Abstract
Host variation in Toll-like receptors and other innate immune signaling molecules alters infection susceptibility. However, only a portion of the variability observed in the innate immune response is accounted for by known genes in these pathways. Thus, the identification of additional genes that regulate the response to Gram positive bacteria is warranted. Bone marrow-derived macrophages (BMMs) from 43 inbred mouse strains were stimulated with lipotechoic acid (LTA), a major component of the Gram positive bacterial cell wall. Concentrations of the proinflammatory cytokines IL-6, IL-12, and TNF-α were measured. In silico whole genome association (WGA) mapping was performed using cytokine responses followed by network analysis to prioritize candidate genes. To determine which candidate genes could be responsible for regulating the LTA response, candidate genes were inhibited using RNA interference (RNAi) and were overexpressed in RAW264.7 macrophages. BMMs from Bdkrb1-deficient mice were used to assess the effect of Bdkrb1 gene deletion on the response to LTA, heat-killed Streptococcus pneumoniae, and heat-killed Staphylococcus aureus. WGA mapping identified 117 loci: IL-6 analysis yielded 20 loci (average locus size = 0.133 Mb; 18 genes), IL-12 analysis produced 5 loci (0.201 Mb average; 7 genes), and TNF-α analysis yielded 92 loci (0.464 Mb average; 186 genes of which 46 were prioritized by network analysis). The follow-up small interfering RNA screen of 71 target genes identified four genes (Bdkrb1, Blnk, Fbxo17, and Nkx6-1) whose inhibition resulted in significantly reduced cytokine production following LTA stimulation. Overexpression of these four genes resulted in significantly increased cytokine production in response to LTA. Bdkrb1-deficient macrophages were less responsive to LTA and heat-killed S. aureus, validating the genetic and RNAi approach to identify novel regulators of the response to LTA. We have identified four innate immune response genes that may contribute to Gram positive bacterial susceptibility.
Keywords: innate immunity, Gram positive bacteria, lipotechoic acid, whole genome association mapping, inbred strains of mice, RNA interference
GRAM positive bacterial infections are a major public health concern, with pneumonia for example, being the most prevalent lower respiratory infection and third leading cause of death around the world (Liu et al. 2015). Around 10% of healthy adults and 20–40% of healthy children have airways colonized by the most common pneumonia pathogen, Streptococcus pneumoniae (van der Poll and Opal 2009) but only a small portion of these individuals become actively infected (Brouwer et al. 2009). Similarly, the rate of Staphylococcus aureus infection is high in hospitals and community settings. However, animal and human studies have shown variable host response and course of disease (Shukla et al. 2015), suggesting genetic factors in the innate immune response to the pathogen are likely at play.
The innate immune response begins with binding of the pathogen-associated molecular pattern (PAMP) to innate immune receptors such as the Toll-like receptor (TLR) (Takeuchi and Akira 2010) and nucleotide-binding domain, leucine rich containing (NLR) (Ting et al. 2008) families. In particular, the TLR2/TLR6 heterodimer on cell surfaces binds to peptidoglycan and the lipotechoic acid (LTA) moieties of Gram positive bacteria, initiating a signaling cascade that leads to activation of NF-κB and production of proinflammatory cytokines.
Novel genes involved in the innate immune response to a variety of PAMPs and pathogens have been identified by our group using comparative genomics approaches (Alper et al. 2008), transcriptional profiling of stimulated macrophages (Yang et al. 2011b) and tissues from animal models (Yang et al. 2011a), and mapping studies in inbred mice (Zaas et al. 2008; Yang et al. 2009). A prior study from our laboratory investigated murine antibacterial defense to streptococcal lung infection in eight strains of mice and found that TLR2-deficient mice had increased bacterial load (Hollingsworth et al. 2007), and a similar study in nine inbred mouse strains identified resistant and susceptible strains of mice (Gingles et al. 2001). To date, no study has examined the cytokine response to TLR2 stimulation, either in vitro or in vivo, on a genome-wide scale across a larger number of mouse strains. Here, we use inbred mouse strains and subsequent loss- and gain-of-function approaches to identify novel regulators of the response to the TLR2/6 PAMP LTA.
Materials and Methods
Animals
Inbred mouse strains were obtained from The Jackson Laboratory (Bar Harbor, ME) and killed for collection of bone marrow. Bdkrb1-deficient mice were created by Michael Bader (Pesquero et al. 2000). Three mice per strain were used. All animal work was reviewed and approved by the Institutional Animal Care and Use Committees at National Jewish Health and the University of Colorado Anschutz Medical Campus.
Bone marrow-derived macrophage culture
Bone marrow-derived macrophages (BMMs) were generated in vitro using standard methodology (Weischenfeldt and Porse 2008). Briefly, bone marrow was harvested from the femurs and tibia, disaggregated, washed, and resuspended in DMEM medium supplemented with 4,500 mg/liter of D-glucose, L-glutamine, 110 mg/liter of sodium pyruvate (high glucose medium; Invitrogen, Carlsbad, CA), 10% heat-inactivated FCS, 100 units/ml penicillin, 100 µg/ml streptomycin (all from Invitrogen), and 25 ng/ml recombinant mouse M-CSF (R&D Systems, Minneapolis, MN). Ten milliliters of this suspension was plated in each of two Petri dishes and cultured at 37° in a 5% CO2 incubator for 6 days. On day 6, nonadherent contaminating cells were removed by three PBS washes. Washed cells were replated at 5 × 105 per well in 96-well plates and incubated at 37° overnight. Following overnight incubation, the cells were stimulated with three concentrations of S. aureus LTA (Sigma, St. Louis, MO) (10, 0.5, and 0.025 µg/ml) in duplicate. Three biological replicates (i.e., three mice per strain) were included. Supernatant was collected 5 hr post-PAMP treatment and cytokine production (IL-6, IL-12p40, and TNF-α) was assayed using the Bio-Plex Kit (Bio-Rad, Hercules, CA) and read on a Luminex reader (Bio-Rad).
Whole genome association mapping analysis
Cytokine concentrations were log10 transformed, duplicate readings for each mouse were averaged, and all further data analysis was performed using triplicate biological replicates. Normality of distribution of cytokine response was assessed using the D’Agostino–Pearson omnibus normality test (GraphPad Prism) and the concentration with the most normally distributed data (0.5 μg/ml) was used for mapping. In silico whole genome association (WGA) mapping was conducted using efficient mixed model association (EMMA) (Kang et al. 2008) using the 4 million SNP data set to identify genetic loci associated with cytokine production in response to LTA stimulation. EMMA corrects for population structure and relatedness between inbred mouse strains using a phylogenetic kinship matrix to avoid false positive associations. We also performed permutation testing with shuffled data sets to empirically determine that the false positives rate was low (Abiola et al. 2003). Pathway and network analysis was performed using Ingenuity pathway analysis (IPA) (Kramer et al. 2014)
RNA interference in RAW264.7 cells
RNA interference (RNAi) assays were carried out as previously described (Alper et al. 2008; Yang et al. 2009; De Arras et al. 2014a). Briefly, small interfering RNAs (siRNAs) (Dharmacon, Lafayette, CO; pools of four siRNA duplexes/gene) were transfected into the mouse macrophage cell line RAW264.7 using the Amaxa Nucleofector Shuttle according to the manufacturer’s instructions. Transfections were carried out in 96-well format using 200,000 cells/well and 2 μm siRNA. Thirty-six hours after siRNA transfection, LTA was added to a final concentration of 2.5 µg/ml LTA. Supernatant was collected 5 hr post-LTA treatment, and cytokine production was assayed using DuoSet ELISA kits (R&D Systems). In experiments monitoring the response to lipopolysaccharide (LPS) instead of LTA, LPS was added at a final concentration of 20 ng/ml (LPS from List Biological Laboratories, Campbell, CA). Cell viability was monitored and cell number normalized using fluorescein diacetate as described (Fernandez-Botran and Vt’vička 2001; Alper et al. 2008). Cytokine production was normalized relative to a negative control siRNA (nontargeting siRNA, Dharmacon). RNA was isolated using the RNAEasy kit (QIAGEN, Valencia, CA) and the extent of gene knockdown was monitored by real-time (RT)-PCR with Taqman gene expression assays on an ABI Viia7 Real-Time PCR System (Applied Biosystems, Foster City, CA). The 384-well plate for qPCR was set up using the Freedom EVO robot (Tecan). siRNAs were initially tested in quadruplicate and siRNAs that affected cytokine production were further tested by transfecting each siRNA duplex in the pool individually to verify that multiple siRNAs targeting each gene could still induce the same phenotype. One-group two-tailed t-tests for siRNA data (testing for deviation from 1) and two-group unpaired two-tailed t-tests for qPCR data were performed in GraphPad Prism.
Gene overexpression in RAW264.7 cells
Plasmids containing full-length complementary DNAs (cDNAs) with stop codon cloned downstream of the cytomegalovirus (CMV) promoter were obtained for Nkx6.1 (Origene); Bdkrb1 (clone ID: 4753410), Blnk (clone ID: 5213221), and Fbxo17 (clone ID: 4486850) (Open Biosystems), and negative control chloramphenicol acetyltransferase (CAT) (Invitrogen). Transient transfections were performed as previously described (De Arras et al. 2014b). Briefly, 100,000 cells plated in 24-well format were transfected with 3.75 μl Fugene HD (Roche), 300 ng NF-κB-AP1-luc (a derivative of the IL-8 promoter) (Mukaida et al. 1989), 100 ng SV40-rluc (Promega, Madison, WI), and 600 ng of overexpression plasmid. Twenty-four hours after transfection, cells were stimulated with LTA for 6 hr and then luciferase activity was monitored using the Dual Luciferase Assay Kit (Promega) using SV40-rluc as a normalization control for transfection efficiency. Unpaired two-group two-tailed t-tests were performed in GraphPad Prism.
Bdkrb1- and Bdkrb2-deficient BMMs
BMMs from Bdkrb1-deficient mice were stimulated with either LTA for 5 hr, heat-killed S. pneumoniae (HKSP) (Invivogen) for 18 hr, or heat-killed S. aureus (HKSA) (Invivogen) for 18 hr, and IL-6 and TNF-α in the supernatant were measured using DuoSet ELISA kits (R&D Systems). ANOVAs with post hoc Tukey tests were performed in GraphPad Prism.
Data availability
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article.
Results
Use of inbred mouse strains to identify candidate regulators of the response to LTA
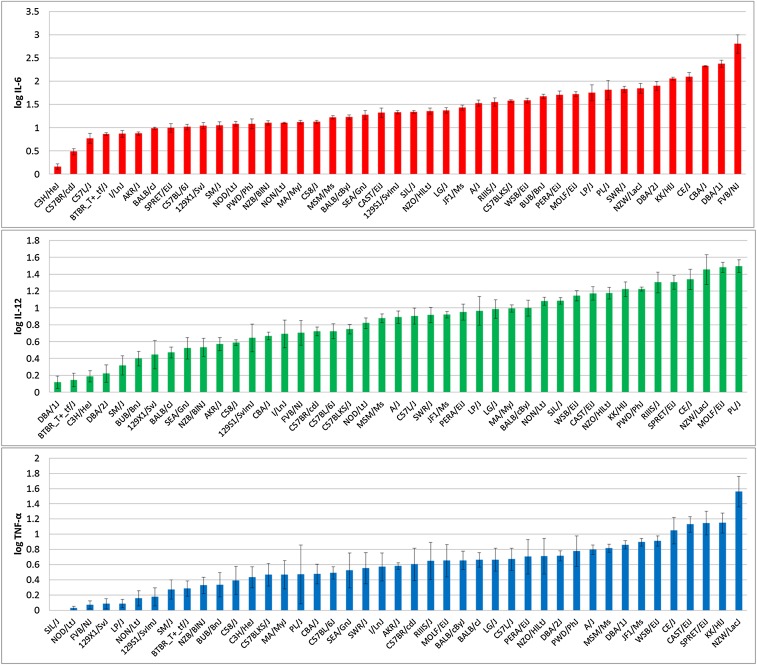
To investigate the differential response of macrophages to TLR2 stimulation, BMMs from 43 inbred strains of mice were stimulated with three concentrations of LTA (10.0, 0.5, and 0.025 µg/ml). To improve the diversity of the genetic background, eight wild-derived strains were included (Supplemental Material, Table S1) (Kirby et al. 2010). Concentrations of IL-6, IL-12, and TNF-α 5 hr post-treatment revealed significant variability in response among the 43 strains. The D’Agostino–Pearson omnibus normality test of log-transformed cytokine concentrations showed the most normal distribution of cytokine response to 0.5 µg/ml LTA stimulation with TNF-α response deviating most from a normal distribution (Table S2). The cytokine response induced by 0.5 µg/ml LTA stimulation across all 43 strains (Figure 1) was chosen for further analyses. To confirm the stability of these phenotypes, six strains of mice (C3H/HeJ, BALB/cByJ, C57BL/6J, DBA/1J, DBA/2J, and SJL/J) were rephenotyped in a separate set of experiments and similar cytokine concentrations with the same ranking of the strains as in the initial screen were observed (data not shown).
Figure 1.
Post-LTA stimulation concentrations of IL-6 (top, red bars), IL-12 (middle, green bars), and TNF-α (bottom, blue bars) secreted by BMMs from 43 inbred mouse strains of mice. BMMs were stimulated with 0.5 µg/ml LTA for 5 hr and cytokine concentrations in the supernatant were measured by ELISA. In all three panels, means of three biological replicates with error bars representing SEMs are plotted.
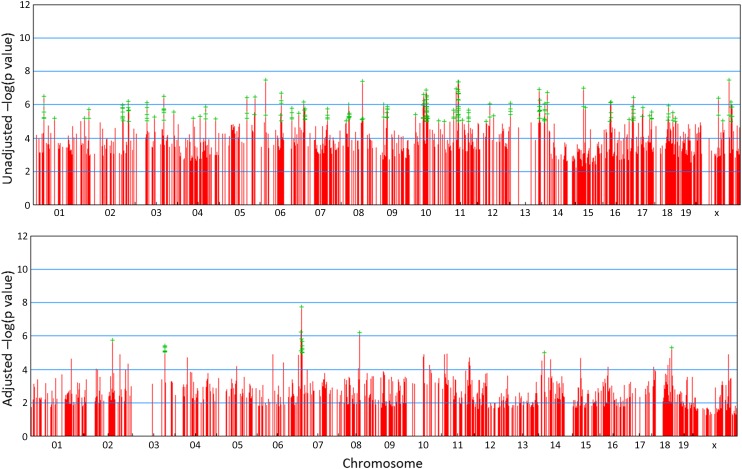
To identify genetic loci associated with cytokine response to LTA stimulation, we performed in silico WGA mapping on cytokine response to 0.5 µg/ml LTA stimulation. We used the EMMA algorithm developed by Kang et al. (2008) because it implements a genetic similarity matrix and a phylogenetic control method to account for population structure among inbred strains of mice, thereby limiting false positive associations (Kang et al. 2008), and we have successfully used it in our previous studies of innate immunity (Yang et al. 2009). The reduction in the false positive associations achieved by implementing the EMMA algorithm to perform WGA mapping of the IL-12 cytokine response data are depicted in Figure 2, A and B, demonstrating the appropriateness of this mapping approach in our study.
Figure 2.
WGA mapping analysis of IL-12 produced by BMMs from 43 strains of mice in response to LTA stimulation. Top plot shows unadjusted P-values while bottom plot shows P-values after adjustment for relatedness among inbred strains of mice. Green plus symbols designate SNPs significantly associated with LTA-induced IL-12 production.
To identify genes in the loci significantly associated with the cytokine response to LTA stimulation, we determined loci boundaries by extending from the most significant single nucleotide polymorphism (SNP) in the analysis to encompass all linked SNPs with P < 1 × 10−4. This resulted in 20 loci (average locus size = 0.133 Mb; 18 known genes) for the IL-6 response, 5 loci (0.201 Mb average; 7 known genes) for the IL-12 response, and 92 loci (0.464 Mb average; 186 known genes) for the TNF-α response (Table 1 and Table S3). The resolution of mapping is poorer (loci are larger), and there are more significant associations for TNF-α production due to the larger deviations from normality compared to IL-6 or IL-12 production. To prioritize genes in the TNF-α response loci for further studies, we performed a systems analysis using IPA. The two top-scoring protein–protein interaction networks identified by IPA contain 46 of the 186 genes that mapped to the TNF-α response (Figure S1). The top network (score = 42) centers on NF-κB, p38 MAPK, and TGF-β while hubs in the second network (score = 36) consist of several kinases (PI3K, ERK1/2, PKA, and PKC), demonstrating the relevance of these networks to the innate immune response (Fukao and Koyasu 2003; Takeuchi and Akira 2010).
Table 1. One-hundred seventeen loci identified as associated with IL-6, IL-12, and TNF-α response of macrophages to LTA by EMMA mapping.
| Cytokine | Chr | Start (bp) | End (bp) | Locus size (bp) |
|---|---|---|---|---|
| IL-6 | chr1 | 173,660,282 | 173,660,282 | |
| IL-6 | chr1 | 174,635,407 | 174,681,425 | 46,018 |
| IL-6 | chr1 | 182,983,026 | 183,092,082 | 109,056 |
| IL-6 | chr13 | 83,926,870 | 84,019,016 | 92,146 |
| IL-6 | chr15 | 26,473,491 | 26,485,688 | 12,197 |
| IL-6 | chr18 | 75,055,572 | 75,055,572 | |
| IL-6 | chr18 | 76,434,758 | 76,444,590 | 9,832 |
| IL-6 | chr19 | 6,607,389 | 6,684,068 | 76,679 |
| IL-6 | chr19 | 6,896,895 | 6,931,773 | 34,878 |
| IL-6 | chr19 | 7,417,909 | 7,626,585 | 208,676 |
| IL-6 | chr19 | 8,039,764 | 8,527,516 | 487,752 |
| IL-6 | chr19 | 8,753,215 | 8,753,215 | |
| IL-6 | chr3 | 63,804,619 | 63,993,407 | 188,788 |
| IL-6 | chr3 | 139,773,695 | 139,929,431 | 155,736 |
| IL-6 | chr3 | 141,191,857 | 141,645,185 | 453,328 |
| IL-6 | chr3 | 141,768,183 | 141,823,620 | 55,437 |
| IL-6 | chr7 | 81,032,677 | 81,075,445 | 42,768 |
| IL-6 | chr8 | 90,554,504 | 90,554,504 | |
| IL-6 | chr8 | 117,778,948 | 117,778,948 | |
| IL-6 | chr8 | 126,224,093 | 126,252,702 | 28,609 |
| IL-12 | chr14 | 20,435,777 | 20,515,227 | 79,450 |
| IL-12 | chr7 | 13,302,434 | 13,302,434 | |
| IL-12 | chr7 | 14,954,483 | 15,009,969 | 55,486 |
| IL-12 | chr7 | 16,857,086 | 17,227,229 | 370,143 |
| IL-12 | chr8 | 79,136,882 | 79,436,459 | 299,577 |
| TNF-α | chr1 | 39,035,119 | 39,181,313 | 146,194 |
| TNF-α | chr1 | 173,661,396 | 173,884,837 | 223,441 |
| TNF-α | chr10 | 50,611,055 | 50,611,055 | |
| TNF-α | chr10 | 50,740,158 | 50,788,326 | 48,168 |
| TNF-α | chr10 | 50,917,983 | 51,023,999 | 106,016 |
| TNF-α | chr10 | 51,135,297 | 51,941,442 | 806,145 |
| TNF-α | chr10 | 52,575,330 | 52,782,128 | 206,798 |
| TNF-α | chr10 | 52,895,811 | 52,977,368 | 81,557 |
| TNF-α | chr10 | 53,086,073 | 53,239,257 | 153,184 |
| TNF-α | chr10 | 68,010,641 | 68,393,947 | 383,306 |
| TNF-α | chr10 | 125,401,945 | 125,908,958 | 507,013 |
| TNF-α | chr11 | 11,674,761 | 11,742,868 | 68,107 |
| TNF-α | chr11 | 47,168,225 | 47,168,244 | 19 |
| TNF-α | chr11 | 72,932,205 | 74,484,806 | 1,552,601 |
| TNF-α | chr11 | 87,114,852 | 87,158,145 | 43,293 |
| TNF-α | chr11 | 90,096,091 | 90,274,475 | 178,384 |
| TNF-α | chr11 | 101,977,726 | 103,331,421 | 1,353,695 |
| TNF-α | chr12 | 5,260,013 | 5,591,949 | 331,936 |
| TNF-α | chr12 | 45,440,710 | 47,752,680 | 2,311,970 |
| TNF-α | chr12 | 106,725,372 | 106,879,407 | 154,035 |
| TNF-α | chr12 | 108,031,321 | 108,249,927 | 218,606 |
| TNF-α | chr12 | 108,381,002 | 108,424,555 | 43,553 |
| TNF-α | chr13 | 40,553,224 | 40,770,110 | 216,886 |
| TNF-α | chr14 | 29,545,056 | 29,545,056 | |
| TNF-α | chr14 | 33,768,861 | 34,187,798 | 418,937 |
| TNF-α | chr14 | 80,516,031 | 80,781,218 | 265,187 |
| TNF-α | chr16 | 51,934,234 | 51,957,839 | 23,605 |
| TNF-α | chr16 | 52,796,410 | 53,140,991 | 344,581 |
| TNF-α | chr18 | 46,359,998 | 46,369,259 | 9,261 |
| TNF-α | chr18 | 61,292,691 | 61,715,155 | 422,464 |
| TNF-α | chr18 | 63,484,322 | 6,3941,799 | 457,477 |
| TNF-α | chr18 | 77,580,880 | 7,7819,968 | 239,088 |
| TNF-α | chr19 | 6,045,940 | 6,045,940 | |
| TNF-α | chr19 | 40,915,624 | 41,163,200 | 247,576 |
| TNF-α | chr19 | 41,960,523 | 42,173,169 | 212,646 |
| TNF-α | chr2 | 136,181,592 | 138,515,186 | 2,333,594 |
| TNF-α | chr2 | 143,850,108 | 143,850,108 | |
| TNF-α | chr2 | 163,054,911 | 163,298,162 | 243,251 |
| TNF-α | chr3 | 4,126,466 | 5,309,431 | 1,182,965 |
| TNF-α | chr3 | 39,534,158 | 41,662,341 | 2,128,183 |
| TNF-α | chr3 | 41,811,467 | 43,139,599 | 1,328,132 |
| TNF-α | chr3 | 44,572,664 | 46,423,886 | 1,851,222 |
| TNF-α | chr3 | 46,532,654 | 47,266,354 | 733,700 |
| TNF-α | chr3 | 50,825,016 | 52,618,750 | 1,793,734 |
| TNF-α | chr3 | 83,640,855 | 83,846,453 | 205,598 |
| TNF-α | chr3 | 157,306,420 | 157,402,557 | 9,6137 |
| TNF-α | chr4 | 7,064,861 | 7,528,602 | 463,741 |
| TNF-α | chr4 | 31,169,777 | 31,331,142 | 161,365 |
| TNF-α | chr4 | 65,675,157 | 66,157,867 | 482,710 |
| TNF-α | chr4 | 79,461,643 | 80,868,868 | 1407,225 |
| TNF-α | chr4 | 103,305,852 | 103,464,317 | 158,465 |
| TNF-α | chr4 | 103,728,271 | 103,902,841 | 174,570 |
| TNF-α | chr4 | 104,241,765 | 104,336,024 | 94,259 |
| TNF-α | chr4 | 143,774,477 | 143,945,145 | 170,668 |
| TNF-α | chr5 | 83,480,859 | 83,768,222 | 287,363 |
| TNF-α | chr5 | 102,050,296 | 102,259,957 | 209,661 |
| TNF-α | chr5 | 102,450,545 | 102,554,777 | 104,232 |
| TNF-α | chr5 | 114,170,021 | 114,364,985 | 194,964 |
| TNF-α | chr5 | 114,660,785 | 114,768,095 | 107,310 |
| TNF-α | chr5 | 128,332,778 | 128,341,406 | 8,628 |
| TNF-α | chr5 | 133,935,870 | 134,009,002 | 73,132 |
| TNF-α | chr6 | 21,142,574 | 21,313,770 | 171,196 |
| TNF-α | chr6 | 30,290,830 | 30,462,108 | 171,278 |
| TNF-α | chr6 | 30,665,722 | 30,671,396 | 5,674 |
| TNF-α | chr6 | 31,059,957 | 31,239,548 | 179,591 |
| TNF-α | chr6 | 79,494,699 | 80,577,083 | 1082,384 |
| TNF-α | chr6 | 81,472,746 | 81,657,303 | 184,557 |
| TNF-α | chr6 | 110,841,305 | 111,262,820 | 421,515 |
| TNF-α | chr6 | 111,442,758 | 112,271,201 | 828,443 |
| TNF-α | chr6 | 130,938,748 | 131,015,928 | 77,180 |
| TNF-α | chr7 | 82,755,139 | 83,269,370 | 514,231 |
| TNF-α | chr7 | 141,656,566 | 141,774,343 | 117,777 |
| TNF-α | chr8 | 72,748,804 | 72,748,804 | |
| TNF-α | chr9 | 22,482,848 | 22,482,848 | |
| TNF-α | chr9 | 30,770,731 | 31,702,031 | 931,300 |
| TNF-α | chr9 | 42,207,980 | 42,490,846 | 282,866 |
| TNF-α | chr9 | 85,402,341 | 85,477,102 | 74,761 |
| TNF-α | chr9 | 93,775,695 | 97,653,544 | 3,877,849 |
| TNF-α | chr9 | 109,384,502 | 109,707,867 | 323,365 |
| TNF-α | chrX | 96,138,670 | 96,211,687 | 73,017 |
| TNF-α | chrX | 112,462,964 | 112,494,950 | 31,986 |
| TNF-α | chrX | 122,722,705 | 122,831,118 | 108,413 |
| TNF-α | chrX | 122,949,728 | 123,652,382 | 702,654 |
| TNF-α | chrX | 122,949,728 | 123,652,382 | |
| TNF-α | chrX | 124,009,011 | 124,073,705 | 64,694 |
| TNF-α | chrX | 124,193,246 | 124,340,106 | 146,860 |
| TNF-α | chrX | 124,477,770 | 124,800,822 | 323,052 |
| TNF-α | chrX | 124,906,257 | 125,118,241 | 211,984 |
| TNF-α | chrX | 126,116,468 | 126,148,778 | 32,310 |
| TNF-α | chrX | 126,389,225 | 126,798,587 | 409,362 |
| TNF-α | chrX | 131,417,076 | 131,646,505 | 229,429 |
| TNF-α | chrX | 131,771,131 | 131,871,707 | 100,576 |
We also performed 10 permutations with shuffled data sets to confirm a low false positive rate in EMMA mapping. We observed only one false positive in 1 of the 10 permutations for IL-6 response, no associations for IL-12 response, and one false positive in three of the 10 permutation for TNF-α response.
Use of RNAi to prioritize candidate genes
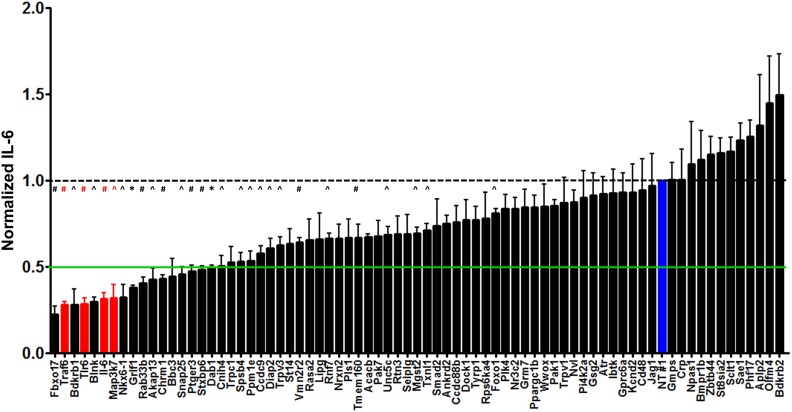
Our analysis identified a total of 71 high-priority candidate genes (18 IL-6, 7 IL-12, and 46 TNF-α + pathway) that are likely to be important in the innate immune response to LTA (Table S4). To functionally test the potential role of the 71 candidate genes identified by our analysis, we used RNAi to inhibit each of these genes in the RAW264.7 mouse macrophage cell line and monitored the resulting effect on LTA-induced IL-6 production. A pool of four siRNA duplexes targeting each gene was transfected into the macrophages, the cells were exposed to LTA, and 5 hr later, cytokines secreted into the supernatant were analyzed by ELISA. As a control, we demonstrated that LTA-induced IL-6 production was decreased when known LTA response genes were inhibited (Tlr6, Traf6, IL-6, and Map3k7) (Figure 3). RNAi-mediated inhibition of 8 of the 71 candidate genes (Fbxo17, Bdkrb1, Blnk, Nkx6-1, Grif1, Rab33b, Akap13, and Chrm1) resulted in a more than twofold reduction in IL-6 production in response to LTA stimulation (Figure 3). In contrast, IL-6 production was moderately increased when Bdkrb2, a gene closely related to Bdkrb1, was inhibited (Figure 3).
Figure 3.
The effect of RNAi-mediated inhibition of 71 genes from WGA mapping in RAW264.7 macrophages on LTA-induced IL-6 production. Pools of four siRNA duplexes per gene were transfected into RAW264.7 cells, LTA was added, and cytokine production was monitored. Shown are the results for negative control siRNA (blue; nontargeting siRNA from Dharmacon), four positive control siRNAs (red; Traf6, Tlr6, Il-6, and Map3k7), and 71 siRNAs targeting genes identified by our eQTL mapping analysis (black). All data are normalized to the NT#1 nontargeting negative control siRNA. Means of four independent measurements with error bars representing SEMs are plotted; green line indicates threshold for twofold reduction in IL-6 response as a result of gene inhibition. *P < 0.0001, #P < 0.001, and ^P < 0.01 by one-group two-tailed t-test testing for deviation from 1.
To confirm that these RNAi treatments were inhibiting the corresponding endogenous gene, we used Taqman gene expression assays to monitor expression of all nine genes whose inhibition resulted in changes in IL-6 production in response to LTA (the eight genes whose inhibition weakened the LTA response: Fbxo17, Bdkrb1, Blnk, Nkx6-1, Grif1, Rab33b, Akap13, Chrm1, and the one gene whose inhibition increased the response: Bdkrb2) (Figure S2). siRNA treatment results in a >2-fold inhibition of gene expression for each gene compared to nontargeting siRNA control, except for Nkx6-1 RNAi, which decreased Nkx6-1 expression 1.8-fold. Comparison of normalized expression levels (ΔCt values) in siRNA targeted cells compared to the nontargeting siRNA pool demonstrated a significant reduction in expression (unpaired two-tailed test P < 0.01) for all genes except for Nkx6-1.
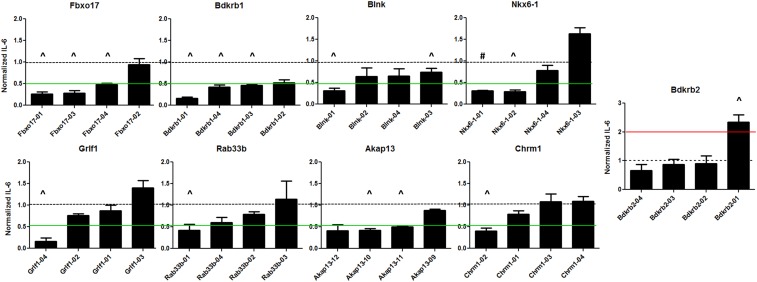
To confirm that the effects of these RNAi treatments were due to inhibition of the corresponding endogenous genes and not due to an off-target RNAi effect, we inhibited each of the nine genes whose inhibition resulted in changes in IL-6 production in response to LTA with four independent siRNA duplexes targeting each gene. At least two of the individual siRNA duplexes resulted in a more than twofold reduction in IL-6 production in response to LTA for four (Fbxo17, Bdkrb1, Blnk, and Nkx6-1) of the nine genes tested (Figure 4). This is consistent with the effect of these RNAi treatments being due to inhibition of that specific target gene. Taken together, these siRNA data support the potential role of Fbxo17, Bdkrb1, Blnk, and Nkx6-1 in the macrophage response to LTA.
Figure 4.
The effect of individual siRNA duplexes for nine candidate genes from the siRNA screen in RAW264.7 macrophages on LTA-induced IL-6 production. Four siRNA duplexes were transfected individually for each of the eight genes whose inhibition resulted in decreased IL-6 production and one gene whose inhibition resulted in higher IL-6 production in the screen presented in Figure 3. Means of four independent measurements with error bars representing SEMs are plotted; green lines indicate threshold for twofold reduction and red twofold increases in IL-6 response as a result of gene inhibition. #P < 0.001 and ^P < 0.01 by one-group two-tailed t-test testing for deviation from 1.
The effect of these four genes was not unique to the macrophage response to LTA. Inhibition of Fbxo17, Bdkrb1, Blnk, and Nkx6-1 using RNAi also greatly weakened IL-6 production induced by the TLR4 agonist LPS (Figure S3). Thus, these four genes are required for a robust innate immune response induced by both Gram positive (LTA) and Gram negative (LPS) bacterial stimuli.
Use of gene overexpression to verify the innate immune regulatory function of candidate genes
RNAi-mediated inhibition of Fbxo17, Bdkrb1, Blnk, and Nkx6-1 led to a weakened response to LTA. To further test the effects of these gene candidates, we overexpressed full-length cDNAs of each gene and monitored the effect using an innate immunity-responsive luciferase reporter. If overexpression induced a phenotype opposite to the RNAi-induced phenotype, that would support a role of that gene in innate immunity regulation. In contrast, lack of an overexpression phenotype is not necessarily indicative of gene function.
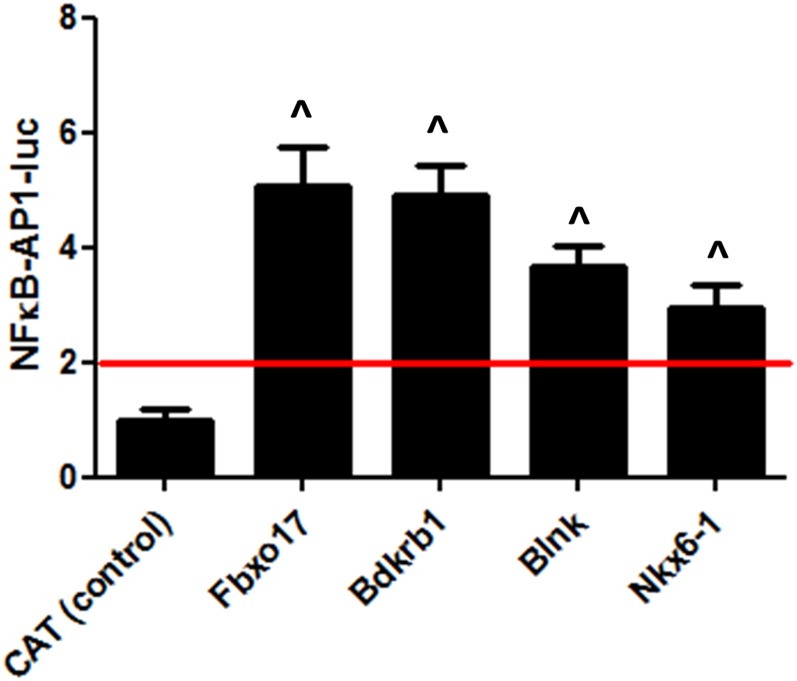
To determine whether overexpression of these four candidate genes led to a more pronounced innate immune response to LTA, we transiently overexpressed each of Fbxo17, Bdkrb1, Blnk, and Nkx6-1 using full-length cDNA clones under control of the CMV promoter, stimulated cells with LTA, and monitored innate immune activation using a NF-κB-AP1-luciferase reporter. As a negative control, cells were transfected with a plasmid overexpressing CAT, which should not alter innate immunity. Overexpression of all four candidate genes tested led to increased NF-кB-AP1 activity (Figure 5), a phenotype opposite to that caused by inhibition of these genes. Thus, these gain-of-function data provide evidence that the wild-type function of these four genes is to amplify the innate immune response induced by LTA.
Figure 5.
Effect of overexpression of four target genes in RAW264.7 cells on NF-κB activation. Plasmids containing full-length cDNAs for the indicated genes (or CAT negative control) cloned downstream of the CMV promoter were cotransfected with NF-kB-AP1-luc and SV40-rluc plasmids. Following transfection, cells were stimulated with 2.5 µg/ml LTA for 5 hr, and innate immune responsiveness was monitored by measuring luciferase production. Means of three independent measurements with error bars representing SEMs are plotted, and red line indicates threshold for twofold increase in IL-6 response as a result of gene overexpression. Compared to CAT (set to 1), ^P < 0.01 by unpaired two-group two-tailed t-test.
BMM Bdrkb1-deficient mice exhibit a weakened response to Gram negative stimuli
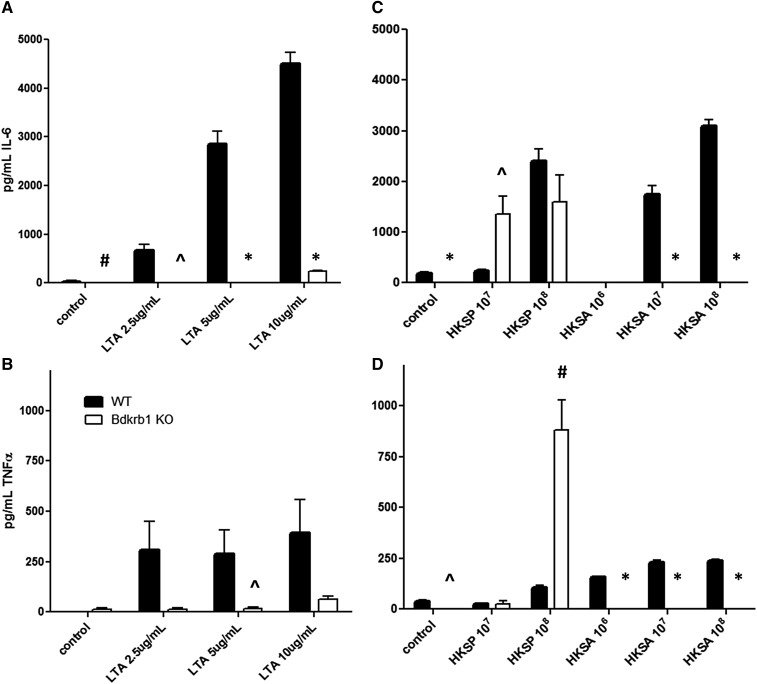
To further verify some of the results from our genetic studies and elucidate the role of Bdkrb1 in the macrophage response to Gram positive bacteria, we exposed BMMs from Bdkrb1-deficient mice (Pesquero et al. 2000) to LTA, HKSP, or HKSA, and monitored resulting inflammatory cytokine production. Bdkrb1-deficient macrophages produce little or no IL-6 or TNF-α when challenged with LTA (for 5 hr) compared to control macrophages from wild-type mice (Figure 6, A and B). These data are consistent with the RNAi and overexpression studies, although complete knockout of Bdrkb1, as expected, induced a much stronger phenotype than RNAi-mediated partial knockdown.
Figure 6.
Effect of Bdkrb1 gene deletion on the macrophage response to Gram positive bacterial stimulation. Depicted are IL-6 (A and C) and TNF-α (B and D) production by Bdkrb1-deficient or wild-type macrophages following stimulation with LTA (A and B), HSKP (C and D), or HKSA (C and D). In all panels, means of three to eight biological replicates with error bars representing SEMs are plotted. *P < 0.0001, #P < 0.001, and ^P < 0.01 by two-group unpaired two-tailed t-test between Bdkrb1-deficient and wild-type macrophages.
Eighteen hours post-HKSA stimulation, Bdkrb1-deficient macrophages also produce less IL-6 and TNF-α than wild-type macrophages (Figure 6, C and D). In contrast, HKSP stimulation of Bdkrb1-deficient macrophages resulted in similar or enhanced cytokine production compared to wild-type control macrophages (Figure 6, C and D), demonstrating the complexity of the response. These data further support the importance of Bdkrb1 in macrophages response to Gram positive bacterial stimulation but also underscore the complexity of this response given the differences in response to HKSP and HKSA.
Discussion
Using a combination of WGA mapping in inbred strains of mice, pathway analysis, and analysis of gene function in cell lines, we have identified specific genes and loci that regulate the macrophage response to LTA, a component of the cell wall of Gram positive bacteria. Four genes (Bdkrb1, Blnk, Fbxo17, and Nkx6-1) emerged from our analysis as high priority candidates due to changes in macrophage response to LTA as a result of their inhibition and overexpression in cell culture. Moreover, we validated the loss- and gain-of-function strategies to identify novel regulators of the response to LTA by demonstrating that Bdkrb1-deficient macrophages were less responsive to LTA and HKSA. Interestingly, all four genes identified in this study also regulated the response to the Gram negative bacterial component LPS, suggesting that these genes may have a more general role in TLR signaling.
Bradykinin 1 receptor (Bdkrb1) has a well-established role in the regulation of inflammation, particularly in the brain (Albert-Weissenberger et al. 2014), cardiovascular system (Couture et al. 2014), and the lung (Yang et al. 2014). Bradykinin receptor genes encode two G-protein-coupled receptors, inducible BDKRB1, and constitutive BDKRB2, that respond to endogenous kinin ligands as well as cytokines via the NF-κB pathway in the case of inducible BDKRB1 (Couture et al. 2014). Both bradykinin receptors were identified in the TNF-α response to LTA in our mapping study. Bdkrb1 gene inhibition led to decreased and Bdkrb2 gene inhibition to increased cytokine production in response to LTA stimulation in cultured macrophages, consistent with the complementary roles of these two receptors (Couture et al. 2014). Our study is the first to demonstrate a role for kinin receptors in the response to Gram positive bacterial stimulation. Interestingly, Bdkrb1-deficient macrophages produced less TNF-α in response to LTA and HKSA but more in response to HKSP, suggesting that other PAMPs may be important in this response.
The Blnk gene (also known as BASH or SLP-65 in mice) (Hayashi et al. 2000) has not been implicated in regulating innate immunity previously, although it is known to regulate the immune system. Blnk encodes a cytoplasmic linker protein that plays a critical role in B-cell development (Jumaa et al. 1999; Pappu et al. 1999). This protein mediates IL-10 production in regulatory B-cells and thus limits the allergic and autoimmune response (Jin et al. 2013). It is expressed by macrophages (Bonilla et al. 2000) and plasmacytoid dendritic cells (pDCs) (Marafioti et al. 2008). Blnk was identified in the TNF-α response to LTA in our mapping study, suggesting a potential role in host response to Gram positive infection. Our RNAi and cDNA overexpression studies indicate that wild-type Blnk is a positive effector of the response to LTA in mouse macrophages.
Fbxo17 encodes a member of the F-box family of proteins, one of the four subunits of the ubiquitin protein ligase complex SCFs (SKP1-cullin-F-box), which function in phosphorylation-dependent ubiquitination, a crticial step in TLR signaling pathways (Takeuchi and Akira 2010). While other members of this gene family have been implicated in the regulation of immunity previously, Fbxo17 has not. Inhibition of another member of this protein family, FBXO3, led to a decreased cytokine response in multiple animal models, suggesting a critical role in the regulation of inflammation (Chen et al. 2013). Our earlier WGA mapping study in mice demonstrated that another member of this protein family, FBXO9, plays a role in the host response to LPS (Yang et al. 2009). Identification of Fbxo17 as associated with IL-6 production by macrophages in response to LTA in the present study provides further support for the role of this protein family in innate immunity and provides the first evidence of its potential importance in the response to Gram positive organisms. Our studies indicate that wild-type Blnk is a positive effector of the response to LTA in mouse macrophages.
Nkx6-1 is a member of the NK homeobox transcription factor gene family that plays an important role in development. Nkx6-1 is specifically required for the development of β-cells in the pancreas (Inoue et al. 1997), but it has no known role in immunity and inflammation. Therefore, our data demonstrating that Nkx6-1 is associated with TNF-α response of macrophages to LTA provide the first evidence for its potential role in innate immunity. Given its role in the development, it is possible that this transcription factor plays a role in the development of the innate immune system, but the pathway and specificity of response to LTA require further investigation. Our studies do demonstrate that Nkx6-1 is a positive regulator of the response induced by LTA in fully differentiated macrophages.
There are several limitations to our study. First, we only mapped the macrophage response to LTA in inbred strains of mice; additional mapping of response to other Gram positive PAMPs, such as peptidoglycan, would provide a more comprehensive view of the macrophage response to Gram positive bacteria. Second, by using a PAMP instead of heat-killed and live bacteria, we likely did not capture the complexity of macrophage response to multiple PAMPs. Third, our siRNA screen was performed only in one cell line; additional cell lines and primary cells would provide additional evidence for a functional role of specific gene candidates. An important future direction of our work will be to test the effect of Bdkrb1 deletion on in vivo susceptibility to S. aureus infection.
Gram positive bacteria are a common cause of pneumonia, and in addition, S. aureus infections are common in hospital settings. These major public health issues will require further study of both host and microbial genetics to identify better and more tailored treatments for patients who become actively infected with Gram positive pathogens. The current study provides important insight into the host genetic basis of the innate immune response to Gram positive bacteria and provides candidate genes that warrant further study in model systems and human populations. We identified four innate immune response genes (Bdkrb1, Blnk, Fbxo17, and Nkx6-1) in our genetic studies that were validated in our RNAi and overexpression studies as genes that regulate the response to LTA. Additionally, we note that some of the other 71 genes identified in our genetic analysis may also contribute to Gram positive bacterial susceptibility, as negative siRNA results in one cell line do not completely rule out a gene’s function in the innate immune response to LTA.
Acknowledgments
This work was funded by National Institutes of Health grants P01-ES18181 (D.A.S. and I.V.Y.), R21-ES019256 (S.A.), and P01-AI060699 (P.B.M.).
Footnotes
Supplemental material is available online at www.genetics.org/lookup/suppl/doi:10.1534/genetics.115.185314/-/DC1.
Communicating editor: D. W. Threadgill
Literature Cited
- Abiola O., Angel J. M., Avner P., Bachmanov A. A., Belknap J. K., et al. , 2003. The nature and identification of quantitative trait loci: a community’s view. Nat. Rev. Genet. 4: 911–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert-Weissenberger C., Mencl S., Hopp S., Kleinschnitz C., Siren A. L., 2014. Role of the kallikrein-kinin system in traumatic brain injury. Front. Cell. Neurosci. 8: 345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alper S., Laws R., Lackford B., Boyd W. A., Dunlap P., et al. , 2008. Identification of innate immunity genes and pathways using a comparative genomics approach. Proc. Natl. Acad. Sci. USA 105: 7016–7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilla F. A., Fujita R. M., Pivniouk V. I., Chan A. C., Geha R. S., 2000. Adapter proteins SLP-76 and BLNK both are expressed by murine macrophages and are linked to signaling via Fcγ receptors I and II/III. Proc. Natl. Acad. Sci. USA 97: 1725–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer M. C., de Gans J., Heckenberg S. G., Zwinderman A. H., van der Poll T., et al. , 2009. Host genetic susceptibility to pneumococcal and meningococcal disease: a systematic review and meta-analysis. Lancet Infect. Dis. 9: 31–44. [DOI] [PubMed] [Google Scholar]
- Chen B. B., Coon T. A., Glasser J. R., McVerry B. J., Zhao J., et al. , 2013. A combinatorial F box protein directed pathway controls TRAF adaptor stability to regulate inflammation. Nat. Immunol. 14: 470–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couture R., Blaes N., Girolami J. P., 2014. Kinin receptors in vascular biology and pathology. Curr. Vasc. Pharmacol. 12: 223–248. [DOI] [PubMed] [Google Scholar]
- De Arras L., Guthrie B. S., Alper S., 2014a Using RNA-interference to investigate the innate immune response in mouse macrophages. J. Vis. Exp. 3: e51306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Arras L., Laws R., Leach S. M., Pontis K., Freedman J. H., et al. , 2014b Comparative genomics RNAi screen identifies Eftud2 as a novel regulator of innate immunity. Genetics 197: 485–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Botran R., Vt’vička V., 2001. Methods in Cell Immunology, CRC, Boca Raton, FL. [Google Scholar]
- Fukao T., Koyasu S., 2003. PI3K and negative regulation of TLR signaling. Trends Immunol. 24: 358–363. [DOI] [PubMed] [Google Scholar]
- Gingles N. A., Alexander J. E., Kadioglu A., Andrew P. W., Kerr A., et al. , 2001. Role of genetic resistance in invasive pneumococcal infection: identification and study of susceptibility and resistance in inbred mouse strains. Infect. Immun. 69: 426–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K., Nittono R., Okamoto N., Tsuji S., Hara Y., et al. , 2000. The B cell-restricted adaptor BASH is required for normal development and antigen receptor-mediated activation of B cells. Proc. Natl. Acad. Sci. USA 97: 2755–2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingsworth J. W., Whitehead G., Berman K. G., Tekippe E. M., Gilmour M. I., et al. , 2007. Genetic basis of murine antibacterial defense to streptococcal lung infection. Immunogenetics 59: 713–724. [DOI] [PubMed] [Google Scholar]
- Inoue H., Rudnick A., German M. S., Veile R., Donis-Keller H., et al. , 1997. Isolation, characterization, and chromosomal mapping of the human Nkx6.1 gene (NKX6A), a new pancreatic islet homeobox gene. Genomics 40: 367–370. [DOI] [PubMed] [Google Scholar]
- Jin G., Hamaguchi Y., Matsushita T., Hasegawa M., Le Huu D., et al. , 2013. B-cell linker protein expression contributes to controlling allergic and autoimmune diseases by mediating IL-10 production in regulatory B cells. J. Allergy Clin. Immunol. 131: 1674–1682. [DOI] [PubMed] [Google Scholar]
- Jumaa H., Wollscheid B., Mitterer M., Wienands J., Reth M., et al. , 1999. Abnormal development and function of B lymphocytes in mice deficient for the signaling adaptor protein SLP-65. Immunity 11: 547–554. [DOI] [PubMed] [Google Scholar]
- Kang H. M., Zaitlen N. A., Wade C. M., Kirby A., Heckerman D., et al. , 2008. Efficient control of population structure in model organism association mapping. Genetics 178: 1709–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby A., Kang H. M., Wade C. M., Cotsapas C., Kostem E., et al. , 2010. Fine mapping in 94 inbred mouse strains using a high-density haplotype resource. Genetics 185: 1081–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer A., Green J., Pollard J., Jr, Tugendreich S., 2014. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 30: 523–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Oza S., Hogan D., Perin J., Rudan I., et al. , 2015. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet 385: 430–440. [DOI] [PubMed] [Google Scholar]
- Marafioti T., Paterson J. C., Ballabio E., Reichard K. K., Tedoldi S., et al. , 2008. Novel markers of normal and neoplastic human plasmacytoid dendritic cells. Blood 111: 3778–3792. [DOI] [PubMed] [Google Scholar]
- Mukaida N., Shiroo M., Matsushima K., 1989. Genomic structure of the human monocyte-derived neutrophil chemotactic factor IL-8. J. Immunol. 143: 1366–1371. [PubMed] [Google Scholar]
- Pappu R., Cheng A. M., Li B., Gong Q., Chiu C., et al. , 1999. Requirement for B cell linker protein (BLNK) in B cell development. Science 286: 1949–1954. [DOI] [PubMed] [Google Scholar]
- Pesquero J. B., Araujo R. C., Heppenstall P. A., Stucky C. L., Silva J. A., Jr, et al. , 2000. Hypoalgesia and altered inflammatory responses in mice lacking kinin B1 receptors. Proc. Natl. Acad. Sci. USA 97: 8140–8145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla S. K., Rose W., Schrodi S. J., 2015. Complex host genetic susceptibility to Staphylococcus aureus infections. Trends Microbiol. 23: 529–536. [DOI] [PubMed] [Google Scholar]
- Takeuchi O., Akira S., 2010. Pattern recognition receptors and inflammation. Cell 140: 805–820. [DOI] [PubMed] [Google Scholar]
- Ting J. P., Willingham S. B., Bergstralh D. T., 2008. NLRs at the intersection of cell death and immunity. Nat. Rev. Immunol. 8: 372–379. [DOI] [PubMed] [Google Scholar]
- van der Poll T., Opal S. M., 2009. Pathogenesis, treatment, and prevention of pneumococcal pneumonia. Lancet 374: 1543–1556. [DOI] [PubMed] [Google Scholar]
- Weischenfeldt J., Porse B., 2008. Bone marrow-derived macrophages (BMM): isolation and applications. CSH Protoc. 2008: pdb.prot5080. [DOI] [PubMed] [Google Scholar]
- Yang I. V., Wade C. M., Kang H. M., Alper S., Rutledge H., et al. , 2009. Identification of novel genes that mediate innate immunity using inbred mice. Genetics 183: 1535–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang I. V., Alper S., Lackford B., Rutledge H., Warg L. A., et al. , 2011a Novel regulators of the systemic response to lipopolysaccharide. Am. J. Respir. Cell Mol. Biol. 45: 393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang I. V., Jiang W., Rutledge H. R., Lackford B., Warg L. A., et al. , 2011b Identification of novel innate immune genes by transcriptional profiling of macrophages stimulated with TLR ligands. Mol. Immunol. 48: 1886–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Zhang N., Lan F., Van Crombruggen K., Fang L., et al. , 2014. Transforming growth factor-beta 1 pathways in inflammatory airway diseases. Allergy 69: 699–707. [DOI] [PubMed] [Google Scholar]
- Zaas A. K., Liao G., Chien J. W., Weinberg C., Shore D., et al. , 2008. Plasminogen alleles influence susceptibility to invasive aspergillosis. PLoS Genet. 4: e1000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article.