Abstract
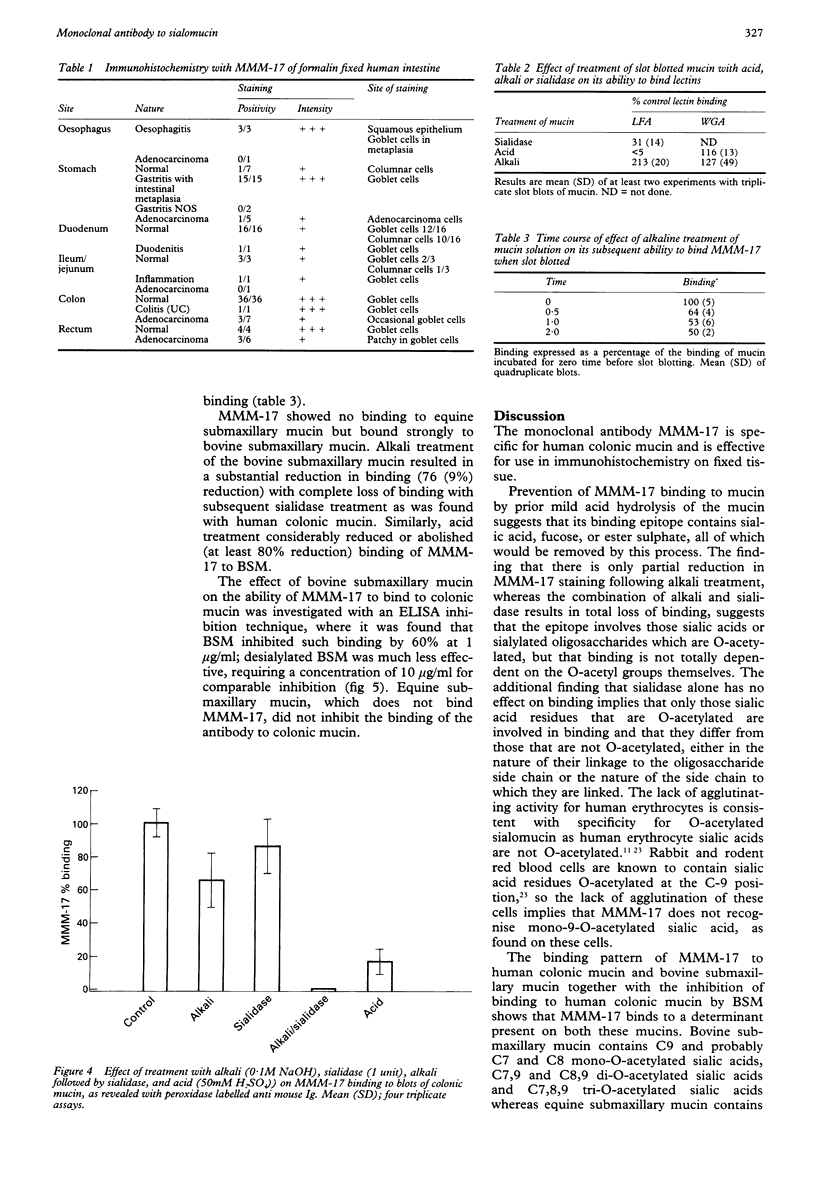
AIMS--To produce and characterise a monoclonal antibody specific for O-acetylated sialomucin and to assess its use in immunohistochemistry on a panel of normal and diseased intestinal tissue samples. METHODS--Mouse monoclonal antibodies were developed following immunisation with highly purified human colonic mucin. One of these (MMM-17) showed strong binding to mucin throughout the normal colon with relative lack of binding to colon cancer tissue. The binding epitope of MMM-17 was then characterised by screening for agglutination activity against a panel of human and animal erythrocytes and by assessment of its binding to a range of normal and chemically treated slot blotted mucins. Further immunohistochemical studies were then performed on formalin fixed, normal, and diseased human intestinal samples. RESULTS--Binding of MMM-17 to slot blotted human colonic mucin was reduced by 38 (SD 14%) (n = 4) by alkali treatment of the mucin, sequential alkali and sialidase treatment completely abolished binding. Sialidase treatment alone, however, caused only an 11 (11%) reduction in binding. MMM-17 failed to agglutinate any human, rabbit, rat or mouse erythrocytes. These findings were compatible with specificity of MMM-17 for sialomucins O-acetylated at the C-7 or C-8 positions on the sialic acid. Strong staining by MMM-17 was found in all goblet cells throughout all 40 normal colonic and rectal samples studied, but staining was absent in seven of 13 colorectal carcinomas. Normal duodenum (n = 16) and normal ileum (n = 3) all showed occasional positive goblet cells. The normal gastric antral mucosa was generally negative B MMM-17, but in all of 15 cases of gastritis with intestinal metaplasia the metaplastic glands were strongly positive for MMM-17. CONCLUSION--Monoclonal antibody MMM-17 has specificity for O-acetylated sialomucins and its binding depends both on the position of O-acetylation and on the adjacent oligosaccharide structure. Preliminary studies using the antibody on archival tissue samples support the previous reports of reduced O-acetylation in colon cancer demonstrated by indirect histochemistry and show the neo-formation of O-acetylated sialomucin in intestinal metaplasia in the stomach.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agawa S., Jass J. R. Sialic acid histochemistry and the adenoma-carcinoma sequence in colorectum. J Clin Pathol. 1990 Jul;43(7):527–532. doi: 10.1136/jcp.43.7.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed H., Gabius H. J. Purification and properties of a Ca2+-independent sialic acid-binding lectin from human placenta with preferential affinity to O-acetylsialic acids. J Biol Chem. 1989 Nov 5;264(31):18673–18678. [PubMed] [Google Scholar]
- Allen D. C., Connolly N. S., Biggart J. D. Mucin profiles in ulcerative colitis with dysplasia and carcinoma. Histopathology. 1988 Oct;13(4):413–424. doi: 10.1111/j.1365-2559.1988.tb02057.x. [DOI] [PubMed] [Google Scholar]
- Atkinson B. F., Ernst C. S., Herlyn M., Steplewski Z., Sears H. F., Koprowski H. Gastrointestinal cancer-associated antigen in immunoperoxidase assay. Cancer Res. 1982 Nov;42(11):4820–4823. [PubMed] [Google Scholar]
- Bresalier R. S., Rockwell R. W., Dahiya R., Duh Q. Y., Kim Y. S. Cell surface sialoprotein alterations in metastatic murine colon cancer cell lines selected in an animal model for colon cancer metastasis. Cancer Res. 1990 Feb 15;50(4):1299–1307. [PubMed] [Google Scholar]
- Cheresh D. A., Varki A. P., Varki N. M., Stallcup W. B., Levine J., Reisfeld R. A. A monoclonal antibody recognizes an O-acylated sialic acid in a human melanoma-associated ganglioside. J Biol Chem. 1984 Jun 25;259(12):7453–7459. [PubMed] [Google Scholar]
- Corfield A. P., Sander-Wewer M., Veh R. W., Wember M., Schauer R. The action of sialidases on substrates containing O-acetylsialic acids. Biol Chem Hoppe Seyler. 1986 May;367(5):433–439. doi: 10.1515/bchm3.1986.367.1.433. [DOI] [PubMed] [Google Scholar]
- Culling C. F., Reid P. E., Dunn W. L. A histochemical comparison of the O-acylated sialic acids of the epithelial mucins in ulcerative colitis, Crohn's disease, and normal controls. J Clin Pathol. 1979 Dec;32(12):1272–1277. doi: 10.1136/jcp.32.12.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culling C. F., Reid P. E. Specific techniques for the identification of O-acylated sialic acids in colonic mucins. J Microsc. 1980 Aug;119(3):415–425. doi: 10.1111/j.1365-2818.1980.tb04113.x. [DOI] [PubMed] [Google Scholar]
- Culling C. F., Reid P. E., Worth A. J., Dunn W. L. A new histochemical technique of use in the interpretation and diagnosis of adenocarcinoma and villous lesions in the large intestine. J Clin Pathol. 1977 Nov;30(11):1056–1062. doi: 10.1136/jcp.30.11.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipe M. I. Mucins in the human gastrointestinal epithelium: a review. Invest Cell Pathol. 1979 Jul-Sep;2(3):195–216. [PubMed] [Google Scholar]
- Gold D. V., Shochat D. Studies on the structure of the organ-specific determinant of human colonic mucin. Mol Immunol. 1989 Aug;26(8):769–777. doi: 10.1016/0161-5890(89)90037-0. [DOI] [PubMed] [Google Scholar]
- Hutchins J. T., Reading C. L., Giavazzi R., Hoaglund J., Jessup J. M. Distribution of mono-, di, and tri-O-acetylated sialic acids in normal and neoplastic colon. Cancer Res. 1988 Jan 15;48(2):483–489. [PubMed] [Google Scholar]
- Jass J. R. Role of intestinal metaplasia in the histogenesis of gastric carcinoma. J Clin Pathol. 1980 Sep;33(9):801–810. doi: 10.1136/jcp.33.9.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macartney J. C., Oriol R., Ciclitira P. J. An immunohistochemical study of a colonic mucus antigen in normal and neoplastic gastrointestinal tissues. J Pathol. 1986 Aug;149(4):279–285. doi: 10.1002/path.1711490403. [DOI] [PubMed] [Google Scholar]
- Manzi A. E., Sjoberg E. R., Diaz S., Varki A. Biosynthesis and turnover of O-acetyl and N-acetyl groups in the gangliosides of human melanoma cells. J Biol Chem. 1990 Aug 5;265(22):13091–13103. [PubMed] [Google Scholar]
- Nicolson G. L. Cell surface molecules and tumor metastasis. Regulation of metastatic phenotypic diversity. Exp Cell Res. 1984 Jan;150(1):3–22. doi: 10.1016/0014-4827(84)90696-7. [DOI] [PubMed] [Google Scholar]
- Parker N., Makin C. A., Ching C. K., Eccleston D., Taylor O. M., Milton J. D., Rhodes J. M. A new enzyme-linked lectin/mucin antibody sandwich assay (CAM 17.1/WGA) assessed in combination with CA 19-9 and peanut lectin binding assay for the diagnosis of pancreatic cancer. Cancer. 1992 Sep 1;70(5):1062–1068. doi: 10.1002/1097-0142(19920901)70:5<1062::aid-cncr2820700509>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Podolsky D. K., Fournier D. A., Lynch K. E. Development of anti-human colonic mucin monoclonal antibodies. Characterization of multiple colonic mucin species. J Clin Invest. 1986 Apr;77(4):1251–1262. doi: 10.1172/JCI112428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raouf A., Parker N., Iddon D., Ryder S., Langdon-Brown B., Milton J. D., Walker R., Rhodes J. M. Ion exchange chromatography of purified colonic mucus glycoproteins in inflammatory bowel disease: absence of a selective subclass defect. Gut. 1991 Oct;32(10):1139–1145. doi: 10.1136/gut.32.10.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid P. E., Culling C. F., Dunn W. L., Clay M. G., Ramey C. W. A correlative chemical and histochemical study of the O-acetylated sialic acids of human colonic epithelial glycoproteins in formalin fixed paraffin embedded tissues. J Histochem Cytochem. 1978 Dec;26(12):1033–1041. doi: 10.1177/26.12.731017. [DOI] [PubMed] [Google Scholar]
- Reid P. E., Dunn W. L., Ramey C. W., Coret E., Trueman L., Clay M. G. Histochemical identification of side chain substituted O-acylated sialic acids: the PAT-KOH-Bh-PAS and the PAPT-KOH-Bh-PAS procedures. Histochem J. 1984 Jun;16(6):623–639. doi: 10.1007/BF01003390. [DOI] [PubMed] [Google Scholar]
- Schauer R. Chemistry, metabolism, and biological functions of sialic acids. Adv Carbohydr Chem Biochem. 1982;40:131–234. doi: 10.1016/s0065-2318(08)60109-2. [DOI] [PubMed] [Google Scholar]
- Schauer R. Sialic acids: metabolism of O-acetyl groups. Methods Enzymol. 1987;138:611–626. doi: 10.1016/0076-6879(87)38055-3. [DOI] [PubMed] [Google Scholar]
- Stramignoni D., Bowen R., Atkinson B. F., Schlom J. Differential reactivity of monoclonal antibodies with human colon adenocarcinomas and adenomas. Int J Cancer. 1983 May 15;31(5):543–552. doi: 10.1002/ijc.2910310504. [DOI] [PubMed] [Google Scholar]
- Yamori T., Ota D. M., Cleary K. R., Hoff S., Hager L. G., Irimura T. Monoclonal antibody against human colonic sulfomucin: immunochemical detection of its binding sites in colonic mucosa, colorectal primary carcinoma, and metastases. Cancer Res. 1989 Feb 15;49(4):887–894. [PubMed] [Google Scholar]




