |
| 9a | Alternaria infectoria | Armadillidium vulgare, Armadillidae (Isopoda) | – |
| 9b | Cladosporium cladosporioides | Armadillidium vulgare, Armadillidae (Isopoda) | nt |
| 9c | Simplicillium sympodiophorum | Armadillidium vulgare, Armadillidae (Isopoda) | nt |
| 10a | Penicillium digitatum | Polydesmidae (Polydesmida) | – |
| 10b | Periconia sp. | Polydesmidae (Polydesmida) | – |
| 11a | Penicillium freii | Pyralidae (Lepidoptera) | – |
| 11b | Talaromyces amestolkiae | Pyralidae (Lepidoptera) | nt |
| 12a | Chaetomium nigricolor | Aphodius sp. Scarabaeidae (Coleoptera) | nt |
| 12b | Chaetomium bostrychodes | Aphodius sp. Scarabaeidae (Coleoptera) | nt |
| 12c | Engyodontium album | Aphodius sp. Scarabaeidae (Coleoptera) | – |
| 13a | Penicillim commune | Araneidae (Araneae) | + |
| 13b | Phoma herbarum | Araneidae (Araneae) | – |
| 14a | Alternaria infectoria | Sarcophagidae (Diptera) | nt |
| 14b | Botrytis cinerea | Sarcophagidae (Diptera) | nt |
| 15a | Embellisia abundans | Araneidae (Araneae) | – |
| 16a | Talaromyces amestolkiae | Capnodis tenebrionis, Buprestidae (Coleoptera) | nt |
| 17a | Penicillium polonicum | Culex sp., Culicidae (Diptera) | – |
| 17b | Talaromyces amestolkiae | Culex sp., Culicidae (Diptera) | – |
Abbreviation: nt not tested
Isolation and identification of fungi from cadavers
In total, from 17 different dead animals, 130 fungal germinations were isolated and purified on PDA plates. The precise identity of the fungal species isolated from dead arthropods was determined by sequencing PCR-amplified Internal Transcribed Spacers (ITSs) and comparing the results to the GenBank database. The list of insects collected and the corresponding fungi is reported in Table 1. Obtained ITS sequences have been deposited in the GenBank database (accession numbers KX394525–KX394566). In a first step, fungi were clustered according to the morphology of their mycelium. Two morphologies were overrepresented and present on several cadavers. The decision was made to sequence one isolate per cadaver for the overrepresented fungi. The genus Cladosporium represented 46 isolates and was found on 12 cadavers (Additional file 1: Table S1). The two other most represented genera were Penicillium and Talaromyces, two very close genera belonging to the order Eurotiales. Talaromyces was isolated 20 times and from four different arthropods. One isolate per insect was sequenced and only one species, Talaromyces amestolkiae, was identified. Four morphological groups of Penicillium were identified; sequencing revealed that they belong to four different species. Penicillium commune was isolated from seven cadavers, P. digitatum and P. frei from two dead animals each. Except for the above mentioned genera for which a selection has been made in order to avoid unnecessary multiple sequencing of the same species, ITSs were PCR-amplified and sequenced from all of the other isolated and purified fungi. All the fungi that were isolated belong to the Dikaria group. Two isolates were basidiomycetes, Fomes fomentarius and Wallemia sp. The remaining species were ascomycete fungi belonging to the most prevalent phyla Dothideomycetes, Eurotiomycetes, Leotiomycetes and Sordariomycetes. Many of the isolated fungi were saprophytes, others had a life style depending on plants. Interestingly, two fungi, Simplicillium sympodiophorum isolated from dead woodlice Armadillidium vulgare, and Engyodontium album isolated from Aphodius sp. (Coleoptera) belong to the Cordycipitaceae, a family comprising the genera of the best studied entomopathogens Metharizium, Chordyceps and Beauveria.
Spore microinjection into Drosophila and survival analysis
After identification, a total of 24 fungal isolates were grown in the laboratory and were able to induce sporulation. Spores were collected, washed, counted and microinjected into wild-type Drosophila to determine the pathogenic potential of each isolate. In parallel, for each experiment, the same number of spores obtained from the well characterized entomopathogen B. bassiana was microinjected as a reference. Fungi that significantly differed from B. bassiana in the rate at which they kill the flies (P < 0.05) were considered negatives; these were 16 isolates corresponding to Aspergillus candidus (2 isolates), Scopulariopsis brevicaulis, Aspergillus glaucus, Chaetomium globosum, Fomes fomentarius, Cladosporium cladosporioides, Alternaria infectoria, Penicillium digitatum, Periconia sp., Penicillium freii, Engyodontium album, Phoma herbarum, Embellisia abundans, Penicillium polonicum, Talaromyces amestolkiae. Among these isolates 13 did not kill more than 25 % of the injected flies while two isolates (P. herbarum and P. polonicum) killed about 30 % and one isolate (A. candidus) killed about 50 %. The isolates that killed with a rate that is not statistically different than that observed with B. bassiana (P > 0.05) were considered positives. Eight fungi correspond to this category: Aspergillus ustus, Aspergillus sclerotium (2 distinct isolates tested), Aspergillus nomius, Wallemia sp., Aspergillus ruber, Alternaria alternata and Penicillium commune. Among these, five isolates (A. ustus, Wallemia sp. A. ruber, A. alternata and P. commune) killed between 50 and 75 % of the injected animals, while only three isolates (A. nomius and A. sclerotium) were able to kill 100 % of the injected flies. It was noted that A. nomius was the only fungus that was able to kill injected Drosophila at an even faster rate than B. bassiana. These results are shown in Fig. 1 and summarized in Table 1.
Fig. 1.
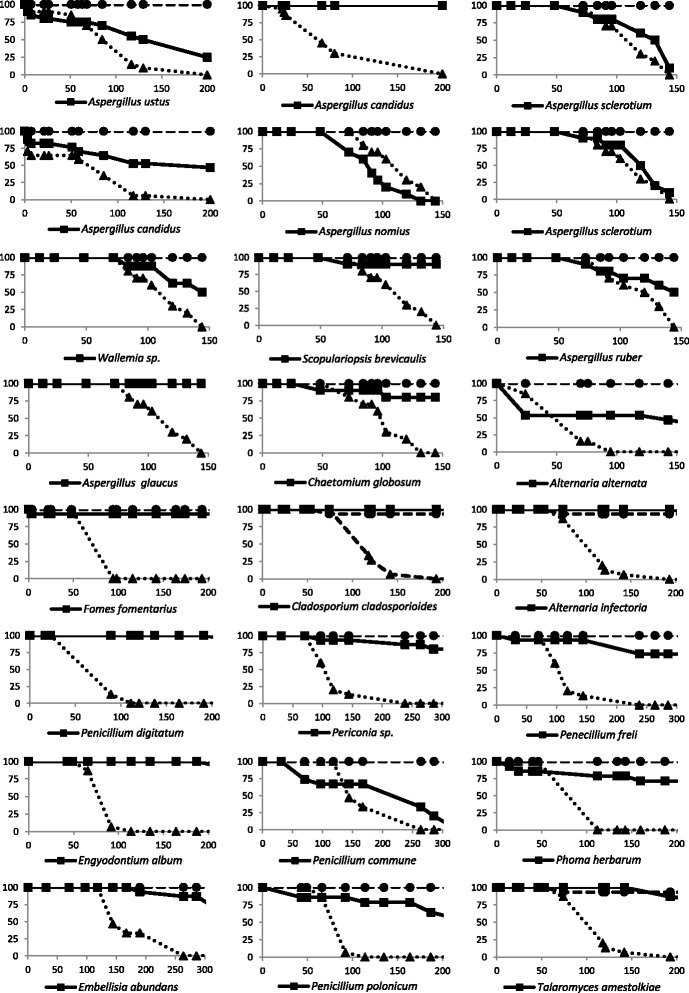
Drosophila susceptibility to the microinjection of spores obtained from the different isolated fungi. Survival of Drosophila following microinjection of fungal spores (plain line with squares) is shown as percentage of flies alive plotted versus time in hours. In each experiment flies microinjected with the same number of B. bassiana spores were used as a reference (dotted line with triangles). In parallel flies microinjected with water are included as control (dashed line with circles). Seven fungi (A. ustus, A. sclerotium, A. nomius, Wallemia sp., A. ruber, A. alternata and P. commune) showed pathogenicity levels that were not statistically different compared to those triggered by B. bassiana (P > 0.05)
Spore microinjection into Aedes spp. and survival analysis
Based on these results, a subset of the fungal isolates (including the 8 that were considered positive and four of the isolates that were not highly pathogenic to Drosophila) was used to microinject Aedes spp. mosquitoes under similar conditions. Aspergillus nomius, A. sclerotium (2 isolates) and A. ruber showed pathogenicity levels that were not statistically different compared to those triggered by B. bassiana (P > 0.05) corroborating the results obtained using Drosophila. Indeed, A. ruber killed about 75 % of injected mosquitoes and A. nomius, A. sclerotium led to a 100 % lethality in Aedes spp. (Fig. 2). On the other hand, Periconia sp., P. herbarum, P. polonicum and T. amestolkiae were not highly pathogenic to mosquitoes in agreement with what has been observed in Fig. 1. However, although A. ustus, Wallemia sp., A. alternata and P. commune injections led to the death of some injected mosquitoes, these isolates were not as pathogenic for Aedes spp. as they were for Drosophila (Fig. 2).
Fig. 2.
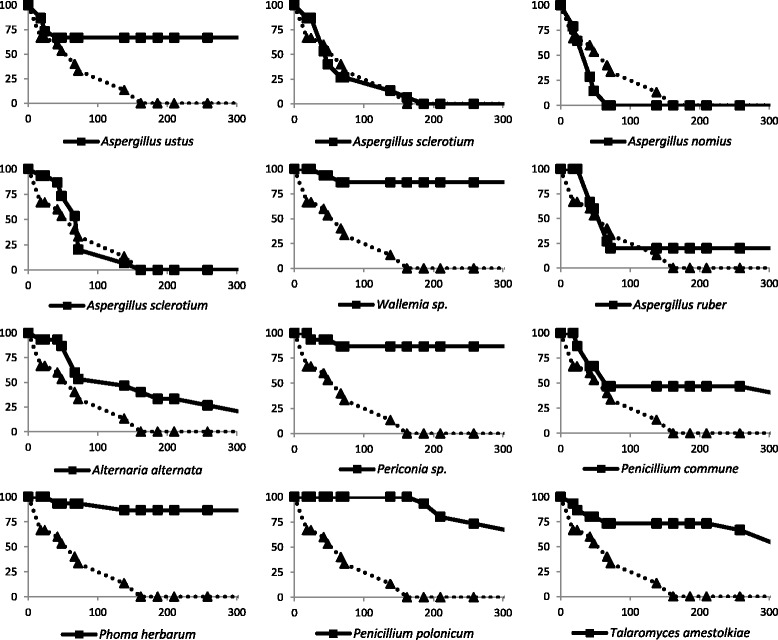
Aedes susceptibility to the microinjection of fungal spores. Survival of Aedes spp. following microinjection of fungal spores is shown. In each experiment flies microinjected with B. bassiana spores were used as a reference (dotted line with triangles). Aedes nomius, A. sclerotium (2 isolates) and A. ruber showed death rates that were not statistically different compared to those triggered by B. bassiana (P > 0.05) indicating that these four isolates are highly pathogenic to Aedes spp. Although A. ustus, Wallemia sp., A. alternata and P. commune injections led to the death of some injected mosquitoes, the results were statistically different when compared to B. bassiana (P < 0.05) reflecting low pathogenicity. Periconia sp., P. herbarum, P. polonicum and T. amestolkiae were not highly pathogenic to mosquitoes
Spore spraying onto Aedes spp. and survival analysis
The fact that an isolate showed high virulence in the microinjection experiment does not imply that it is a natural pathogen of insects. Indeed, the insect cuticle is an important barrier that needs to be breached by the germinating fungal spores. Therefore, before concluding that a fungus is a real entomopathogen, it is important to test it in a system that is close to natural infection setting. This can be achieved by spraying spores on the insects without injuring the cuticle. For this reason, we wanted to assay the pathogenicity of A. nomius - along with a selection of other isolates - in comparison to B. bassiana after spore spraying. In this experiment, A. albopictus mosquitoes were used as model. Among nine isolates tested with this mode of infection (including A. nomius, Wallemia sp., A. ruber, A. alternata and P. commune of the fungi that were pathogenic by microinjection and P. digitatum, Periconia sp., P. freii and T. amestolkiae of the ones that were not highly pathogenic by microinjection) A. nomius was the only fungus that killed at a very similar rate compared to B. bassiana (Fig. 3). Interestingly, only in the case of A. nomius (in addition to B. bassiana), irrespectively of whether the exposure to the spores was by microinjection or via spraying, the dead flies were completely covered by fungal mycelia confirming that the cause of death is due to the development of the spores in the insect and that the spores were able to germinate and probably pierce the mosquito cuticle (Fig. 4). Infection by spraying was also performed with A. nomius using another mosquito (Culex pipiens) and the results confirmed that this isolate is as pathogenic as B. bassiana to mosquitoes (Additional file 4: Figure S2).
Fig. 3.
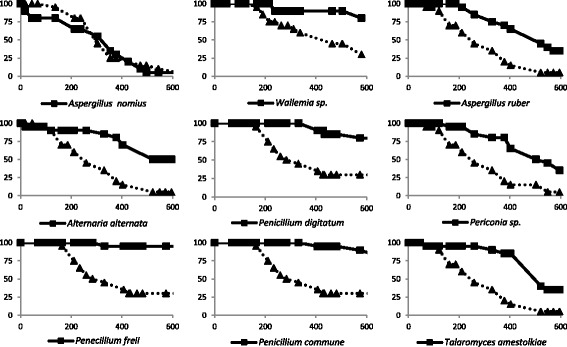
Survival of Aedes albopictus mosquitoes after infection by spraying the insects with a suspension of fungal spores (plain line with squares) is shown as the percentage of mosquitoes alive plotted versus time in hours. In each experiment the same number of B. bassiana spores was sprayed on control mosquitoes as a reference (dotted line with triangles). Only A. nomius was able to kill the mosquitoes at a very similar rate compared to B. bassiana. None of the mosquitoes that were mock-sprayed with water under the same conditions succumbed to the treatment (not shown)
Fig. 4.
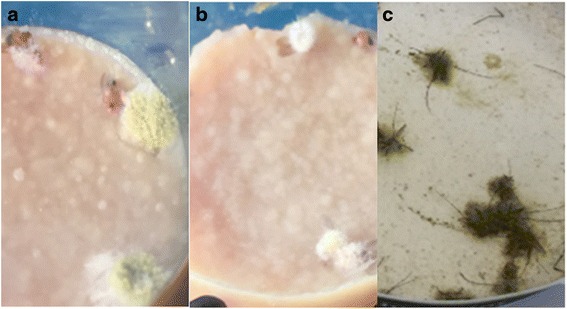
Photos of dead insects after microinjection or spraying with A. nomius spores. a Drosophila cadavers following A. nomius spores microinjection. c Aedes mosquitoes after spraying with the same fungus. The dead insects are completely covered by fungal growth indicating that the cause of death is the development of the spores within the animal. Drosophila cadavers after B. bassiana spores microinjection are shown for comparison (b)
Discussion
In the aim of recovering novel entomopathogens, we isolated fungi from dead arthropods and a subset of isolates per cadaver underwent ITS-sequencing and identification as well as pathogenicity testing. Several isolated fungi are likely to be airborne contaminant and/or saprotrophic fungi that may have developed on the arthropod carcass after the death of the animal has occurred. Examples of such possible contaminants are Penicillium, Talaromyces and Cladosporium isolates that have been oversampled in the course of the survey. Fomes fomentarius is known for its role in wood decay and for causing white-rot in plants according to some reports [25]; it has also been used in traditional medicine mostly for its anti-inflammatory and pain-killing properties [26–28]. Wallemia sp. has a saprophyte life-style and has been shown in some cases to be involved in food spoilage [29, 30].
Noteworthy, two human-related fungi were isolated in our study: Aspergillus fumigatus and S. brevicaulis. Aspergillus fumigatus is considered an opportunistic human-pathogen. However, it is primarily a ubiquitous saprophyte fungus present in many natural environments [31]. Although aspergilli are well-known airborne contaminants or soil inhabitants, A. nomius proved to be of considerable interest in our survey. Indeed, this fungus was as pathogenic as B. bassiana both by microinjection into Drosophila and A. albopictus or by infection via spore spraying onto A. albopictus and C. pipiens. Moreover, a study focusing on stonebrood, a fungi-caused disease that affects honey bee larvae, has detected the presence of A. nomius in affected hives. Indeed, among the ten Aspergillus species identified in honey bee hives, A. flavus, A. phoenicis and A. nomius were shown to be pathogenic to the larvae [32].
In the present study, A. nomius was isolated from a dead beetle (Coleoptera: Buprestidae) and was able to develop on and kill both Drosophila and mosquitoes (Diptera) indicating that it is a general entomopathogen with a broad host range. Targeting different insects can be considered an advantage, since the same fungus can be used to target several pests. However, fungi with a broad range of target insects can lead to the undesirable killing of non-target species and they should be used with caution [33, 34]. In contrast, bacteria can be used to kill insects in a very specific manner, due to the presence of toxin receptors on their epithelial cells in the target species [35].
Host-range specificity could also be correlated to differences in the immune systems of the target insects. Therefore, in addition to a potential use as biocontrol agent, A. nomius could be used as elicitor of insect immune responses in model organisms to decipher the pathways involved in the recognition of fungal infections. Indeed, although the major antifungal players have been characterized such as GNBP3 which plays different roles, both activating Toll pathway and assembling effector complexes that directly attack fungi, some aspects of insect antifungal responses remain unknown [36, 37].
Differences in the immune system between Aedes and Drosopila could explain the fact that from the eight fungal isolates that were pathogenic to Drosophila, only four (including A. nomius) were pathogenic to the mosquito, while A. ustus, Wallemia sp., A. alternata and P. commune were not as pathogenic to the mosquitoes as B. bassiana. It is worth mentioning that these four isolates were relatively “mild” in Drosophila (killing between 50 and 75 % of injected flies) as compared to the four that killed both Drosophila and Aedes (killing 75–100 % of injected flies). However, these differences are not surprising if we take into consideration that even between Drosphila species there are differences in antifungal defenses [38].
The mildly pathogenic fungi too can be interesting as biocontrol agent if they show more restricted host range as compared to the virulent ones. Also, the slow killing rate can allow more time for the infected animals to spread the spores within a population, especially because it has been reported that Anopheles female mosquito are attracted to dead insect carrying B. bassiana spores [39] and because transmission of B. bassiana from male to female Aedes mosquitoes has been observed [40].
Conclusions
The identification of A. nomius as a new natural insect pathogen and a potential disease-vector control agent is encouraging. More importantly, this pilot study demonstrates the feasibility of a simple approach for the identification of potential mosquito killers, especially that this may provide a solution to pest control from within the ecosystem rather than utilizing toxic substances. Indeed, it is essential to anticipate and prepare biocontrol methods to fight the expansion of mosquitoes’ habitat predicted in certain geographical areas in association with the occurring climatic changes. A larger scale screen could be conducted in the aim of identifying more entomopathogens with perhaps some fungi that are specific to certain host families and to give a more precise idea about saprophyte fungi that decompose arthropod cadavers in nature.
Acknowledgments
We are very grateful to P. Silar for his advice and help in the isolation of fungi and to M. Janeh for her help with the statistical analysis. SB acknowledges the technical assistance of C. Boucher and C. Clairet.
Funding
This study was supported by a CEDRE grant (Project N°30887ZH) awarded to SB and ZK and by URB funds (102848 - Project N°21712) received by ZK.
Availability of data and material
ITS sequences for isolated fungi were deposited in the GenBank database under the accession numbers KX394525–KX394566.
Authors’ contributions
SJ, AM, KK, SB and ZK performed experiments and analyzed the data. SB and ZK designed the experiments and wrote the manuscript. All authors read and approved the final version of the manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Abbreviations
- PCR
Polymerase chain reaction
- PDA
Potato dextrose agar
Additional files
Number of occurrences of each isolated fungus in the collected cadavers. (DOCX 22 kb)
Statistical analysis for survival experiments. (DOCX 23 kb)
Photos of the dead arthropods from which fungi were isolated. (DOCX 505 kb)
Effects of A. nomius on another species of mosquitoes: Culex pipiens. (PPTX 90 kb)
Contributor Information
Sana Jaber, Email: saj@mail.aub.edu.
Alex Mercier, Email: alex.mercier.2508@gmail.com.
Khouzama Knio, Email: kknio@aub.edu.lb.
Sylvain Brun, Email: sylvain.brun@univ-paris-diderot.fr.
Zakaria Kambris, Email: zk28@aub.edu.lb.
References
- 1.Stone W, Gonçalves BP, Bousema T, Drakeley C. Assessing the infectious reservoir of falciparum malaria: past and future. Trends Parasitol. 2015;31(7):287–96. doi: 10.1016/j.pt.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 2.Furuya-Kanamori L, Liang S, Milinovich G, Soares Magalhaes RJ, Clements AC, Hu W, et al. Co-distribution and co-infection of chikungunya and dengue viruses. BMC Infect Dis. 2016;16:84. doi: 10.1186/s12879-016-1417-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saiz JC, Vázquez-Calvo Á, Blázquez AB, Merino-Ramos T, Escribano-Romero E, Martín-Acebes MA. Zika virus: the latest newcomer. Front Microbiol. 2016;7:496. doi: 10.3389/fmicb.2016.00496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schaffner F, Mathis A. Dengue and dengue vectors in the WHO European region: past, present, and scenarios for the future. Lancet Infect Dis. 2014;14(12):1271–80. doi: 10.1016/S1473-3099(14)70834-5. [DOI] [PubMed] [Google Scholar]
- 5.Haddad N, Mousson L, Vazeille M, Chamat S, Tayeh J, Osta MA, et al. Aedes albopictus in Lebanon, a potential risk of arboviruses outbreak. BMC Infect Dis. 2012;12:300. doi: 10.1186/1471-2334-12-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akiner MM, Demirci B, Babuadze G, Robert V, Schaffner F. Spread of the invasive mosquitoes Aedes aegypti and Aedes albopictus in the Black Sea region increases risk of chikungunya, dengue, and Zika outbreaks in Europe. PLoS Negl Trop Dis. 2016;10(4):e0004664. doi: 10.1371/journal.pntd.0004664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roiz D, Boussès P, Simard F, Paupy C, Fontenille D. Autochthonous chikungunya transmission and extreme climate events in southern France. PLoS Negl Trop Dis. 2015;9(6):e0003854. doi: 10.1371/journal.pntd.0003854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hemingway J, Ranson H. Insecticide resistance in insect vectors of human disease. Annu Rev Entomol. 2000;45:371–91. doi: 10.1146/annurev.ento.45.1.371. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen TH, Nguyen HL, Nguyen TY, Vu SN, Tran ND, Le TN. Field evaluation of the establishment potential of wmelpop Wolbachia in Australia and Vietnam for dengue control. Parasit Vectors. 2015;8:563. doi: 10.1186/s13071-015-1174-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iturbe-Ormaetxe I, Walker T, O’ Neill SL. Wolbachia and the biological control of mosquito-borne disease. EMBO Rep. 2011;12(6):508–18. doi: 10.1038/embor.2011.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joanne S, Vythilingam I, Yugavathy N, Leong CS, Wong ML, AbuBakar S. Distribution and dynamics of Wolbachia infection in Malaysian Aedes albopictus. Acta Trop. 2015;148:38–45. doi: 10.1016/j.actatropica.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 12.Jeffries CL, Walker T. Biocontrol strategies for arboviral diseases and the potential influence of resident strains in mosquitoes. Curr Trop Med Rep. 2016;3:20–25. doi: 10.1007/s40475-016-0066-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.St Leger RJ, Wang C. Genetic engineering of fungal biocontrol agents to achieve greater efficacy against insect pests. Appl Microbiol Biotechnol. 2010;85(4):901–7. doi: 10.1007/s00253-009-2306-z. [DOI] [PubMed] [Google Scholar]
- 14.Lemaitre B, Hoffmann J. The host defense of Drosophila melanogaster. Annu Rev Immunol. 2007;25:697–743. doi: 10.1146/annurev.immunol.25.022106.141615. [DOI] [PubMed] [Google Scholar]
- 15.Hoffmann JA, Kafatos FC, Janeway CA, Ezekowitz RA. Phylogenetic perspectives in innate immunity. Science. 1999;284(5418):1313–8. doi: 10.1126/science.284.5418.1313. [DOI] [PubMed] [Google Scholar]
- 16.Gottar M, Gobert V, Matskevich AA, Reichhart JM, Wang C, Butt TM. Dual detection of fungal infections in Drosophila via recognition of glucans and sensing of virulence factors. Cell. 2006;127(7):1425–37. doi: 10.1016/j.cell.2006.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gomes SA, Paula AR, Ribeiro A, Moraes CO, Santos JW, Silva CP, Samuels RI. Neem oil increases the efficiency of the entomopathogenic fungus Metarhizium anisopliae for the control of Aedes aegypti (Diptera: Culicidae) larvae. Parasit Vectors. 2015;8:669. doi: 10.1186/s13071-015-1280-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mnyone LL, Ng’habi KR, Mazigo HD, Katakweba AA, Lyimo IN. Entomopathogenic fungi, Metarhizium anisopliae and Beauveria bassiana reduce the survival of Xenopsylla brasiliensis larvae (Siphonaptera: Pulicidae) Parasit Vectors. 2012;5:204. doi: 10.1186/1756-3305-5-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paula AR, Carolino AT, Paula CO, Samuels RI. The combination of the entomopathogenic fungus Metarhizium anisopliae with the insecticide Imidacloprid increases virulence against the dengue vector Aedes aegypti (Diptera: Culicidae) Parasit Vectors. 2011;4:8. doi: 10.1186/1756-3305-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Usda A. Collection of the US Department of Agriculture. 2014. [Google Scholar]
- 21.Abdo C, Nemer N, Nemer G, Abou-Jawdah Y, Atamian H, Kawar NS. Isolation of Beauveria species from Lebanon and evaluation of its efficacy against the cedar web-spinning sawfly, Cephalcia tannourinensis. BioControl. 2008;53(2):341–52. doi: 10.1007/s10526-006-9062-0. [DOI] [Google Scholar]
- 22.Lecellier G, Silar P. Rapid methods for nucleic acids extraction from Petri dish-grown mycelia. Curr Genet. 1994;25(2):122–3. doi: 10.1007/BF00309536. [DOI] [PubMed] [Google Scholar]
- 23.White TJ, Brun T, Lee S, Taylor JW. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR protocols: a guide to methods and applications. New York: Academic; 1990. p. 38. [Google Scholar]
- 24.NCBI. GenBank. 2016. http://www.ncbi.nlm.nih.gov/genbank/.
- 25.Kachlishvili E, Asatiani M, Kobakhidze A, Elisashvili V. Trinitrotoluene and mandarin peels selectively affect lignin-modifying enzyme production in white-rot basidiomycetes. Springerplus. 2016;5:252. doi: 10.1186/s40064-016-1895-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ito H, Sugiura M, Miyazaki T. Antitumor polysaccharide fraction from the culture filtrate of Fomes fomentarius. Chem Pharm Bull (Tokyo) 1976;24(10):2575. doi: 10.1248/cpb.24.2575. [DOI] [PubMed] [Google Scholar]
- 27.Park YM, Kim IT, Park HJ, Choi JW, Park KY, Lee JD. Anti-inflammatory and anti-nociceptive effects of the methanol extract of Fomes fomentarius. Biol Pharm Bull. 2004;27(10):1588–93. doi: 10.1248/bpb.27.1588. [DOI] [PubMed] [Google Scholar]
- 28.Roussel B, Rapior S, Charlot C, Masson CL, Boutié P. History of the therapeutic uses of the tinder polypore, Fomes fomentarius (L.) Rev Hist Pharm (Paris) 2002;50(336):599–614. doi: 10.3406/pharm.2002.5432. [DOI] [PubMed] [Google Scholar]
- 29.Zalar P, Sybren de Hoog G, Schroers HJ, Frank JM, Gunde-Cimerman N. Taxonomy and phylogeny of the xerophilic genus Wallemia (Wallemiomycetes and Wallemiales, cl. et ord. nov.). Antonie Van Leeuwenhoek. J Microbiol. 2005;87(4):311–28. doi: 10.1007/s10482-004-6783-x. [DOI] [PubMed] [Google Scholar]
- 30.Pitt JI, Hocking AD. Fungi and food spoilage. 2. London, New York: Blackie Academic & Professional; 1997. pp. 61–444. [Google Scholar]
- 31.Tekaia F, Latgé JP. Aspergillus fumigatus: saprophyte or pathogen? Curr Opin Microbiol. 2005;8(4):385–92. doi: 10.1016/j.mib.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 32.Foley K, Fazio G, Jensen AB, Hughes WO. The distribution of Aspergillus spp. opportunistic parasites in hives and their pathogenicity to honey bees. Vet Microbiol. 2014;169(3–4):203–10. doi: 10.1016/j.vetmic.2013.11.029. [DOI] [PubMed] [Google Scholar]
- 33.Ortiz-Urquiza A, Luo Z, Keyhani NO. Improving mycoinsecticides for insect biological control. Appl Microbiol Biotechnol. 2015;99(3):1057–68. doi: 10.1007/s00253-014-6270-x. [DOI] [PubMed] [Google Scholar]
- 34.Paterson RR. Fungi and fungal toxins as weapons. Mycol Res. 2006;110(Pt 9):1003–10. doi: 10.1016/j.mycres.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 35.Bravo A, Gill SS, Soberón M. Mode of action of Bacillus thuringiensis Cry and Cyt toxins and their potential for insect control. Toxicon. 2007;49(4):423–35. doi: 10.1016/j.toxicon.2006.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matskevich AA, Quintin J, Ferrandon D. The Drosophila PRR GNBP3 assembles effector complexes involved in antifungal defenses independently of its Toll-pathway activation function. Eur J Immunol. 2010;40(5):1244–54. doi: 10.1002/eji.200940164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu HL, St Leger RJ. Insect immunity to entomopathogenic fungi. Adv Genet. 2016;94:251–85. doi: 10.1016/bs.adgen.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 38.Seto Y, Tamura K. Extensive differences in antifungal immune response in two Drosophila species revealed by comparative transcriptome analysis. Int J Genomics. 2013;2013:542139. doi: 10.1155/2013/542139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.George J, Jenkins NE, Blanford S, Thomas MB, Baker TC. Malaria mosquitoes attracted by fatal fungus. PLoS One. 2013;8(5):e62632. doi: 10.1371/journal.pone.0062632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.García-Munguía AM, Garza-Hernández JA, Rebollar-Tellez EA, Rodríguez-Pérez MA, Reyes-Villanueva F. Transmission of Beauveria bassiana from male to female Aedes aegypti mosquitoes. Parasit Vectors. 2011;4:24. doi: 10.1186/1756-3305-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]


