Abstract
During the last two decades numerous research teams demonstrated that skeletal muscles function as an exercise-dependent endocrine organ secreting dozens of myokines. Variety of physiological and pathophysiological implications of skeletal muscle myokines secretion has been described; however, upstream signals and sensing mechanisms underlying this phenomenon remain poorly understood. It is well documented that in skeletal muscles intensive exercise triggers dissipation of transmembrane gradient of monovalent cations caused by permanent activation of voltage-gated Na+ and K+ channels. Recently, we demonstrated that sustained elevation of the [Na+]i/[K+]i ratio triggers expression of dozens ubiquitous genes including several canonical myokines, such as interleukin-6 and cyclooxygenase 2, in the presence of intra- and extracellular Ca2+ chelators. These data allowed us to suggest a novel [Na+]i/[K+]i-sensitive, Ca2+i-independent mechanism of excitation-transcription coupling which triggers myokine production. This pathway exists in parallel with canonical signaling mediated by Ca2+i, AMP-activated protein kinase and hypoxia-inducible factor 1α (HIF-1α). In our mini-review we briefly summarize data supporting this hypothesis as well as unresolved issues aiming to forthcoming studies.
Keywords: Myokines, Secretion, Skeletal muscle, Transcription, Translation
Skeletal muscles represent up to 40% of the total body mass and contain 50–75% of all body proteins. As a part of the musculoskeletal system it maintains posture and provides locomotion.1 During the last two decades it was shown that skeletal muscles also function as an exercise-dependent endocrine organ secreting dozens of cytokines, regulatory glycoproteins with molecular weights of 15–30 kDa2 called myokines by analogy with adipokines and hepatokines, i.e. proteins secreted by adipocytes and hepatocytes, respectively.3 Myokines exert auto-, para- or endocrine effects communicating with other organs, such as adipose tissue, liver, bone, and immune system.4, 5
In 1990th–2000th several investigations demonstrated that during exercise plasma interleukin-6 (IL-6) was transiently increased up to 100-fold.6, 7, 8, 9 Importantly, unlike sepsis-induced production of this cytokine the sharp increment of IL-6 evoked by exercise was not preceded by elevation of circulating tumor necrosis factor TNF-α. Pedersen and co-workers were the first who found that exercise does not affect IL-6 mRNA content in monocytes thus ruling out the possible implication of immune system cells.6 Keller and co-workers reported that both IL-6 mRNA and immunoreactive protein content are increased in human contracting skeletal muscle. They also found augmented IL-6 transcription rate in nuclei isolated from human muscle biopsies after the onset of exercise.10 Viewed collectively, these experiments demonstrated that myoblasts rather than other type of cells presented in skeletal muscle and neighboring tissues are the major source of IL-6.
Recent proteomics studies identified more than 500 proteins secreted by human and rodent skeletal muscle cells.11, 12, 13 Along with IL-6, the highest exercise-dependent up-regulation of transcription and secretion exhibited IL-7, IL-8, murine chemokine CXC ligand-1 (CXCL-1), leukemia inhibitory factor (LIF). Irisin, a recently discovered myokine, is suggested to mediate beneficial effect of exercise by inducing browning in adipose tissue.14 Contrary to above-listed myokines, sustained training resulted in attenuation of expression of few other peptides including myostatin.4, 5
Side-by-side with above-listed peptides, numerous research teams observed exercise-dependent production of prostaglandins (PGEs). Thus, in situ microdialysis of human skeletal muscle detected ∼5-fold increment of interstitial PGE2 concentration after 60 min of dynamic exercise.15 Importantly, exercise-induced PGE2 production was suppressed by cyclooxygenase (COX) inhibitors16 suggesting activation and/or de novo expression of this enzyme. At least two isoforms of COX have been identified, COX-1 and COX-2. COX-1 is considered as a constitutively expressed enzyme while COX-2 is induced by diverse cell type-specific stimuli.17 In humans exercise increases activity of both isoforms and selectively increases COX-2 mRNA and protein content in contracting skeletal muscle of humans.18, 19, 20
It should be stressed that the increment of plasma content of some myokines triggered by exercises might be caused by their release from cells distinct of skeletal muscle. Thus, along with skeletal muscle cells intense exercise augmented IL-8 production by peripheral blood mononuclear cells.21 Cocks and co-workers using quantitative immunofluorescence demonstrated that elevation of the content of endothelial nitric oxide synthase (eNOS) in the human vastus lateralis biopsy evoked by endurance and sprint interval training is caused by its elevation in microvasculature endothelial cells.22 Considering this, mouse skeletal muscle cell lines, C2C12 myoblasts, and primary human myotubes subjected to electrical pulse stimulation (EPS) are widely employed as an in vitro exercise model for the study of myokine production.23, 24, 25 Using this approach it was shown that 24 h exposure of human myotubes to EPS resulted in 183 differentially expressed transcripts with the highest secretion level of IL-6, IL-8, CXCL-1, and LIF.26
Here, we briefly summarized the data on the upstream intermediates of intracellular signaling involved in the exercise-dependent regulation of myokine production with emphasis on a novel mechanism of excitation-transcription triggered by elevation of intracellular [Na+]i/[K+]i ratio.27 Physiological and pathophysiological implications of myokines were considered in several comprehensive reviews.2, 28, 29, 30, 31, 32
Search for upstream intermediates of exercise-dependent myokine transcription
Intracellular Ca2+
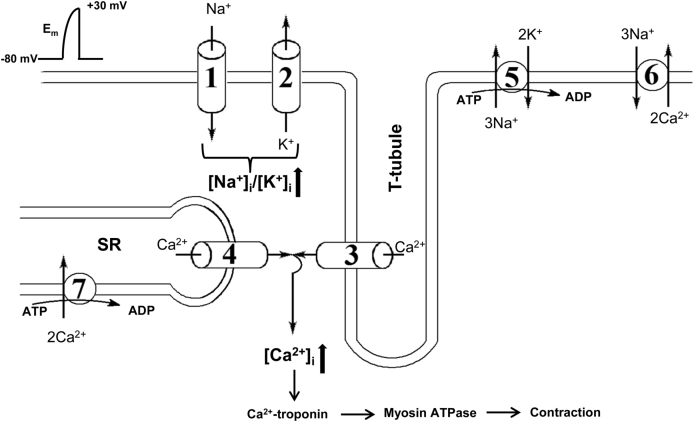
Contraction of skeletal muscle is induced by propagation of action potential along T-tubule evoked by opening of voltage-sensitive Na+ channels (Nav) and sarcolemma depolarization from the resting potential (Em) of −80 mV to +30 mV. Conformation transition of the skeletal muscle isoform of voltage-sensitive L-type Ca2+ channels (Cav), also known as dihydropyridine receptors (DHPR), leads to physical interaction with the skeletal muscle isoform of the ryanodine receptor Ca2+ release channels (RyR) (Fig. 1). Activation of RyR triggers Ca2+ release from the sarcoplasmic reticulum, elevation of intracellular Ca2+ concentration ([Ca2+]i), Ca2+i binding to troponin that, in turn, results in activation of myosin ATPase and shortening of sarcomeres.33, 34
Fig. 1.
Major ion transporters involved in skeletal muscle excitation-contraction coupling. 1 – voltage-gated Na+ channels; 2 – K+ channels; 3 – voltage-sensitive L-type Ca2+ channels; 4 – Ca2+ release channels; 5 – Na+,K+-ATPase, 6 – Na+/Ca2+ exchanger; 7 – Ca2+-ATPase; SR – sarcoplasmic reticulum; Em – electrical membrane potential. For more details, see text.
Besides triggering muscle contraction, elevation of [Ca2+]i from ∼0.1 to 1 μM affects the expression of hundreds of genes, i.e. phenomenon termed excitation-transcription coupling.35, 36, 37 It was shown that Ca2+i affects transcription via at least three signaling pathways. i) Elevation of [Ca2+]i promotes translocation of nuclear factor kappa-light-chain enhancer of activated B cells (NFκB) from cytosol to the nucleus. NFκB translocation is triggered by activation of Ca2+/calmodulin-sensitive protein kinase (CaMKI, II or III), leading to phosphorylation of the inhibitor of κB (IκB) by phosphorylated IκB kinase. Phosphorylated IκB dissociates from NFκB, which evokes its translocation into the nucleus. ii) [Ca2+]i elevation also leads to translocation of activated T-cells nuclear factor (NFAT) from cytosol to the nucleus. However, in contrast to NFκB, NFAT translocation is evoked by its dephosphorylation by the (Ca2+/calmodulin)-dependent phosphatase calcineurin.38 iii) The rise of cytosolic and nucleoplasmic Ca2+ concentrations lead to phosphorylation of cAMP response element-binding protein (CREB) by CaMKII and CaMKIV, respectively. Phosphorylated CREB and its co-activator CREB-binding protein regulate transcription via their binding to the (Ca2++cAMP)-response element (CRE) sequences of DNA (for comprehensive review, see Ref. 35, 39).
Both CRE and NFκB response elements were found in several myokines, including IL-62, 40 suggesting implication of Ca2+i-mediated signaling in exercise-induced myokine production. It should be noted, however, that NFκB signaling pathway is activated by contraction in rodents41 but not in humans.42, 43 In mice, IκB kinase had no effect on IL-6 transcription44 whereas in human skeletal muscle exercise did not affect the nuclear abundance with NFAT.42 Treatment of rat soleus muscle with Ca2+ ionophore ionomycin for one hour resulted in 5-fold elevation of IL-6 mRNA content.45 Later on, Whitham and co-workers detected that exposure of C2C12 myotubes to less selective Ca2+ ionophore A23187 sharply increased IL-6 transcription that was not affected by inhibitors of NFκB signaling.46 Using the same in vitro exercise model it was shown that extracellular Ca2+ chelator EGTA diminishes by 2-fold EPS-induced accumulation of CXL chemokines. It should be noted, however, that calcineurin inhibitor cyclosporine A did not affect the increment of these cytokines production.47 Recently, we reported that extracellular Ca2+ chelators sharply increase permeability of the plasma membrane for monovalent ions (for more details, see below).48 Thus, additional experiments should be performed to clarify the relative impact of Ca2+i-mediated signaling in the transcription of myokines as well as mechanisms of its modulation by Ca2+ ionophores and chelators.
Partial oxygen pressure
The drop of partial oxygen pressure (PO2) results in elevation of local blood flow via several mechanisms including NO-dependent relaxation of vascular smooth muscle cells triggered by ATP release from erythrocytes.49, 50 Because of this, oxygen delivery to skeletal muscle is subjected to strong feed-back regulation thus buffering the decrease of intracellular partial oxygen pressure (PO2i) caused by augmented exercise-induced increment of oxygen consumption and providing the tight linkage of oxygen demand and supply during exercise.51 Using 1H magnetic resonance spectrometry of myoglobin it was shown that in spite of this regulatory feedback PO2i is decreased during intensive exercise up to 5 fold52 with the prevalence in fast/glycolytic fibers as compared to slow/oxidative ones.53
Hypoxia-inducible factor 1alpha (HIF-1α), considered to be a major oxygen sensor, regulating gene expression in hypoxic conditions via interaction of HIF-1α/HIF-1β heterodimer with hypoxia response elements (HREs) in promoter/enhancer regions of the target genes. In normoxia, HIF-1α is hydroxylated by oxygen-dependent prolyl hydrolase that elicits its proteasomal degradation. In contrast, under hypoxic conditions, HIF-1α is translocated to the nucleus, where it forms HIF-1α/HIF-1β complex. The list of HIF-1-sensitive genes comprises Hif-1α per se, and others related to vasomotor control (nitric oxide synthase-2, adrenomedulin, endothelin-1), angiogenesis (vascular endothelial growth factor (VEGF) and its receptor FLT1), erythropoiesis and iron metabolism (erythropoietin, transferrin, transferrin receptor, ceruloplasmin), cell proliferation (IGF1, IGFBP1, TGFb), energy metabolism (glucose transporters GLUT1-GLUT3, phosphoenolpyruvate carboxylase, lactate dehydrogenase A, aldose, phosphoglucokinase-1, -L and -C, endolase, tyrosine hydroxylase and plasminogen activator inhibitor-1) (for review see,54, 55, 56, 57, 58).
It was shown that eccentric exercise increased the content of VEGF and endothelial nitric oxide synthase (eNOS) mRNA and protein in rat skeletal muscle as well as prompted the binding of HIF-1α to promoters of VEGF and eNOS genes thus indicating HIF-1α-mediated mechanism of this phenomenon.59 It should be noted, however, that hypoxic microvasculature rather than skeletal muscle per se might be the source of over-expression of these genes.22 Indeed, exercise-induced production of VEGF and eNOS seen in muscle biopsy was accompanied by elevation of capillary density.59 Importantly, our recent studies demonstrated that in vascular smooth muscle cells hypoxia-induced transcriptomic changes are at least partially triggered by HIF-1α-independent, [Na+]i/[K+]i-mediated, excitation-transcription coupling.60 The role of this novel mechanism of excitation-transcription coupling in myokine production by contracting skeletal muscle is considered below.
AMP-activated protein kinase
Independent of HIF-1α, hypoxia can affect gene expression via decline of intracellular ATP content that, in turn, leads to accumulation of AMP and activation of AMP-sensitive protein kinase (AMPK). AMPK is a phylogenetically conserved αβγ heterodimeric enzyme activated by phosphorylation the α subunit under elevation of AMP/ATP ratio. AMPK acts as a “metabolic master switch” and a cellular energy sensor whose activation results in increased catabolism and augmented ATP production.61 It might be proposed that intensive exercise is accompanied by elevation of intracellular AMP content due to high activity myosin ATPase, Na+,K+-ATPase and Ca2+-ATPase (Fig. 1) which together account for 90% of ATP use.62
The role of AMPK in exercise-induced myokine expression is supported by several observations: i) the increment of IL-6 mRNA during contraction is sharply attenuated in skeletal muscle with high content of glycogen as well as by glucose ingestion during exercise (for review, Ref. 2) suggesting the role of energy metabolism in myokine transcription regulation; ii) administration of AMPK agonist activated expression of dozens of metabolic genes in skeletal muscle and enhanced running endurance by almost 2-fold63; iii) both in human and experimental animals, exercises evoked fiber type specific activation of AMPK64, 65, 66; iv) exercise-induced IL-15 production was decreased in mice lacking both β1 and β2 AMPK subunits in skeletal muscle.67
It should be noted, however, that in contrast to myokines mentioned above contraction-mediated IL-6 expression was normal in muscle-specific AMPK α2 knock out mice.68 Importantly, because effective feedback regulation of metabolic and ATP consuming pathways ATP content in skeletal muscle during intensive exercise is decreasing by only 20–25%.62 Considering this it might be assumed that AMPK activation is caused by distal stimuli such as augmented production of NO69, 70 rather than by elevated AMP/ATP ratio per se. More recently, Benziane and co-workers demonstrated that AMPK stimulates rather than inhibits Na+,K+-ATPase activity71 thus providing negative regulation of Na+i/K+i-sensitive mechanism of excitation-transcription coupling considered in the next section.
Intracellular [Na+]i/[K+]i ratio
Sustained excitation of skeletal muscle results in dissipation of transmembrane gradient of monovalent cations due to Na+ influx via Nav that, in turn, leads to depolarization and K+ efflux via voltage-gated K+ channels (Kv), Ca2+-activated K+ channels (KCa) and voltage-insensitive inwardly rectifying K+ channels (Fig. 1).33 Using distinct experimental approaches it was shown that both in humans and in experimental animals intensive exercise contribute to increases of [Na+]i by 3–4-fold and decreases of [K+]i by up to 50% in skeletal muscles through activation of ion channels as well as through partial inactivation of the Na+,K+-ATPase. It was also demonstrated that K+ efflux from myotubes during exercise resulted in elevation of [K+] in skeletal muscle interstitial fluid from 4 to 5 to 11–15 mM. In humans, intensive dynamic and static exercises lead to up to 2-fold elevation of venous [K+] due to its release from skeletal muscle, i.e. a major source of intracellular K+ (for comprehensive reviews, Ref. 72, 73, 74, 75, 76).
These findings allow us to hypothesize that elevation of the [Na+]i/[K+]i ratio per se is sufficient to trigger myokine production. This hypothesis is based on several observations. First, employing Affymetrix-based technology, we detected up to 60-fold changes in the expression levels of 684, 737 and 1839 transcripts in HeLa cells, human umbilical vein endothelial cells (HUVEC) and rat aorta smooth muscle cells (RASMC), respectively, that were highly correlated in cells subjected to 3 h Na+,K+-ATPase inhibition with ouabain or K+-free medium. Among these Na+i/K+i-sensitive genes, 80 transcripts were common (ubiquitous) for all three of cell types.77 Importantly, almost half of ubiquitous Na+i,K+i-sensitive transcripts was represented by immediate response genes (IRG) and other genes involved in the regulation of transcription/translation which was ∼7-fold higher than in the total human genome. Second, we demonstrated that several myokines, including IL-6, as well as prostaglandin producing COX-2 are among the ubiquitous genes whose expression is strongly increased by elevation of the [Na+]i/[K+]i ratio.77 Recently, Broholm and co-workers reported that side-by-side with canonical myokines resistance exercise triggers profound accumulation of several IRG in human skeletal muscle biopsies including ∼4-fold elevation of JUNB.78 We noted that this gene is also subjected to sharp up-regulation by sustained elevation of the [Na+]i/[K+]i ratio in all cell types being under investigation.77 Third, several research teams reported that myokine secretion is accompanied by upstream activation of ERK1/2-, JNK- and NF-κB-dependent pathways.26, 46 These signaling pathways might be also activated by elevation of the [Na+]i/[K+]i ratio triggered by Na+,K+-ATPase inhibition.79, 80
In RASMC and HeLa cell lines inhibition of the Na+,K+-ATPase by ouabain resulted in expression of several IRG including 10- and 4-fold increment of immunoreactive c-Fos and c-Jun.81, 82 A 4-fold increment of c-Fos mRNA was detected in 30 min after ouabain addition. Within this time interval, [Na+]i was increased by ∼5-fold whereas [K+]i was decreased by only 10–15%. These results show that [Na+]i augmentation rather than [K+]i attenuation generates a signal that leads to c-Fos expression. Uddin and co-workers demonstrated that in human cytotrophoblasts IL-6 secretion might be triggered by ouabain and marinobufagenin,83 i.e. potent Na+,K+-ATPase inhibitors causing different structural changes in its a1-subunit.84 Viewed collectively, these data strongly suggest that these cardiotonic steroids trigger IL-6 expression via elevation the [Na+]i/[K+]i ratio rather than Na+i/K+i-independent signaling pathways.
To examine relative contribution of Ca2+i-mediated and -independent signaling, we compared transcriptomic changes triggered by elevation of the [Na+]i/[K+]i ratio in control and Ca2+-depleted cells. Surprisingly, Ca2+-depletion increased rather than decreased the number of ubiquitous and cell-type specific Na+i/K+i-sensitive genes.77 Among the ubiquitous Na+i/K+i-sensitive genes up-regulated independently of the presence of Ca2+ chelators, we found canonical myokine IL-6 as well as JUNB and COX-2. To further examine the role of Ca2+i, we studied action of Ca2+ chelators on intracellular monovalent ion handling. In vascular smooth muscle cells, addition of 50 μM EGTA to Ca2+-free medium led to ∼3-fold elevation of [Na+]i and 2-fold attenuation of [K+]i. Ca2+-depletion resulted in almost 3-fold elevation of the rate of 22Na and 86Rb influx measured in the presence of inhibitors of Na+,K+-ATPase and Na+,K+,2Cl− cotransport.48, 85 The augmented permeability for monovalent cations seen in Ca2+-depleted cells is probably caused by attenuation of extra-rather than intracellular Ca2+. Indeed, in contrast to extracellular Ca2+ chelator EGTA, neither the [Na+]i/[K+]i ratio nor permeability of VSMC for Na+ were affected by Ca2+-free medium lacking Ca2+ chelators as well as by addition of intracellular Ca2+ chelator BAPTA-AM alone.48 Importantly, the list of genes up regulated in Ca2+-depleted cells by more than 4-fold was abundant with genes whose expression was also affected by inhibition of the Na+,K+-ATPase in K+-free medium. In additional experiments, we found that dissipation of transmembrane gradients of Na+ and K+ in high-K+, low-Na+-medium abolished the increment of the [Na+]i/[K+]i ratio as well as sharp elevation of Atf3, Nr4a1 and Erg3 mRNA content triggered by 3-hr incubation of VSMC in Ca2+-free, EGTA containing medium.48 Thus, alternative approaches should be developed to clarify relative impact of Ca2+i-independent and Ca2+i-mediated mechanisms of excitation-transcription coupling in transcriptomic changes triggered by elevation of the [Na+]i/[K+]i ratio.
Does exercise affect myokine translation?
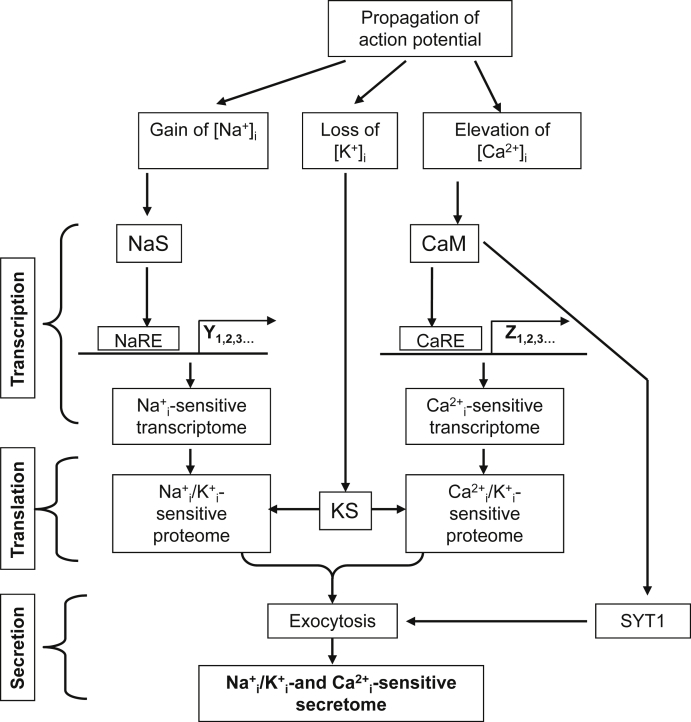
Comparative analysis of exercise-sensitive secretome of skeletal muscle cells revealed little correlation between mRNA and protein levels,12, 86 indicating pronounced modulation of myokine translation and/or secretion. Data considered below strongly suggest that besides of transcription elevation of the [Na+]i/[K+]i ratio seen in contracting skeletal muscles affects myokine translation. Almost 50 years ago, it was demonstrated that protein synthesis in prokaryotes is sharply inhibited in the absence of K+.87 Later on, the requirement of K+ for protein synthesis was detected in animal cells of different origins (for review, Ref. 88). In human fibroblasts, sustained Na+/K+-ATPase inhibition suppresses translation without any impact on transcription, ATP content and amino acid transport,89 indicating a direct influence of [K+]i on the protein synthesis machinery. In reticulocytes, globin contributes to more than 90% of total protein synthesis. In these cells, it was found that K+i depletion inhibits the elongation step without any impact on ribosome subunit assembly. Half-maximal activation of globin synthesis by reticulocyte lysate in medium containing 60, 90 and 125 mM Na+ was observed at [K+] of 15, 25 and 40 mM, respectively.90 These data indicate that elevation of [Na+]i diminishes the efficiency of protein synthesis regulation by K+i via attenuation of K+ interaction with its hypothetical sensor (Fig. 2). As alternative hypothesis it might be proposed that elevation of [Na+]i diminishes the transcription of elongation factors.91, 92, 93 This hypothesis is currently being examined in our laboratory.
Fig. 2.
Ionic mechanisms of excitation-contraction, excitation-transcription, excitation-translation and excitation-secretion coupling involved in exercise-induced myokine production by skeletal muscles. CaM – calmodulin and other intracellular Ca2+-sensors; CaRE – Ca2+-sensitive response elements in gene promoters; KS and NaS – intracellular K+ and Na+ sensors, respectively; NaRE – Na+-sensitive response elements in gene promoters; SYT1 – synaptotagmin 1. For more details, see text.
Does exercise affect myokine secretion?
It is generally accepted that myokine secretion is mediated by exocytosis.35 Exocytosis consists of multiple kinetically defined stages such as recruitment, targeting, tethering and docking of secretory vesicles with the sarcolemma, priming the fusion machinery and finally membrane fusion. The final stage is triggered by Ca2+ and involves several secretory vesicle proteins including Ca2+-sensing protein synaptotagmin 1 (SYT1).94 These data suggest that elevation of [Ca2+]i in contracting muscle may affect myokine secretion independently on regulation of their transcription and translation (Fig. 2). Indeed, using confocal and green fluorescent protein to visualize intracellular targets, Lauritzen and co-workers found that contraction stimulates IL-6 vesicle depletion from mouse muscle fibers in vivo.68
In addition to Ca2+, exocytosis may be regulated by intermediates of intracellular signaling such as cAMP-binding protein EPAC, guanine-exchange factors (Rap1-CEFs).95 Importantly, in vascular smooth muscle and endothelial cells, sustained inhibition of the Na+,K+-ATPase affected expression of dozen proteins involved in these signaling cascades (data prepared for publication). The role of elevated [Na+]i/[K+]i ratio in regulation of myokine secretion by altered expression of [Na+]i/[K+]i-sensitive genes involved in the secretory machinery remains unknown.
Search for intracellular monovalent ion sensors
Our model suggests that elevation of the [Na+]i/[K+]i ratio affects myokine transcription and translation independently via interaction of Na+i and K+i with their hypothetical sensors: NaS and KS, respectively (Fig. 2). The molecular origin of monovalent cation sensors distinct from ion transporters is still a mystery. This uncertainty is in contrast with rapid progress in the identification of Ca2+i sensors. It should be noted, however, that high-affinity binding sites, initially detected in parvalbumins and calmodulin, are formed by a highly conservative linear amino acid sequence consisting of 14 amino acid residues (the so-called “EF-hand” domain). This knowledge led to the rapid identification of more than 30 other Ca2+i sensors by the screening of cDNA libraries.96 In contrast, monovalent ion sensors are probably formed by 3D protein structures and recruit space-separated amino acid residues.88 In addition, high-affinity Ca2+i sensors are almost completely saturated at [Ca2+]i of 1 μM. This feature led to the identification of amino acid residues by 45Ca binding assay. Unlike Ca2+, monovalent cations affect cellular function in the millimolar range that complicates their identification by screening with radioisotopes.
It is generally accepted that transcription is under the control of transcription factors interacting with specific response elements. Considering this, we tried to find Na+ response element (NaRE) within c-Fos promoter. With the construct containing CRE and all other known transcription elements of the c-Fos promoter, we failed to detect any significant elevation of luciferase expression in HeLa cells subjected to 6-hr inhibition of Na+/K+-ATPase that contrasted with massive accumulation of endogenous c-Fos mRNA and immunoreactive protein in ouabain-treated HeLa cells.82
Several hypotheses could be proposed to explain negative results obtained in this study. (i) NaRE is located within introns or/and the c-Fos 3′-UTR. (ii) [Na+]i/[K+]i ratio elevation affects gene expression via epigenetic modification of the DNA, histones or nucleosome remodeling, i.e. regulatory mechanism having a major impact on diverse cellular functions.97 Importantly, the epigenetic mechanism of gene expression does not contribute to the regulation of L-luc transcription in the plasmid employed in our experiments.82 (iii) Increasing evidence indicates that gene activation or silencing is under the complex control of three-dimensional (3D) positioning of genetic materials and chromatin in the nuclear space (for review, Ref. 98). It may be proposed that augmented [Na+]i/[K+]i ratio affects gene transcription by changing the chromatin structure. (iv) Ono and co-workers reported that at the baseline level of [Ca2+]i (∼100 nM), Na+ interacts with calpain Ca2+-binding sites, and this enzyme functions as Na+i-dependent protease with K0.5 of 15 mM for Na+.99 Additional experiments should be performed to examine the role of Ca2+-binding proteins as potential monovalent cation sensors involved in transcriptomic and proteomic changes triggered by elevation of the [Na+]i/[K+]i ratio.
It should be underlined that side-by-side with transcription, translation and secretion stages myokines can affects their production by autocrine receptor-mediated mechanisms.100 Thus, IL-15 augments expression of peroxisome proliferator-activated receptor δ (PPARδ) and silent regulator of transcription-1 (SIRT1) via interaction with its receptor IL-15Rα,101 PGE2 triggers accumulation of IL-640, 102 whereas CXL-1 expression is regulated by IL-6.103
Conclusion and unresolved issues
During the last two decades it was shown that skeletal muscles function as an exercise-dependent endocrine organ secreting numerous myokines. In spite of diverse physiological and pathophysiological implications, upstream signals and sensing mechanisms underlying this phenomenon remain poorly understood. Data summarized in our mini-review show that side-by-side with canonical Ca2+i-AMPK- and HIF-1α-mediated signaling pathways, myokine production by contracting skeletal muscle may be mediated by the novel [Na+]i/[K+]i-sensitive, Ca2+i-independent mechanism of excitation-transcription coupling. Comparative analysis of HUVEC, RASMC and HeLa cells demonstrated that elevation of the [Na+]i/[K+]i ratio triggers cell type-specific transcriptomic changes via Ca2+i-mediated and -independent signaling.77 What is the relative impact of [Na+]i/[K+]i-sensitive genes in overall exercise-induced transcriptomic changes in fast and slow skeletal muscles? What is the relative impact on myokine production of [Na+]i/[K+]i-sensitive, Ca2+i-mediated and -independent mechanisms of excitation-transcription, excitation-translation and excitation-secretion coupling? What is the molecular origin of [Na+]i and [K+]i sensors involved in Ca2+i-independent regulation of gene transcription and translation? We address these questions to forthcoming studies.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgements
This work was supported by grants from the Canadian Institutes for Health Research (MOP-81392), the Kidney Foundation of Canada, the Heart and Stroke Foundation of Canada, Russian Foundation for Fundamental Research (15-04-00101) and the Russian Scientific Foundation (14-15-00006). Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR000430 (N.O.D). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Frontera W.R., Ochala J. Skeletal muscle: a brief review of structure and function. Calcif Tissue Int. 2015;96:183–195. doi: 10.1007/s00223-014-9915-y. [DOI] [PubMed] [Google Scholar]
- 2.Pedersen B.K., Febbraio M.A. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev. 2008;88:1379–1406. doi: 10.1152/physrev.90100.2007. [DOI] [PubMed] [Google Scholar]
- 3.Pedersen B.K., Akerstrom T.C., Nielson A.R., Fischer C.P. Role of myokines in exercise and metabolism. J Appl Phys. 2007;103:1093–1098. doi: 10.1152/japplphysiol.00080.2007. [DOI] [PubMed] [Google Scholar]
- 4.Pedersen B.K., Febbraio M.A. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol. 2012;8:457–465. doi: 10.1038/nrendo.2012.49. [DOI] [PubMed] [Google Scholar]
- 5.Pedersen B.K. Muscle as a secretory organ. Compr Physiol. 2013;3:1337–1362. doi: 10.1002/cphy.c120033. [DOI] [PubMed] [Google Scholar]
- 6.Ullum H., Haahr P.M., Diamant M., Palmo J., Halkjaer Kristensen J., Pedersen B.K. Bicycle exercise enhances plasma IL-6 but does not change Il-1α, Il-1β, Il-6 or TNF-αlpha mRNA in BMNC. J Appl Physiol. 1994;77:93–97. doi: 10.1152/jappl.1994.77.1.93. [DOI] [PubMed] [Google Scholar]
- 7.Febbraio M.A., Pedersen B.K. Muscle-derived interleikin-6: mechanisms for activation and possible biological roles. FASEB J. 2002;16:1335–1347. doi: 10.1096/fj.01-0876rev. [DOI] [PubMed] [Google Scholar]
- 8.Fisher C.P. Interleikin-6 in acute exercise and training: what is the biological relevance? Exerc Immunol Rev. 2006;12:6–33. [PubMed] [Google Scholar]
- 9.Pedersen B.K., Steensberg A., Schjerling P. Muscle-derived interleukin-6: possible biological effects. J Physiol. 2001;536:329–337. doi: 10.1111/j.1469-7793.2001.0329c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keller C., Steensberg A., Pilegaard H., Osada T., Saltin B., Pedersen B.K. Transcriptional activation of the IL-6 gene in human contracting skeletal muscle: influence of muscle glycogen content. FASEB J. 2001;15:2748–2750. doi: 10.1096/fj.01-0507fje. [DOI] [PubMed] [Google Scholar]
- 11.Hartwig S., Raschke S., Knebel B. Secretome profiling of primary human muscle cells. Biochim Biophys Acta. 2014;1844:1011–1047. doi: 10.1016/j.bbapap.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Henningsen J., Rigbolt K.T., Blagoev B., Pedersen B.K., Kratchmarova I. Dynamics of the skeletal muscle secretome during myoblast differentiation. Mol Cell Proteomics. 2010;9:2482–2496. doi: 10.1074/mcp.M110.002113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan C.Y., Masui O., Krakovska O. Identification of differentially regulated secretome components during skeletal myogenesis. Mol Cell Proteomics. 2011;10 doi: 10.1074/mcp.M110.004804. M110.004804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bostrom P.A., Wu J., Jedrychowski M.P. A PGC1-a-dependent myokine that drives brow-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karamouzis M., Landberg H., Skovgaard D., Bulow J., Kjaer M., Saltin B. In situ microdialysis of intramuscular prostaglandin and thromboxane in contracting skeletal muscle in humans. Acta Physiol Scand. 2001;171:71–76. doi: 10.1046/j.1365-201X.2001.00775.x. [DOI] [PubMed] [Google Scholar]
- 16.Trappe T.A., Fluckey J.D., White F., Lambert C.P., Evans W.J. Skeletal muscle PGE2alpha and PGE2 in response to eccentric resistance exercise: influence of ibuprofen and acetaminophen. J Clin Endocrin Metab. 2001;86:5067–5070. doi: 10.1210/jcem.86.10.7928. [DOI] [PubMed] [Google Scholar]
- 17.Vane J.R., Bakhle Y.S., Botting R.M. Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol. 1998;38:97–120. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- 18.Carroll C.C., O'Connor D.T., Steinmeyer R. The influence of acute resistant exercise on cyclooxygenase-1 and -2 activity and protein levels in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2013;305:R24–R30. doi: 10.1152/ajpregu.00593.2012. [DOI] [PubMed] [Google Scholar]
- 19.Weinheimer E.M., Jemiolo B., Carroll C.C. Resistance exercise and cyclooxygenase (COX) expression in human skeletal muscle: implications for COX-inhibiting drugs and protein synthesis. Am J Physiol Regul Integr Comp Physiol. 2007;292:R2241–R2248. doi: 10.1152/ajpregu.00718.2006. [DOI] [PubMed] [Google Scholar]
- 20.Buford T.W., Cooke M.B., Willoughby D.S. Resistance exercise-induced changes of inflammatory gene expression within human skeletal muscle. Eur J Appl Physiol. 2009;107:463–471. doi: 10.1007/s00421-009-1145-z. [DOI] [PubMed] [Google Scholar]
- 21.Kimsa M.C., Strzalka-Mrozik B., Kimsa M.W., Kochanska-Dziurowicz A., Zebrowska A., Mazurek U. Differential expression of inflammation-related genes after intense exercise. Prague Med Rep. 2014;115:24–32. doi: 10.14712/23362936.2014.3. [DOI] [PubMed] [Google Scholar]
- 22.Cocks M., Shaw C.S., Shepherd S.O. Sprint interval and endurance training are equally effective in increasing muscle microvascular density and eNOS content in sedentary males. J Physiol. 2013;591:641–656. doi: 10.1113/jphysiol.2012.239566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nedachi T., Fujita H., Kanzaki M. Contractile C2C12 myotube model for studying exercise-inducible responses in skeletal muscle. Am J Physiol Endocrinol Metab. 2008;295:E1191–E1204. doi: 10.1152/ajpendo.90280.2008. [DOI] [PubMed] [Google Scholar]
- 24.Lambernd S., Taube A., Schober A. Contractile activity of human skeletal muscle cells prevents insulin resistance by inhibiting pro-inflammatory signaling pathways. Diabetologia. 2012;55:1128–1139. doi: 10.1007/s00125-012-2454-z. [DOI] [PubMed] [Google Scholar]
- 25.Nikolic N., Bakke S.S., Kase E.T. Electrical pulse stimulation of cultured human skeletal muscle cells as an in vitro model of exercise. PLoS One. 2012;7:e33203. doi: 10.1371/journal.pone.0033203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scheler M., Irmler M., Lehr S. Cytokine response of primary human myotubes in an in vitro exercise model. Am J Physiol Cell Physiol. 2013;305:C877–C886. doi: 10.1152/ajpcell.00043.2013. [DOI] [PubMed] [Google Scholar]
- 27.Orlov S.N., Hamet P. Salt and gene expression: evidence for Na+i,K+i-mediated signaling pathways. Pflugers Arch. 2015;467:489–498. doi: 10.1007/s00424-014-1650-8. [DOI] [PubMed] [Google Scholar]
- 28.Iizuka K., Machida T., Hirafuji M. Skeletal muscle is an endocrine organ. J Pharmacol Sci. 2014;125:125–131. doi: 10.1254/jphs.14r02cp. [DOI] [PubMed] [Google Scholar]
- 29.Benatti F.B., Pedersen B.K. Exercise as an anti-inflammatory therapy for rheumatic diseases – myokine regulation. Nat Rev Rheumatol. 2015;11:86–97. doi: 10.1038/nrrheum.2014.193. [DOI] [PubMed] [Google Scholar]
- 30.Migliaccio S., Greco E.A., Wannenes F., Donini L.M., Lenzi A. Adipose, bone and muscle tissues as new endocrine organs: role of reciprocal regulation for osteoporosis and obesity development. Horm Mol Biol Clin Investig. 2014;17:39–51. doi: 10.1515/hmbci-2013-0070. [DOI] [PubMed] [Google Scholar]
- 31.Eckardt K., Gorgens S.W., Raschke S., Eckel J. Myokines in insulin resistance and type 2 diabetes. Diabetologia. 2014;57:1087–1099. doi: 10.1007/s00125-014-3224-x. [DOI] [PubMed] [Google Scholar]
- 32.Bostrom P.A., Fernandez-Real J.M., Mantzoros C. Irisin in humans: recent advances and questions for future research. Metabolism. 2014;63:178–180. doi: 10.1016/j.metabol.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 33.Jurkat-Rott K., Fauler M., Lehmann-Horn F. Ion channels and ion transporters of the transverse tubular system of skeletal muscle. J Muscle Res Cell Motil. 2006;27:275–290. doi: 10.1007/s10974-006-9088-z. [DOI] [PubMed] [Google Scholar]
- 34.Rebbeck R.T., Karunasekara Y., Board P.G., Beard N.A., Casarotto M.G., Dulhunty A.F. Skeletal muscle excitation-contraction coupling: who are the dancing partners? Int J Biochem Cell Biol. 2014;48:28–38. doi: 10.1016/j.biocel.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 35.Gundersen K. Excitation-transcription coupling in skeletal muscle: the molecular pathways of exercise. Biol Rev. 2011;86:564–600. doi: 10.1111/j.1469-185X.2010.00161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma H., Groth R.D., Wheeler D.G., Barrett C.F., Tsien R.W. Excitation-transcription coupling in sympathetic neurons and the molecular mechanism of its initiation. Neurosci Res. 2011;70:2–8. doi: 10.1016/j.neures.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Santana L.F. NFAT-dependent excitation-transcription coupling in heart. Circ Res. 2008;103:681–683. doi: 10.1161/CIRCRESAHA.108.185090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McDonald T.F., Pelzer S., Trautwein W., Pelzer D.J. Regulation and modulation of calcium channels in cardiac, skeletal, and smooth muscle cells. Physiol Rev. 1994;74:365–512. doi: 10.1152/physrev.1994.74.2.365. [DOI] [PubMed] [Google Scholar]
- 39.Hardingham G.E., Chawla S., Johnson C.M., Bading H. Distinct functions of nuclear and cytoplasmic calcium in the control of gene expression. Nature. 1997;385:260–265. doi: 10.1038/385260a0. [DOI] [PubMed] [Google Scholar]
- 40.Dendorfer U., Oettgen P., Libermann T.A. Multiple regulatory elements in the interleukin-6 gene mediate induction by prostaglandins, cyclic AMP, and lipopolysaccharide. Mol Cell Biol. 1994;14:4442–4454. doi: 10.1128/mcb.14.7.4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ji L.L., Gomez-Cabrera M.C., Steinhafel N., Vina J. Acute exercise activates nuclear factor (NF)-kappaB signaling pathway in rat skeletal muscle. FASEB J. 2004;18:1499–1506. doi: 10.1096/fj.04-1846com. [DOI] [PubMed] [Google Scholar]
- 42.Chan M.H., Carey A.L., Watt M.J., Febbraio M.A. Altering dietary nutrient intake that reduces glycogen content leads to phosphorylation of nuclear p38 MAPK in human skeletal muscle: association with IL-6 gene transcription during contraction. FASEB J. 2004;18:1785–1787. doi: 10.1096/fj.03-1039fje. [DOI] [PubMed] [Google Scholar]
- 43.Steensberg A., Keller C., Hillig T. Nitric oxide production is a proximal signaling event controlling exercise-induced mRNA expression in human skeletal muscle. FASEB J. 2007;21:2683–2694. doi: 10.1096/fj.06-7477com. [DOI] [PubMed] [Google Scholar]
- 44.Cai D., Frantz J.D., Tawa N.E. IKKbeta/NF-kappaB activation causes severe muscle wasting in mice. Cell. 2004;119:285–298. doi: 10.1016/j.cell.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 45.Holmes A.G., Watt M.J., Carey A.L., Febbraio M.A. Ionomycin, but not physiological doses of epinephrine, stimulates skeletal muscle interleukin-6 mRNA expression and protein release. Metabolism. 2004;53:1492–1495. doi: 10.1016/j.metabol.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 46.Whitham M., Chan M.H.S., Pal M. Contraction-induced interleukin-6 gene transcription in skeletal muscle is regulated by c-Jun terminal kinase/activator protein-1. J Biol Chem. 2012;287:10771–10779. doi: 10.1074/jbc.M111.310581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nedachi T., Hatakeyama H., Kono T., Sato M., Kanzaki M. Characterization of contraction-inducible CXC chemokines and their roles in C2C12 myocytes. Am J Physiol Endocrinol Metab. 2009;297:E866–E878. doi: 10.1152/ajpendo.00104.2009. [DOI] [PubMed] [Google Scholar]
- 48.Koltsova S.V., Tremblay J., Hamet P., Orlov S.N. Transcriptomic changes in Ca2+-depleted cells: role of elevated intracellular [Na+]/[K+] ratio. Cell Calcium. 2015;58:317–324. doi: 10.1016/j.ceca.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 49.Gonzalez-Alonso J. ATP as a mediator of erythrocyte-dependent regulation of skeletal muscle blood flow and oxygen delivery in humans. J Physiol. 2012;590:5001–5013. doi: 10.1113/jphysiol.2012.235002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Luneva O.G., Sidorenko S.V., Maksimov G.V., Grygorczyk R., Orlov S.N. Erythrocytes as regulators of blood vessel tone. Biochem (Mosc) Suppl Ser A Membr Cell Biol. 2015;9:161–171. [Google Scholar]
- 51.Joyner M.J., Casey D.P. Regulation of increased blood flow (hyperemia) to muscles during exercise: a hierarchy of competing physiological needs. Physiol Rev. 2015;95:549–601. doi: 10.1152/physrev.00035.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Richardson R.S., Newcomer S.C., Noyszewski E.A. Skeletal muscle intracellular PO2 assessed by myoglobin desaturation: response to graded exercise. J Appl Physiol. 2001;91:2679–2685. doi: 10.1152/jappl.2001.91.6.2679. [DOI] [PubMed] [Google Scholar]
- 53.McDonough P., Behnke B.J., Padilla D.J., Musch T.I., Poole D.C. Control of microvascular oxygen pressures during recovery in rat fast-twitch muscle of differing oxidative capacity. Exp Physiol. 2007;92:731–738. doi: 10.1113/expphysiol.2007.037721. [DOI] [PubMed] [Google Scholar]
- 54.Sharp F.R., Ran R., Lu A. Hypoxic preconditioning protects against ischemic brain injury. NeuroEx. 2004;1:26–35. doi: 10.1602/neurorx.1.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maxwell P.H., Wiesener M.S., Chang G.W. The tumor suppressor protein VHL targets hypoxia-inducible factor for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 56.Kallio P.J., Pongratz I., Gradin K., McGuire J., Poellinger L. Activation of hypoxia-inducible factor 1a: posttranslational regulation and conformational change by recruitment of the Arnt transcription factor. Proc Natl Acad Sci USA. 1997;94:5667–5672. doi: 10.1073/pnas.94.11.5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Semenza G.L., Jiang B.H., Leung S.W. Hypoxia response elements in the aldolase A, enolase 1, and lactate dehydrogenase A gene promoters contain essential binding sites for hypoxia-inducible factor 1. J Biol Chem. 1996;271:32529–32537. doi: 10.1074/jbc.271.51.32529. [DOI] [PubMed] [Google Scholar]
- 58.Ke Q., Costa M. Hypoxia-inducible factor-1 (HIF-1) Mol Pharmacol. 2006;70:1469–1480. doi: 10.1124/mol.106.027029. [DOI] [PubMed] [Google Scholar]
- 59.Rodriguez-Miguelez P., Lima-Cabello E., Martinez-Florez S., Almar M., Cuevas M.J., Gonzalez-Gallego J. Hypoxia-inducible factor-1 modulates the expression of vascular endothelial growth factor and endothelial nitric oxide synthase induced by eccentric exercise. J Appl Physiol. 2015;118:1075–1083. doi: 10.1152/japplphysiol.00780.2014. [DOI] [PubMed] [Google Scholar]
- 60.Koltsova S.V., Shilov B., Burulina J.G. Transcriptomic changes triggered by hypoxia: evidence for HIF-1α-independent, [Na+]i/[K+]i-mediated excitation-transcription coupling. PLoS One. 2014;9:e110597. doi: 10.1371/journal.pone.0110597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kahn B.B., Alquier T., Carling D., Hardie D.G. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 62.MacIntosh B.R., Holash R.J., Renaud J.-M. Skeletal muscle fatigue – regulation of excitation-contraction coupling to avoid metabolic catastrophe. J Cell Sci. 2012;125:2105–2114. doi: 10.1242/jcs.093674. [DOI] [PubMed] [Google Scholar]
- 63.Narkar V.A., Downes M., Yu R.T. AMPK and PPARPdelta agonists are exercise mimetics. Cell. 2008;134:405–415. doi: 10.1016/j.cell.2008.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee-Young R.S., Canny B.J., Myers D.E., McConell G.K. AMPK activation is fiber type specific in human skeletal muscle: effects of exercise and short-term exercise training. J Appl Physiol. 2009;107:283–289. doi: 10.1152/japplphysiol.91208.2008. [DOI] [PubMed] [Google Scholar]
- 65.Lee-Young R.S., Ayala J.F., Hunley C.F. Endothelial nitric oxide synthase is central to skeletal muscle metabolic regulation and enzymatic signaling during exercise in vivo. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1399–R1408. doi: 10.1152/ajpregu.00004.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Magnoni L.J., Palstra A.P., Planas J.P. Fueling the engine: induction of AMP-activated protein kinase in trout skeletal muscle by swimming. J Exp Biol. 2014;217:1649–1652. doi: 10.1242/jeb.099192. [DOI] [PubMed] [Google Scholar]
- 67.Crane J.D., MacNeil L.G., Lally J.S. Exercise-stimulated interleukin-15 is controlled by AMPK and regulates skin metabolism and aging. Aging Cell. 2015;14:625–634. doi: 10.1111/acel.12341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lauritzen H.P., Brandauer J., Schjerling P. Contraction and AICAR stimulate IL-6 vesicle depletion from skeletal muscle fibers in vivo. Diabetes. 2013;62:3081–3092. doi: 10.2337/db12-1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lira V.A., Soltow Q.A., Long J.H.D., Betters J.L., Sellman J.E., Criswell D.S. Nitric oxide increases GLUT4 expression and regulates AMPK signaling in skeletal muscle. Am J Physiol Endocrinol Metab. 2007;293:E1062–E1068. doi: 10.1152/ajpendo.00045.2007. [DOI] [PubMed] [Google Scholar]
- 70.Zhang J., Xie Z., Dong Y., Wang S., Liu C., Zou M.H. Identification of nitric oxide as an endogenous activator of the AMP-activated protein kinase in vascular endothelial cells. J Biol Chem. 2008;283:27461. doi: 10.1074/jbc.M802578200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 71.Benziane B., Bjornholm M., Pirkmajer S. Activation of AMP-activated protein kinase stimulates Na+,K+-ATPase activity in skeletal muscle cells. J Biol Chem. 2012;287:23451–23463. doi: 10.1074/jbc.M111.331926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sejersted O.M., Sjogaard G. Dynamics and consequences of potassium shifts in skeletal muscle and heart during exercise. Physiol Rev. 2000;80:1411–1481. doi: 10.1152/physrev.2000.80.4.1411. [DOI] [PubMed] [Google Scholar]
- 73.McDonough A.A., Thompson C.B., Youn J.H. Skeletal muscle regulates extracellular potassium. Am J Physiol Ren Physiol. 2002;282:F967–F974. doi: 10.1152/ajprenal.00360.2001. [DOI] [PubMed] [Google Scholar]
- 74.McKenna M.J., Bangsbo J., Renaud J.M. Muscle K+, Na+, and Cl- disturbances and Na+-K+ pump inactivation: implications for fatigue. J Appl Phys. 2008;104:288–295. doi: 10.1152/japplphysiol.01037.2007. [DOI] [PubMed] [Google Scholar]
- 75.Murphy K.T., Nielsen O.B., Clausen T. Analysis of exercise-induced Na+-K+ exchange in rat skeletal muscle. Exp Physiol. 2008;93:1249–1262. doi: 10.1113/expphysiol.2008.042457. [DOI] [PubMed] [Google Scholar]
- 76.Cairns S.P., Lindinger M.I. Do multiple ionic interactions contribute to skeletal muscle fatigue? J Physiol. 2008;586:4039–4054. doi: 10.1113/jphysiol.2008.155424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Koltsova S.V., Trushina Y., Haloui M. Ubiquitous [Na+]i/[K+]i-sensitive transcriptome in mammalian cells: evidence for Ca2+i-independent excitation-transcription coupling. PLoS One. 2012;7:e38032. doi: 10.1371/journal.pone.0038032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Broholm C., Laye M.J., Brandt C. LIF is a contraction-induced myokine stimulating human myocyte proliferation. J Appl Physiol. 2011;111:251–259. doi: 10.1152/japplphysiol.01399.2010. [DOI] [PubMed] [Google Scholar]
- 79.Schoner W., Scheiner-Bobis G. Endogenous and exogenous cardiac glycosides: their role in hypertension, salt metabolism, and cell growth. Am J Physiol Cell Physiol. 2007;293:C509–C536. doi: 10.1152/ajpcell.00098.2007. [DOI] [PubMed] [Google Scholar]
- 80.Akimova O.A., Tverskoi A.M., Smolyaninova L.V. Critical role of the a1-Na+,K+-ATPase subunit in insensitivity of rodent cells to cytotoxic action of ouabain. Apoptosis. 2015;20:1200–1210. doi: 10.1007/s10495-015-1144-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Taurin S., Dulin N.O., Pchejetski D. c-Fos expression in ouabain-treated vascular smooth muscle cells from rat aorta: evidence for an intracellular-sodium-mediated, calcium-independent mechanism. J Physiol. 2002;543:835–847. doi: 10.1113/jphysiol.2002.023259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Haloui M., Taurin S., Akimova O.A. Na+i-induced c-Fos expression is not mediated by activation of the 5′-promoter containing known transcriptional elements. FEBS J. 2007;274:3257–3267. doi: 10.1111/j.1742-4658.2007.05885.x. [DOI] [PubMed] [Google Scholar]
- 83.Uddin M., Horvat D., Glaser S.S., Mitchell B.M., Puschett J.B. Examination of the cellular mechanisms by which marinobufagenin inhibits cytoblast function. J Biol Chem. 2008;283:17946–17953. doi: 10.1074/jbc.M800958200. [DOI] [PubMed] [Google Scholar]
- 84.Klimanova E.A., Petrushenko I.Y., Mitkevich V.A. Binding of ouabain and marinobufagenin leads to different structural changes in Na,K-ATPase and depends on the enzyme conformation. FEBS Lett. 2015;589:2668–2674. doi: 10.1016/j.febslet.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 85.Orlov S.N., Aksentsev S.L., Kotelevtsev S.V. Extracellular calcium is required for the maintenance of plasma membrane integrity in nucleated cells. Cell Calcium. 2005;38:53–57. doi: 10.1016/j.ceca.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 86.Broholm C., Mortensen O.H., Nielsen S. Exercise induces expression of leukaemia inhibitory factor in human skeletal muscle. J Physiol. 2008;586:2195–2201. doi: 10.1113/jphysiol.2007.149781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lubin M., Ennis H.L. On the role of intracellular potassium in protein synthesis. Biochim Biophys Acta. 1964;80:614–631. doi: 10.1016/0926-6550(64)90306-8. [DOI] [PubMed] [Google Scholar]
- 88.Orlov S.N., Hamet P. Intracellular monovalent ions as second messengers. J Membr Biol. 2006;210:161–172. doi: 10.1007/s00232-006-0857-9. [DOI] [PubMed] [Google Scholar]
- 89.Ledbetter M.L.S., Lubin M. Control of protein synthesis in human fibroblasts by intracellular potassium. Exp Cell Res. 1977;105:223–236. doi: 10.1016/0014-4827(77)90120-3. [DOI] [PubMed] [Google Scholar]
- 90.Cahn F., Lubin M. Inhibition of elongation steps of protein synthesis at reduced potassium concentrations in reticulocytes and reticulocyte lysate. J Biol Chem. 1978;253:7798–7803. [PubMed] [Google Scholar]
- 91.Jennings M.D., Pavitt G.D. eIF5 is a dual function GAP and GDI for eukaryotic translational control. Small GTPases. 2010;1:118–123. doi: 10.4161/sgtp.1.2.13783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cao J., He L., Lin G. Cap-dependent translation initiation factor, eIF4E, is the target for ouabain-mediated inhibition of HIF-1a. Biochem Pharmacol. 2014;89:20–30. doi: 10.1016/j.bcp.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 93.Klann E., Dever T.E. Biochemical mechanisms for translation regulation in synaptic plasticity. Nat Rev Neurosci. 2004;5:931–942. doi: 10.1038/nrn1557. [DOI] [PubMed] [Google Scholar]
- 94.Messenger S.W., Falokowski M.A., Groblewski G.E. Ca2+-regulated secretory granule exocytosis in pancreatic and parotid acinar cells. Cell Calcium. 2014;55:369–375. doi: 10.1016/j.ceca.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tomes C.N. The proteins of exocytosis: lessons from the sperm model. Biochem J. 2015;465:359–370. doi: 10.1042/BJ20141169. [DOI] [PubMed] [Google Scholar]
- 96.Heizmann C.W., Hunziker W. Intracellular calcium-binding proteins: more sites than insights. TiBS. 1991;16:98–103. doi: 10.1016/0968-0004(91)90041-s. [DOI] [PubMed] [Google Scholar]
- 97.Graff J., Kim D., Dobbin M.M., Tsai L.-H. Epigenetic regulation of gene expression in physiological and pathological brain processes. Physiol Rev. 2011;91:603–649. doi: 10.1152/physrev.00012.2010. [DOI] [PubMed] [Google Scholar]
- 98.Lanctot C., Cheutin T., Cremer M., Cavalli G., Cremer T. Dynamic genome architecture in the nuclear space: regulation of gene expression in three dimensions. Nat Rev Genet. 2007;8:104–115. doi: 10.1038/nrg2041. [DOI] [PubMed] [Google Scholar]
- 99.Ono Y., Ojimam K., Torii F. Skeletal muscle-specific calpain is an intracellular Na+-dependent protease. J Biol Chem. 2010;285:22986–22998. doi: 10.1074/jbc.M110.126946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Peake J.M., Gatta P.D., Suzuki K., Nieman D.C. Cytokine expression and secretion by skeletal muscle cells: regulatory mechanisms and exercise effects. Exerc Immunol Rev. 2015;21:8–25. [PubMed] [Google Scholar]
- 101.Quinn L.S., Anderson B.G., Conner J.D., Wolden-Hanson T., Marcell T.J. Il-15 required for postexercise induction of the pro-oxidative mediators PPARd and SIRT1 in male mice. Endocrinology. 2014;155:143–155. doi: 10.1210/en.2013-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Standley R.A., Liu S.Z., Jemiolo B., Trappe S.W., Trappe T.A. Prostaglandin E2 induces transcription of skeletal muscle mass regulators interleukin-6 and muscle RING finger-1 in humans. Prostagl Leukot Essent Fat Acids. 2013;88:361–364. doi: 10.1016/j.plefa.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pedersen L., Pilegaard H., Hansen J. Exercise-induced liver chemokine expression is linked to muscle-derived interleukin-6 expression. J Physiol. 2011;589:1409–1420. doi: 10.1113/jphysiol.2010.200733. [DOI] [PMC free article] [PubMed] [Google Scholar]