Abstract
Objective
The aim of this study was to understand the relationships among depressive symptoms, cognition, and functional performance in a community-based sample of older adults.
Method
Older adults (N=885) from the Staying Keen in Later Life study completed tests of executive function, speed of processing, and memory. The Center for Epidemiological Depression Scale assessed depressive symptoms. The Timed Instrumental Activities of Daily Living Test assessed participants’ everyday functional performance.
Results
Depressive symptoms had significant associations with measures of executive function, speed of processing, memory, and everyday functional performance. Cognitive performance completely mediated the association between depressive symptoms and everyday function.
Discussion
Among community-dwelling older adults, depressive symptoms were associated with impaired cognition across multiple domains, which detrimentally affected everyday function. Healthcare providers should be aware of these associations to monitor and manage changes in depressive symptoms and cognitive performance and thereby potentially mitigate functional decline.
Keywords: depression, cognition, functional performance, instrumental activities of daily living
Depression is a major public health concern affecting approximately seven million adults 65 years and older (Steinman et al., 2007). The prevalence of depression among older adults is estimated at 15% to 19% (Cahoon, 2012). Depressive symptoms frequently co-occur with cognitive impairment in community-residing older adults (Arve, Tilvis, Lehtonen, Valvanne, & Sairanen, 1999; Butters et al., 2004; Ganguli, Snitz, Bilt, & Change, 2009; Rosenberg, Mielke, Xue, & Carlson, 2010). This co-occurrence of depression and cognitive impairment is estimated to double every five years after age 70, with about 25% of adults older than age 85 experiencing both depression and cognitive impairment (Arve et al., 1999). Depressive symptoms are associated with worsening cognitive impairment (e.g., Wilson et al., 2014) and can lead to the loss of everyday function and independence (Yen, Rebok, Gallo, Jones, & Tennestedt, 2011). As such, it is important to understand the relationship between depressive symptoms and cognitive performance in community-dwelling older adults and to examine whether this relationship can affect everyday functional performance.
While research has shown that depressive symptoms are associated with cognitive impairment (e.g., Wilson et al., 2014), the mechanisms of this relationship are still unexplained. Depressive symptoms could result from cognitive decline, though some studies have discounted this possibility (Bennett & Thomas, 2014; Carpenter et al., 2008; Wilson, Mendes de Leon, Bennett, Bienias, & Evans, 2004). On the other hand, depressive symptoms and cognitive decline could be manifestations of the same underlying neuropathology (Panza et al., 2010). Still yet, depressive symptoms could be an independent risk factor for cognitive decline (Kohler, van Boxtel, et al., 2010; Wilson et al., 2014). Studies have suggested that the negative thoughts associated with depression compete with (Christopher & MacDonald, 2005) or interfere with (Joormann & Gotlib, 2008) the resources needed to complete cognitive tasks.
Another unclear aspect of the relationship between depressive symptoms and cognitive function is whether any particular cognitive domain is more affected by depressive symptoms. Several studies have reported associations between depressive symptoms in older adults and poor performance on tests of executive function (EF), speed of processing (SOP), and memory. Some studies show that depressive symptoms among older adults are associated primarily with poor EF (Ganguli et al., 2009; Royall, Palmer, Chiodo, & Polk, 2011; Walter, Wolf, Spitzer, & Vasic, 2007). Other studies, however, have failed to show that depressive symptoms influence EF and have asserted that cognitive deficits among depressed older adults are evidenced primarily by slowed SOP (Bunce, Batterham, Mackinnon, & Christensen, 2012; Butters et al., 2004; Köhler, Thomas, Barnett, & O’Brien, 2010; Kohler, van Boxtel, et al., 2010). Similarly, depressive symptoms may be associated with worse memory (Kohler, Thomas, Barnett, & O’Brien, 2010; Pantzar et al., 2013). However, Sexton et al. (2012) noted that although memory was initially associated with depressive symptoms, the relationship disappeared when EF and SOP were added as covariates. Thus, the effects of depressive symptoms across domains of cognition are unclear.
Depressive symptoms are also associated with impaired functional status in older adults, which is most often measured through self-reported Instrumental Activities of Daily Living (IADL). Tomita and Burns (2013) found that the odds of self-reported functional difficulty were several times higher for young-old (65 to 74 years) community-residing participants with depressive symptoms. However, depressive symptoms did not influence functional difficulty in the oldest-old (over 85 years). Yamazaki, Nakano, Saito, and Yasumura (2012) who did not stratify their participants based on age, noted an association between depression, self-reported cognitive impairment, and lower self-reported IADL functioning in a community-based sample of 783 elders. In a study involving older adults with mild cognitive impairment, Brown et al. (2013) found that self-reported difficulties with IADL were associated with more depressive symptoms, memory problems, and slowed SOP, and that the SOP deficits fully mediated the relationship between EF and IADL and partially mediated the relationship between depression and self-reported IADL. None of these studies examined functional performance and only one (Brown et al., 2013) was carried out in the U.S.
To our knowledge, only two studies have measured depression, cognitive function, and everyday functional performance among community-residing older adults in the U.S. Performance of IADL, by definition, relies on cognitive function. Given evidence that depression precedes cognitive impairment (Devanand et al., 1996; Paterniti, Verdier-Taillefer, Dufouil, & Alperovitch, 2002), it is likely that the effects of depression on functional performance are mediated by cognition. Gallo et al. (2003) reported an association between depressive symptoms and performance-based measures of functioning (i.e., Everyday Problems Test and Observed Tasks of Daily Living) that was mediated by memory and reasoning abilities. Using these same two functional outcomes, Yen et al. (2011) also found an association between depressive symptoms and impaired everyday performance, which was mediated by memory and reasoning. SOP was associated with depressive symptoms, but not with everyday functional performance. However, these studies used performance-based functional measures that are most closely related to memory and reasoning (Jobe et al., 2001). In contrast, our study examined a different performance-based IADL measure that is timed and therefore may be more closely related to SOP (Owsley, McGwin, Sloane, Stalvey, & Wells, 2001). The impact of depressive symptoms on this performance-based measure of IADL has not been previously examined.
The aim of this study was to determine the association between depressive symptoms and cognitive performance across the domains of EF, SOP, and memory among community-residing older adults. We further sought to clarify whether depressive symptoms were associated with functional performance, and, if so, whether cognitive performance mediated this relationship. We hypothesized that depressive symptoms would be associated with cognitive and functional performance, primarily in SOP, and that SOP would mediate the association between depression and functional performance.
Method
Participants
The Staying Keen in Later Life (SKILL) study investigated interrelationships among sensory, cognitive and functional abilities of community-residing older adults from Bowling Green, KY, Birmingham, AL, and surrounding areas (Wood et al., 2005). Inclusion criteria were ≥ 60 years of age and far visual acuity (with correction) of 20/80 or better. Inclusion criteria were minimal with the goal of including a representative sample of community-dwelling older adults with a wide range of cognitive and functional abilities. Of the 1052 screened, 894 (85%) met the inclusion criteria and agreed to participate further (See Edwards, Wadley, Vance, Roenker, & Ball, 2005; Wood et al., 2005 for more details of the SKILL study).
We included SKILL participants who completed a measure of depressive symptoms, the Center for Epidemiology Studies Depression Scale (CES-D; n=885). Participants were mostly female (58%) and either White (89%) or Black (10.2%). The average age was 73.4 years (SD=6), and average education was “some vocational or technical training”. See Table 1 for more details.
Table 1.
Descriptive Statistics of Analytic Sample
| Variable | M (n) |
SD or (%) |
Minimum | Maximum |
|---|---|---|---|---|
| Participant Demographics | ||||
| Female | (513) | (58) | ||
| Caucasian | (788) | (89) | ||
| Black | (90) | (10) | ||
| Age (Years) | 73.47 (885) | 6.00 | 62 | 97.73 |
| Education | 6th grade | Doctorate | ||
| 11th grade or less | (71) | (8) | ||
| High school graduate | (217) | (33) | ||
| Technical or vocational training | (240) | (27) | ||
| College degree | (142) | (16) | ||
| Mini-Mental Status Exam (Score) | 28.18 (885) | 1.87 | 14 | 30 |
| CES-D (Score)+ | 7.63 (885) | 6.89 | 0 | 51 |
| Cognition Factor Composites | ||||
| Executive Function+ | 0 | 1 | −1.53 | 5.53 |
| Speed of Processing | 0 | 1 | −3.52 | 2.43 |
| Memory | 0 | 1 | −4.07 | 3.37 |
| Functional Performance | ||||
| Timed Instrumental Activities of Daily Living (z-scores composite)+ | −0.01 (872) | 0.59 | −0.66 | 4.66 |
Note: CES-D – Center for Epidemiologic Studies Depression Scale. Executive Function Composite: Stroop Color Word Test, Trail Making Test Part A, and Trail Making Test Part B are included in the factor analysis derived score. Memory Composite: Digit Span Forward, Spatial Span Forward, Hopkins Verbal Learning Test are included in the factor analysis derived score. Speed of Processing Composite: Useful Field of View, Pattern Comparison, Letter Comparison, and Digit Symbol Substitution are included in the factor analysis derived score.
Lower scores reflect better performance.
Measures
Depressive Symptoms
The CES-D (Radloff, 1977) is a 20-item self-report measure asking respondents to rate frequency of experiencing depressive symptoms in the past week on a 4-point scale from 0 (rarely or none of the time) to 3 (most of the time). Possible scores range from 0–60. Scores of 16 or higher indicate risk for clinical depression (Radloff, 1977); 14% of our sample scored within this range. The incidence of risk for clinical depression in the study sample approximates the prevalence rate of depression in the older adult population, which ranges from 15 to 19% in a community-dwelling population (Blazer, 2003; Cahoon, 2012).
Executive Function
A composite of EF was created from the Stroop and Trail Making Tests (TMT). The computerized Stroop Test measures the time to read color words (e.g., red, blue), identify color blocks, and name the color of ink in which color words appear (Spreen & Strauss, 1991). In TMT Part A, participants connect numbered circles in numerical order as fast as possible. In TMT Part B, participants draw a line connecting numbers and letters in sequential order as fast as possible (Spreen & Strauss, 1991).
Speed of Processing
A SOP composite comprised the Useful Field of View (UFOV), Letter and Pattern Comparison, and Digit Symbol Substitution Tests. The computerized UFOV measures cognitive SOP for visual attention tasks and summary scores reflect display speed for 75% accurate performance. Further details can be found elsewhere (Edwards, Vance, et al., 2005). Letter and Pattern Comparison, adapted from (Salthouse & Babcock, 1991), require participants to quickly compare sets of letters (3, 6, or 9 letters) or patterns (3, 6, or 9 lines). The Digit Symbol Substitution Test presents participants with an empty grid of boxes, with a number above each box, and a corresponding key pairing each number with a symbol (Wechsler, 1981). The score is the number of correct substitutions completed within 90s.
Memory
The memory composite included Wechsler Memory Scale–III Digit and Spatial Span Forward Tests (Wechsler, 1987) and the Hopkins Verbal Learning Test (Brandt, 1991). In Digit Span, participants listen to number sequences and then verbally recall the numbers in order. Spatial Span gauges spatial memory by requiring replication of touching a sequence of blocks in order. The number of correctly replicated sequences from Digit and Spatial Span was recorded. The Hopkins Verbal Learning Test consists of a list of 12 related words read aloud to the participants who then freely recalls the words. The average number of words recalled across three trials was recorded.
Everyday Function
Functional performance was assessed by the Timed IADL Test, which includes several tasks for the IADL domains of communication, finances, food, shopping, and medicine. Some examples of the tasks include making change, reading food labels, and reading directions on a prescription medicine bottle (for each of 3 bottles). For all tasks, the examiner used a digital stop watch to record the time taken (to one-tenth second) to perform the task and also determined whether the task was completed accurately, with minor error, with major errors, or whether the subject failed to complete the task for any reason. Timed IADL were scored as a composite z- score by both time and accuracy with lower scores reflecting better performance (Edwards, Vance, et al., 2005; Owsley et al., 2001; Owsley, Sloane, McGwin Jr, & Ball, 2002).
Procedure
Participants were first screened for eligibility in the SKILL study during a university lab testing visit lasting 1.5 hours. If the inclusion criteria were satisfied, participants completed a second lab assessment of cognitive and functional abilities (~2.5 hours).
Analyses
Descriptive analyses were performed. A composite score was computed for each cognitive domain, derived by factor analysis. Age, sex (female=0), and education were used as covariates in the analysis because they have been previously associated with depressive symptoms (e.g., Raji, Reyes-Ortiz, Kuo, Markides, & Ottenbacher, 2007).
Correlations among the variables were examined. Simple linear regression analyses, using pairwise deletion for missing values, were performed with composites of EF, SOP, memory, and Timed IADL as outcomes, and depressive symptoms and the covariates as independent variables. If depressive symptoms significantly predicted both cognitive and Timed IADL performance, we planned to examine whether cognitive performance mediated the ability of depressive symptoms to predict everyday functional performance. To do so, we conducted separate hierarchical linear regressions with depressive symptoms, each cognitive domain, and Timed IADL performance. Sobel’s test (1982) was used to determine the portion of the depression – Timed IADL relationship that was explained by each mediator. According to Preacher and Kelley (2011), this proportional measure may be an inefficient estimator and be unstable with small samples. Therefore, we also compared the indirect effects using the partially standardized indirect effect formula proposed by MacKinnon (2008), ab=ab/sd (y) where ab is the product of the paths from depressive symptoms to each cognitive domain and each cognitive domain to Timed IADL performance and sd (y) is the standard deviation of Timed IADL performance.
Results
From the original sample (N=894), participants who completed the CES-D (n=885) were included in analyses. The sample had a mean CES-D score of 7.6 (SD=6.9; range=0–51). See Table 1 for descriptives.
Correlations are reported in Table 2. Females and those with less education reported more depressive symptoms. Poor EF, SOP and memory performance were associated with more depressive symptoms. Age, sex, and education were added as covariates to the subsequent regression analyses. The addition of these covariates made the association between depressive symptoms and everyday functional performance significant in the multivariate models.
Table 2.
Correlations among Depressive Symptoms, Covariates, Cognition, and Everyday function
| Variable Name | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| 1. CES-D (score) | - | |||||||
| 2. Age (years) | −.04 | - | ||||||
| 3. Sex (female=0) | −.14** | .03 | - | |||||
| 4. Education (years) | −.15** | .03 | .14** | - | ||||
| 5. Executive Function Composite+ | .18** | .27** | .02 | −.25** | - | |||
| 6. Speed of Processing Composite | −.20** | −.39** | −.09** | .28** | −.70** | - | ||
| 7. Memory Composite | −.19** | −.20** | −.10** | .26** | −.54** | .57** | - | |
| 8. Timed Instrumental Activities of Daily Living Composite+ |
.05 | .37** | .13** | −.15** | .49** | −.42** | −.56** | - |
Note: CES-D – Center for Epidemiologic Studies Depression Scale;
Lower scores reflect better performance; Executive function, speed of processing, and memory composite scores derived from factor analyses
p < .05,
p < .001
Cognition
Results of hierarchical regressions showed that the demographic variables entered at Step 1 accounted for 14% of the variance in EF, 25% of the variance in SOP, and 12% of the variance in memory. Older age and less education were associated with worse cognitive performance. Male sex was associated with poorer SOP and memory. The addition of depressive symptoms at Step 2 added a significant 3% to the prediction of the EF, SOP, and memory composites. Depressive symptoms statistically contributed to cognitive performance after accounting for covariates (see Table 3).
Table 3.
Hierarchical Regression Models for Cognitive and Functional Performance Outcomes
| Variables | Model 1 | Model 2 | ||||||
|---|---|---|---|---|---|---|---|---|
| β |
95% CI (LB,UB) |
R2 | t | β | 95% CI (LB,UB) | R2 | t | |
| Executive Function Composite+ | ||||||||
| Age | .29 | (.04, .06) | 9.14** | .30 | (.04, .06) | 9.49** | ||
| Sex | .05 | (−.03, .23) | 1.57 | .07 | (.05,.31) | 2.25* | ||
| Education | −.26 | (−.12,−.07) | −8.02** | −.24 | (−.11, −.07) | −7.43** | ||
| CES-D+ | .17 | (.02,.03) | 5.17** | |||||
| R2 | .14 | .17 | ||||||
| ΔR2 | .03 | |||||||
| Speed of Processing Composite | ||||||||
| Age | −.39 | (−.07, −.06) | −13.37** | −.40 | (−.08, −.06) | −13.86** | ||
| Sex | −.12 | (−.36, −.12) | −4.00* | −.14 | (−.40, −.17) | −4.83** | ||
| Education | .30 | (.08, .13) | 8.61** | .28 | (.08, .12) | 9.43** | ||
| CES-D+ | −.19 | (−.04, −.02) | −6.36** | |||||
| R2 | .25 | .28 | ||||||
| ΔR2 | .03 | |||||||
| Memory Composite | ||||||||
| Age | −.21 | (−.05, −.02) | −6.55** | −.21 | (−.05, −.03) | −6.85** | ||
| Sex | −.13 | (−.39, −.14) | −4.10** | −.15 | (−.44, −.18) | −4.86** | ||
| Education | .27 | (.08, .13) | 8.62** | .25 | (.07, .12) | 7.91** | ||
| CES-D+ | −.18 | (−.04, −.02) | −5.79** | |||||
| R2 | .12 | .15 | ||||||
| ΔR2 | .03 | |||||||
| Functional Performance | ||||||||
| Timed Instrumental Activities of Daily Living Composite+ | ||||||||
| Age | .38 | (.03, .04) | 12.27** | .38 | (03, .04) | 12.35** | ||
| Sex | .14 | (.09, .24) | 4.47** | .15 | (.10, .25) | 4.70** | ||
| Education | −.17 | (−.05, −.03) | −5.56** | −.16 | (−.05, −.02) | −5.25** | ||
| CES-D+ | .06 | (.00, .01) | 2.02* | |||||
| R2 | .18 | .19 | ||||||
| ΔR2 | .01 | |||||||
Note: CES-D – Center for Epidemiologic Studies Depression Scale;
Lower scores reflect better performance; Executive function, speed of processing, and memory composite scores derived from factor analyses
p ≤ .05,
p < .001
Everyday Function
Hierarchical regression results showed that the demographic variables entered at Step 1 accounted for 18% of the variance in Timed IADL performance. Older age, male sex, and less education were associated with lower Timed IADL performance. The addition of depressive symptoms at Step 2 significantly increased the prediction of Timed IADL performance by 1%. Depressive symptoms statistically contributed to performance on Timed IADL after accounting for covariates (see Table 3).
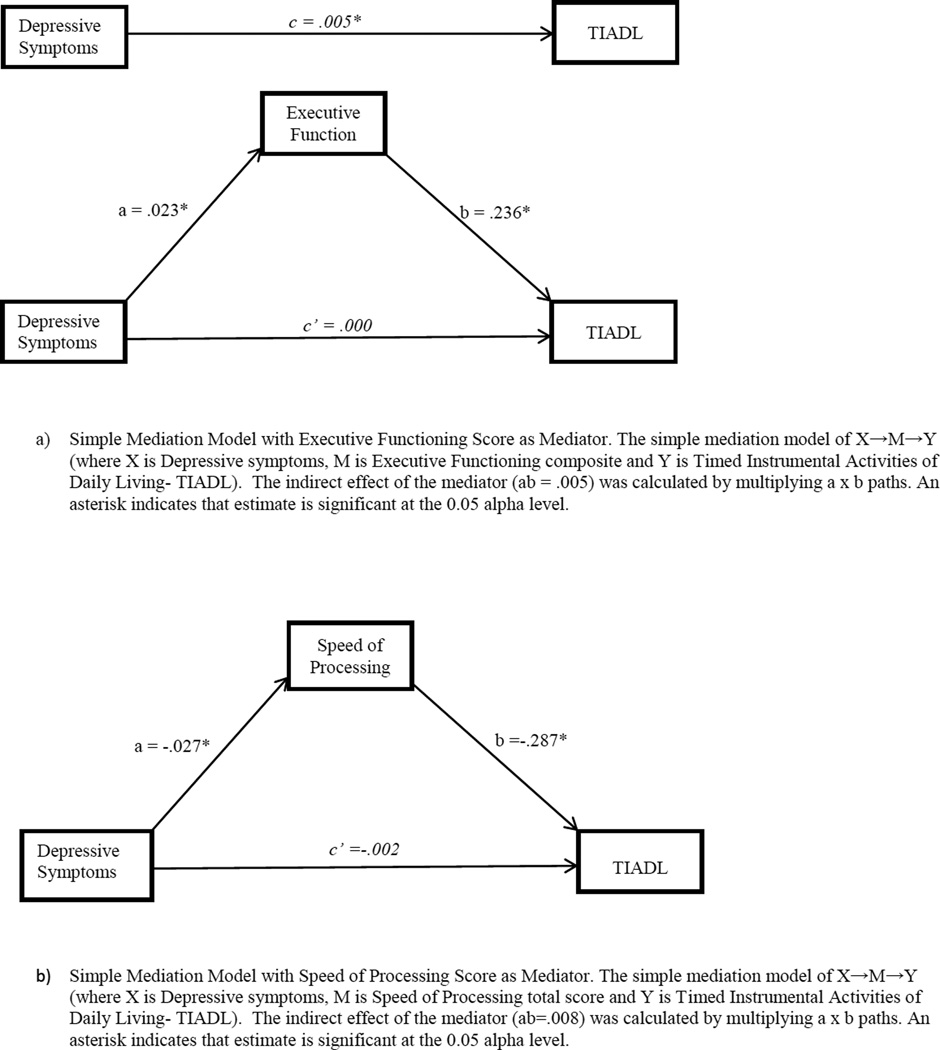
Mediation analyses
Since depressive symptoms significantly predicted everyday functional performance, we conducted meditation analyses (see Table 4 and Figure 1). The total effect of depressive symptoms on Timed IADL performance was significant, c=.005, t(832)=2.01, p=.05. Each of the cognitive composites (EF, SOP, or memory) were individually examined as a mediator in three separate models. In each model (See Figure 1), a represents the path between depressive symptoms and cognitive mediator, b indicates the path between cognitive mediator and Timed IADL performance, and c represents the independent relationship between depressive symptoms and Timed IADL performance. Depressive symptoms were significantly associated with each cognitive domain (a paths), and each cognitive domain was significantly associated with Timed IADL performance (b paths).
Table 4.
Hierarchical Regression Models for Depressive Symptoms, Cognition, and Functional Performance
| Variables | Model 1 | Model 2 | Model 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| β | R2 | t | β | R2 | t | β | R2 | t | |
| Age | .374 | 11.939** | .376 | 12.014** | .266 | 8.829* | |||
| Sex | .145 | 4.584** | .153 | 4.798** | .125 | 4.265** | |||
| Education | −.179 | −5.667** | −.171 | −5.360** | −.078 | −2.574** | |||
| CES-D+ | .063 | 1.960* | .000 | −.004 | |||||
| Executive Function Composite+ | .392 | 12.474** | |||||||
| R2 | .18 | .19 | .32 | ||||||
| ΔR2 | .01 | .13 | |||||||
| Age | .374 | 12.209** | .376 | 12.286** | .188 | 6.228* | |||
| Sex | .145 | 4.688** | .153 | 4.907** | .085 | 3.025** | |||
| Education | −.179 | −5.795** | −.171 | −5.482** | −.038 | −1.314 | |||
| CES-D+ | .063 | 2.005* | −.027 | −.930 | |||||
| Speed of Processing Composite | −.476 | −14.776** | |||||||
| R2 | .18 | .19 | .35 | ||||||
| ΔR2 | .01 | .16 | |||||||
| Age | .374 | 12.209** | .376 | 12.286** | .309 | 10.403** | |||
| Sex | .145 | 4.688** | .153 | 4.907** | .104 | 3.477** | |||
| Education | −.179 | −5.795** | −.171 | −5.482** | −.089 | −2.928** | |||
| CES-D+ | .063 | 2.005* | .004 | .141 | |||||
| Memory Composite | −.320 | −10.160** | |||||||
| R2 | .18 | .19 | .28 | ||||||
| ΔR2 | .01 | .09 | |||||||
Note: CES-D = Center for Epidemiologic Studies Depression Scale;
Lower scores reflect better performance; Executive function, speed of processing, and memory composite scores derived from factor analyses
p ≤ .05,
p < .001
Figure 1.
Simple Mediation Models
When examining EF as a mediator between depressive symptoms and Timed IADL, the model’s a and b paths were significant (95% CI, .199, .273) (see Figure 1). The direct effect of depressive symptoms on TIADL, after adjusting for the mediator, was not significant, c’<.001, t(833) = −.004, p = .997. Similarly, we found that when SOP was examined as a mediator, the model’s a and b paths were significant (95% CI -.325, −.248) (see Figure 1). The direct effect of depressive symptoms on Timed IADL, after adjusting for the mediators, was not significant, c’= −.002, t(871) = −.930, p= .352. Subsequently, the memory composite was examined as a mediator and the model’s a and b paths were significant (95% CI, −.230, −.156) (see Figure 1). After adjusting for the mediators, the direct effect of depressive symptoms on Timed IADL performance was not significant, c’< .001, t(871) = .141, p=.888. In each of the models, cognition completely mediated the relationship between depressive symptoms and Timed IADL performance such that there was no longer a significant association (c paths).
Sobel’s test for each of the cognitive domains indicated the relationship between depressive symptoms and Timed IADL performance was completely mediated by cognitive performance. Based on the partially standardized indirect effect calculation (MacKinnon, 2008), everyday functional performance as measured by Timed IADL scores are worse by .009, .013, and .009 for every one-unit increase in depressive symptoms indirectly through EF, SOP and memory, respectively. Effect sizes were thus similar for each cognitive domain.
Discussion
We examined the associations between depressive symptoms and cognitive performance across cognitive domains of EF, SOP, and memory among community-residing older adults. We further sought to clarify whether a relationship existed between depressive symptoms and functional performance, and if cognitive performance mediated such a relationship. After controlling for age, sex, and education, the cross-sectional analysis showed that depressive symptoms significantly accounted for 3% of the variance across each cognitive domain. Our results are similar to those of Ganguli et al. (2009) in that depressive symptoms among a community-residing population accounted for a small amount of the variance in EF and memory. Our study went further to examine SOP performance, with depressive symptoms similarly accounting for 3% of the variance.
Our hypothesis that depressive symptoms would be associated with cognitive and functional performance, primarily in SOP, was not supported. We did not find stronger associations between depressive symptoms and SOP, as demonstrated in prior studies (e.g., Butters et al., 2004; Ganguli et al., 2009). We did find that SOP mediates the relationship between depressive symptoms and functional performance as measured by Timed IADL, in support of our hypothesis. However, EF and memory also had similar significant mediation effects.
We examined the association between depressive symptoms and functional performance using Timed IADL (Owsley et al., 2001). Time is an important component of everyday performance given our society’s emphasis on efficiency. Depressive symptoms accounted for a significant but small portion of the variance in Timed IADL. Previous studies of depressive symptoms and functional performance have shown an association mediated by memory and reasoning, but not SOP (Gallo et al., 2003; Yen et al., 2011). However, the outcome measures did not depend on speed (Jobe et al., 2001). We found that EF, SOP, and memory mediated the relationship between depressive symptoms and Timed IADL performance. The relationship with SOP was expected, given the time constraints on performance. Memory is also important for performance on the Timed IADL tasks in that one has to recall information such as the items for which they are searching. EF also plays a role in everyday functioning in that one must plan and initiate appropriate behaviors to complete tasks.
To examine whether the significant effects of depressive symptoms on cognition and functional impairments could be attributed to those in the sample who were at-risk for clinical depression, subsample analyses included 123 participants with CES-D scores ≥ 16 (Radloff, 1977). Depressive symptoms were not associated with either cognitive or everyday function in this subsample. Thus, our findings cannot be attributed only to those with clinical depression. Our results indicate that in a community-dwelling sample of older adults, depressive symptoms affect cognitive functioning to a degree that also detrimentally affects everyday functional performance.
We acknowledge that this study has limitations. Secondary data has advantages in that it saves time, expense, and offers pre-established validity and reliability, but data are limited. Some cognitive tests may have overlapping domains, possibly blurring domain-specific differences in the effects of depressive symptoms. This problem is difficult to avoid (e.g., Clark et al., 2014). It is important to understand more about the EF and SOP domains in the context of depressive symptoms, and to try to disentangle which impairment is most prominent because if the impairment is one of EF, it may influence how well a patient responds to a particular treatment (Alexopoulos et al., 2005). Although the inclusion criteria were minimal to ensure that our sample included a wide-range of cognitive and functional abilities, as in most psychological research, our sample was likely more highly educated, healthier, and less depressed than the general population. Thus, the relationships between depressive symptoms, cognition, and everyday functional performance are likely underestimated in our sample. Although our sample included females and African-Americans in proportion to the US older adult population, participants were under-representative of the Hispanic-American population. Our study was cross-sectional and longitudinal analyses are needed to confirm findings.
Because these data were cross-sectional, it is not clear whether depressive symptoms impair cognition, or conversely if cognitive decline causes depression. The interplay of depressive symptoms and cognitive function is complex, and studies have offered varied explanations (Bennett & Thomas, 2014; Panza et al., 2009; Wilson et al., 2014). While some studies have discounted the hypothesis that depressive symptoms are a consequence of cognitive decline (Bennett & Thomas, 2014; Carpenter et al., 2008; Wilson et al., 2004), others have suggested that they are linked to the same underlying neuropathology (Panza et al., 2010). Still others have proposed that depressive symptoms could be an independent risk factor for cognitive decline (Kohler, van Boxtel, et al., 2010; Wilson et al., 2014). One recent longitudinal study (Wilson et al., 2014) found that depressive symptoms did not worsen as cognitive function declined, but that higher levels of depressive symptoms were associated with a faster rate of cognitive decline, after adjusting for neuropathology. This and other studies (Bennett & Thomas, 2014; Kohler, van Boxtel, et al., 2010) have suggested that interventions targeting depressive symptoms have the potential to help maintain cognitive health in old age. The present study further indicates that such interventions may preclude everyday functional declines.
The present study did not find differential effects of depression across cognitive domains, but did indicate varying effects of depression may be found on everyday functional performance depending on the tasks measured. Interestingly, there is evidence that cognitive SOP training can prevent depressive symptoms from worsening into clinically relevant depression (Wolinsky, Mahncke, et al., 2009; Wolinsky, Vander Weg, et al., 2009). Such training also improves Timed IADL performance (Ball et al., 2002; Edwards, Wadley, Myers, et al., 2005). However, performance on the UFOV test, the primary outcome for SOP training, did not mediate the training effect on depression, indicating that the training effect on depressive symptoms is mediated by brain functions or processes other than SOP.
In summary, our results show that depressive symptoms in a community-residing sample are significantly associated with impairment across the cognitive domains of EF, memory, and SOP, and negatively affect everyday functional performance. We did not find that these associations could be attributed to those who exceeded the clinical cut-point for depression. Implications are that low-levels of depressive symptoms in a community-based sample of older adults may significantly affect cognition and everyday function. The effect of depressive symptoms on everyday functional performance may be mediated by cognition. These effect sizes were small, and it is not certain if they are clinically meaningful. However, what defines a “clinically meaningful effect” varies based on numerous factors including the outcome of interest and the stakeholders (Keefe et al., 2013). Harvey and Keefe (2001) noted that there is currently no consensus on clinically-meaningful cognitive effect sizes. Maintained everyday function is vital to older adults’ quality of life and any change that reduces patients’ quality of life should be considered clinically meaningful (Keefe et al., 2013). Longitudinal studies suggest that depressive symptoms may be a precursor or risk factor for later dementia (e.g., Bunce et al., 2012) and are recommended to explore the trajectories of cognitive and functional decline related to depression and assess the value of depression treatment on these outcomes.
Acknowledgments
This research was supported in part by the National Institutes of Health/National Institute on Aging grant 5 R37 AG05739-16, Improvement of Visual Processing in Older Adults, Karlene K. Ball, principal investigator. The authors also wish to acknowledge Dr. Karlene K. Ball, who was awarded the MERIT grant to conduct the SKILL study, and the investigators of SKILL, Drs. Daniel Roenker, Lesley Ross, David Roth, Virginia Wadley, and David Vance.
Dr. Glenna Brewster was supported by the National Hartford Center of Gerontological Nursing Excellence.
Contributor Information
Glenna S. Brewster, College of Nursing, University of South Florida.
Lindsay Peterson, School of Aging Studies, University of South Florida.
Rosalyn Roker, School of Aging Studies, University of South Florida.
Michelle L. Ellis, School of Aging Studies, University of South Florida.
Jerri D. Edwards, School of Aging Studies, University of South Florida.
References
- Alexopoulos GS, Kiosses DN, Heo M, Murphy CF, Shanmugham B, Gunning-Dixon F. Executive dysfunction and the course of geriatric depression. Biological Psychiatry. 2005;58(3):204–210. doi: 10.1016/j.biopsych.2005.04.024. [DOI] [PubMed] [Google Scholar]
- Arve S, Tilvis RS, Lehtonen A, Valvanne J, Sairanen S. Coexistence of lowered mood and cognitive impairment of elderly people in five birth cohorts. Aging. 1999;11(2):90–95. [PubMed] [Google Scholar]
- Ball KK, Berch DB, Helmers KF, Jobe JB, Leveck MD, Marsiske M, Willis SL. Effects of cognitive training interventions with older adults: A randomized controlled trial. JAMA. 2002;288:2271–2281. doi: 10.1001/jama.288.18.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett S, Thomas AJ. Depression and dementia: Cause, consequence or coincidence? Maturitas. 2014;79(2):184–190. doi: 10.1016/j.maturitas.2014.05.009. [DOI] [PubMed] [Google Scholar]
- Blazer DG. Depression in late life: review and commentary. J Gerontol A Biol Sci Med Sci. 2003;58(3):249–265. doi: 10.1093/gerona/58.3.m249. [DOI] [PubMed] [Google Scholar]
- Brandt J. The Hopkins Verbal Learning Test: Development of a new memory test with six equivalent forms. Clinical Neuropsychology. 1991;5:125–142. [Google Scholar]
- Brown PJ, Liu XL, Sneed JR, Pimontel MA, Devanand DP, Roose SP. Speed of processing and depression affect function in older adults with mild cognitive impairment. Journal of the American Geriatrics Society. 2013;21(7):675–684. doi: 10.1016/j.jagp.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunce D, Batterham PJ, Mackinnon AJ, Christensen H. Depression, anxiety and cognition in community-dwelling adults aged 70 years and over. Journal of Psychiatric Research. 2012;46:1662–1666. doi: 10.1016/j.jpsychires.2012.08.023. [DOI] [PubMed] [Google Scholar]
- Butters MA, Whyte EM, Nebes RD, Begley AE, Dew MA, Mulsant BH, Bhalla R. The nature and determinants of neurpsychological functioning in late-life depression. Archives of General Psychiatry. 2004;61(6):587–595. doi: 10.1001/archpsyc.61.6.587. [DOI] [PubMed] [Google Scholar]
- Cahoon CG. Depression in older adults. American Journal of Nursing. 2012;112(11):22–30. doi: 10.1097/01.NAJ.0000422251.65212.4b. [DOI] [PubMed] [Google Scholar]
- Carpenter BD, Xiong C, Porensky EK, Lee MM, Brown PJ, Coats M, Morris JC. Reaction to a dementia diagnosis in individuals with Alzheimer’s disease and mild cognitive impairment. Journal of the American Geriatrics Society. 2008;36(3):405–412. doi: 10.1111/j.1532-5415.2007.01600.x. [DOI] [PubMed] [Google Scholar]
- Christopher G, MacDonald J. The impact of clinical depression on working memory. Cogn Neuropsychiatry. 2005;10(5):379–399. doi: 10.1080/13546800444000128. [DOI] [PubMed] [Google Scholar]
- Clark CA, Nelson JM, Garza J, Sheffield TD, Wiebe SA, Espy KA. Gaining control: Changing relations between executive control and processing speed and their relevance for mathematics achievement over course of the preschool period. Frontiers in Psychology. 2014;5:107. doi: 10.3389/fpsyg.2014.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devanand DP, Sano M, Tang MX, Taylor S, Gurland BJ, Wilder D, Mayeux R. Depressed mood and the incidence of Alzheimer’s disease in the elderly living in the community. Arch Gen Psychiatry. 1996;53(2):175–182. doi: 10.1001/archpsyc.1996.01830020093011. [DOI] [PubMed] [Google Scholar]
- Edwards JD, Vance DE, Wadley VG, Cissell GM, Roenker DL, Ball KK. Reliability and validity of useful field of view test scores as administered by personal computer. Journal of Clinical and Experimental Neuropsychology. 2005;27:529–543. doi: 10.1080/13803390490515432. [DOI] [PubMed] [Google Scholar]
- Edwards JD, Wadley VG, Myers R, Roenker DL, Cissell GM, Ball KK. Transfer of a speed of processing intervention to near and far cognitive functions. Gerontology. 2005;48:329–340. doi: 10.1159/000065259. [DOI] [PubMed] [Google Scholar]
- Edwards JD, Wadley VG, Vance DE, Roenker DL, Ball KK. The impact of speed of processing training on cognitive and everyday performance. Aging & Mental Health. 2005;9:262–271. doi: 10.1080/13607860412331336788. [DOI] [PubMed] [Google Scholar]
- Gallo JJ, Rebok GW, Tennsted S, Wadley VG, Horgas A, The Advanced Cognitive Training for, I., & Vital Elderly Study, I Linking depressive symptoms and functional disability in late life. Aging & Mental Health. 2003;7(6):469–480. doi: 10.1080/13607860310001594736. [DOI] [PubMed] [Google Scholar]
- Ganguli M, Snitz B, Bilt JV, Change CH. How much do depressive symptoms affect cognition at the population level? The Monogahela-Youghiogheny Health Aging Team (MYHAT) study. International Journal of Geriatric Psychiatry. 2009;24:1277–1284. doi: 10.1002/gps.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PD, Keefe RS. Studies of cognitive change in patients with schizophrenia following novel antipsychotic treatment. Am J Psychiatry. 2001;158(2):176–184. doi: 10.1176/appi.ajp.158.2.176. [DOI] [PubMed] [Google Scholar]
- Jobe JB, Smith DM, Ball K, Tennstedt SL, Marsiske M, Willis SL, Kleinman K. ACTIVE: A cognitive intervention trial to promote independence in older adults. Controlled Clinical Trials. 2001;22(4):453–479. doi: 10.1016/s0197-2456(01)00139-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J, Gotlib IH. Updating the contents of working memory in depression: interference from irrelevant negative material. J Abnorm Psychol. 2008;117(1):182–192. doi: 10.1037/0021-843X.117.1.182. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Kraemer HC, Epstein RS, Frank E, Haynes G, Laughren TP, Leon AC. Defining a clinically meaningful effect for the design and interpretation of randomized controlled trials. Innovations in clinical neuroscience. 2013;10(5–6 Suppl A):4S. [PMC free article] [PubMed] [Google Scholar]
- Kohler S, Thomas AJ, Barnett NA, O’Brien JT. The pattern and course of cognitive impairment in late-life depression. Psychological Medicine. 2010;40(4):591–602. doi: 10.1017/S0033291709990833. [DOI] [PubMed] [Google Scholar]
- Köhler S, Thomas AJ, Barnett NA, O’Brien JT. The pattern and course of cognitive impairment in late-life depression. Psychological Medicine. 2010;40(4):591–602. doi: 10.1017/S0033291709990833. [DOI] [PubMed] [Google Scholar]
- Kohler S, van Boxtel MPJ, van Os J, Thomas AJ, O’Brien JT, Jolles J, Allardyce J. Depressive symptoms and cognitive decline in community-dwelling older adults. Journal of the American Geriatrics Society. 2010;58:573–579. doi: 10.1111/j.1532-5415.2010.02807.x. [DOI] [PubMed] [Google Scholar]
- MacKinnon DP. Introduction to statistical mediation analysis. Mahwah, N.J.: Erlbaun; 2008. [Google Scholar]
- Owsley C, McGwin G, Sloane ME, Stalvey BT, Wells JW. Timed instrumental activities of daily living tasks: Relationship to visual function in older adults. Optometry and Vision Science. 2001;78(5):350–359. doi: 10.1097/00006324-200105000-00019. [DOI] [PubMed] [Google Scholar]
- Owsley C, Sloane M, McGwin G, Jr, Ball K. Timed Instrumental Activities of Daily Living Tasks: Relationship to Cognitive Function and Everyday Performance Assessments in Older Adults. Gerontology. 2002;48(4):254–265. doi: 10.1159/000058360. [DOI] [PubMed] [Google Scholar]
- Pantzar A, Lukka EJ, Atti AR, Fastbom J, Fratiglioni L, Backman L. Cognitive deficits in unipolar old-age depression: A population based-study. Psychological Medicine. 2013 doi: 10.1017/S0033291713001736. [DOI] [PubMed] [Google Scholar]
- Panza F, D’Introno A, Colacicco AM, Capurso C, Del Parigi A, Caselli R, Solfrizzi V. Temporal relationship between depressive symptoms and cognitive impairment: The Italian Longitudinal Study. Journal of Alzheimer’s Disease. 2009;17(4):899–911. doi: 10.3233/JAD-2009-1111. [DOI] [PubMed] [Google Scholar]
- Panza F, Frisardi V, Capurso C, D’Introno A, Colacicco AM, Imbimbo BP, Solfrizzi V. Late-life depression, mild cognitive impairment, and dementia: Possible continuum? American Journal of Geriatric Psychiatry. 2010;18(2):98–116. doi: 10.1097/JGP.0b013e3181b0fa13. [DOI] [PubMed] [Google Scholar]
- Paterniti S, Verdier-Taillefer MH, Dufouil C, Alperovitch A. Depressive symptoms and cognitive decline in elderly people. Longitudinal study. Br J Psychiatry. 2002;181:406–410. doi: 10.1192/bjp.181.5.406. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Kelley K. Effect size measures for mediation models: quantitative strategies for communicating indirect effects. Psychol Methods. 2011;16(2):93–115. doi: 10.1037/a0022658. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Raji M, Reyes-Ortiz C, Kuo Y, Markides K, Ottenbacher K. Depressive symptoms and cognitive changes in older Mexican Americans. Journals of Geriatric Psychiatry and Neurology. 2007;20(3):145–152. doi: 10.1177/0891988707303604. [DOI] [PubMed] [Google Scholar]
- Rosenberg PB, Mielke MM, Xue QL, Carlson MC. Depressive symptoms predict incident cognitive impairment in cognitive healthy older women. American Journal of Geriatric Psychiatry. 2010;18(3):204. doi: 10.1097/JGP.0b013e3181c53487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royall DR, Palmer R, Chiodo LK, Polk MJ. Depressive symptoms predict longitudinal change in executive control but not memory. International Journal of Geriatric Psychiatry. 2011;27:89–96. doi: 10.1002/gps.2697. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Babcock RL. Decomposing adult age differences in working memory. Developmental Psychology. 1991;27:763–776. [Google Scholar]
- Sexton CE, McDermott L, Kalu UG, Herrmann LL, Bradley KM, Allan CL, Ebmeier KP. Exploring the pattern and neural correlates of neuropsychological impairment in late-life depression. Psychological Medicine. 2012;42(06):1195–1202. doi: 10.1017/S0033291711002352. [DOI] [PubMed] [Google Scholar]
- Sobel ME. Asymptotic confidence intervals for indirect effects in structural equation models. Sociological methodology. 1982;13(1982):290–312. [Google Scholar]
- Spreen O, Strauss E. A compendium of neuropsychological tests: Administration norms and commentary. New York: Oxford University; 1991. [Google Scholar]
- Steinman LE, Frederick JT, Prohaska T, Satariano WA, Dornberg-Lee S, Fisher R, Presby K. Recommendations for treating depression in community-based older adults. American Journal of Preventive Medicine. 2007;33(3):175–181. doi: 10.1016/j.amepre.2007.04.034. [DOI] [PubMed] [Google Scholar]
- Tomita A, Burns JK. Depression, disability and functional status among community-dwelling older adults in South Africa: evidence from the first South African National Income Dynamics Study. Int J Geriatr Psychiatry. 2013;28(12):1270–1279. doi: 10.1002/gps.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter H, Wolf RC, Spitzer M, Vasic N. Increased left prefrontal activation in patients with unipolar depression: an event-related, parametric, performance-controlled fMRI study. J Affect Disord. 2007;101(1–3):175–185. doi: 10.1016/j.jad.2006.11.017. [DOI] [PubMed] [Google Scholar]
- Wechsler D. WAIS-R Manual. New York: The Psychological Corporation; 1981. [Google Scholar]
- Wechsler D. Wechsler Memory Scale-Revised Manual. San Antonio: The Psychological Corporation; 1987. [Google Scholar]
- Wilson RS, Capuano AW, Boyle PA, Hoganson GM, Hizel LP, Shah RC, Bennett DA. Clinical-pathologic study of depressive symptoms and cognitive decline in old age. Neurology. 2014;83(8):702–709. doi: 10.1212/WNL.0000000000000715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Mendes de Leon CF, Bennett DA, Bienias JL, Evans DA. Depressive symptoms and cognitive decline in a community population of older people. Journal of Neurology, Neurosurgery and Psychiatry. 2004;75(1):126–129. [PMC free article] [PubMed] [Google Scholar]
- Wolinsky FD, Mahncke HW, Vander Weg MW, Martin R, Unverzagt FW, Ball KK, Tennstedt SL. The ACTIVE cognitive training interventions and the onset of and recovery from suspected clinical depression. Journals of Gerontology: Psychological Sciences. 2009;5:577–585. doi: 10.1093/geronb/gbp061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolinsky FD, Vander Weg MW, Martin R, Unverzagt FW, Ball KK, Jones RN, Tennestedt SL. The effect of speed-of-processing training on depressive symptoms in ACTIVE. Journal of Gerontology: Medical Sciences. 2009;64A(4):468–472. doi: 10.1093/gerona/gln044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood KM, Edwards JD, Clay OJ, Wadley VG, Roenker DL, Ball KK. Sensory and cognitive factors influencing functional ability in older adults. Gerontology. 2005;51:131–141. doi: 10.1159/000082199. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Nakano K, Saito E, Yasumura S. Prediction of functional disability by depressive state among community-dwelling elderly in Japan: a prospective cohort study. Geriatr Gerontol Int. 2012;12(4):680–687. doi: 10.1111/j.1447-0594.2012.00841.x. [DOI] [PubMed] [Google Scholar]
- Yen Y, Rebok GW, Gallo JJ, Jones RN, Tennestedt SL. Depressive symptoms impaired everyday problem-solving ability through cognitive abilities in late life. American Journal of Geriatric Psychiatry. 2011;19(2):142–150. doi: 10.1097/JGP.0b013e3181e89894. [DOI] [PMC free article] [PubMed] [Google Scholar]